Abstract
Background
Mucins are glycoproteins that act as a selective molecular barrier and its alterations usually accompany the carcinogenesis.
Aim
To evaluate the transition of mucins in the grades of oral epithelial dysplasia (OED) and oral squamous cell carcinoma (OSCC) using histochemical stains.
Materials & Method
A total of 66 samples of variable grades of OED and OSCC and each section was stained with PAS, Alcian blue- PAS (AB-PAS) and Aldehyde fuschin – Alcian blue (AF-AB). Mucins pattern and intensity were examined at 5 randomly selected fields on 10x magnification.
Results
1. PAS stain – Predominantly OED and OSCC showed a diffuse pattern with a gradual decrease in intensity in OED and overall a weak intensity in OSCC. 2. AB-PAS stain – Neutral mucins showed gradual increase in its intensity in grades of OED and OSCC with no predominant pattern. The intensity for the acid mucins remains weak in all the grades of OED and OSCC with diffuse distribution, except in higher grades of OED and OSCC. 3. AF- AB stain - For sulphated mucins, in OED a focal and diffuse pattern was observed in OSCC with minimal intensity. The carboxylated mucin was absent in both.
Conclusion
Mucins undergo change in its pattern and intensity in varying grades of OED/OSCC. Although in GIT and other mucosa, the expression of altered mucin is a recognized factor, seldom research has been done in OED and OSCC. Thus, the present study could be the stepping stone in the exploration of mucinous alteration in OED and OSCC.
Keywords: Histochemistry, Mucin, Oral epithelial dysplasia, Oral squamous cell carcinoma, Stroma
1. Introduction
Worldwide oral squamous cell carcinoma (OSCC) is a major health problem and it is the most common oral cancer in India with a high incidence rate (30–40%).1 OSCC starts as an oral epithelial dysplasia (OED) and is characterized by an altered proliferation of squamous dysplastic cells of the epithelium. General and widespread changes occur in the distribution and quantity of the basement membrane during the transition from benign to carcinoma generating a local and distant invasion.2,3 These dysplastic tumor cells invade the stroma and are surrounded by an extracellular matrix (ECM) thereby producing reactive change in the stroma.4,5
Thus, the behaviour of carcinoma is not only dependent on the genetics of the tumor cells but also on the surrounding environment which is required for the tumour cell survival, growth, proliferation and a metastasis. This evolving concept is defined as tumour microenvironment. Considering that genetic and epigenetic factors are capable of affecting the entire tissue (epithelium and stroma), it would be logical to assume that carcinogenesis and its progression result from a defective response of both compartments.6
Over the past decade, several studies have shown that the stroma of the neoplastic tissues plays an active role in tumor progression. Concurrent with the conversion of non-diseased epithelial tissue to pre-cancerous epithelium to carcinoma, the stroma also changes from normal to primed to activated or tumor associated. The main focus of the various studies remains the cells and the fibrous components of stroma.4, 5, 6 The changes in the stroma of oral epithelial dysplasia (OED)/oral squamous cell carcinoma (OSCC) by various immunohistochemistry (IHC) and genetic studies have also been done, since the histochemical method which is an easy and inexpensive tool, is not yet explored.
Mucins, one of the main components of glycoprotein, are produced by the cells of the epithelium or the tissue that lines cavities and structure of the body. Deregulation of mucin production has provided an important link between inflammation and cancer.7 Moreover, carcinoma of the breast, prostate, lung and pancreas commonly overexpress mucins to exploit their role in promoting growth and survival but the expression of the mucins in OED and OSCC still remains to be defined.
Taking into the consideration the modifications occurring in the connective tissue mucins, the present study was taken to evaluate the alterations in the mucins in different grades of OED and OSCC and also to observe the staining intensity and patterns of glycoproteins, acids mucins, neutral mucins, sulphated mucins and carboxylated mucins using stain in different grades of OED and OSCC using different histochemical stains.
2. Materials and methods
This retrospective study was conducted wherein total of 66 histopathological confirmed cases of OED and OSCC were obtained from the archival formalin-fixed paraffin-embedded specimens. From these samples, 4 sections of 4μ were cut and each section was stained with H and E using the standard protocol and was OED and OSSC were graded by two independent observers using the WHO (1978) and Broder's system (1920) respectively. The results showed that the 32 OED cases consisted of 12 Mild, 9 Moderate and 11 Severe OED and 34 Primary OSCC cases were of 14 Well, 13 Moderate and 7 Poorly differentiated OSCC. After grading, each section of each sample were stained with PAS, Alcian blue- PAS (AB-PAS) stain and Aldehyde Fuschin – Alcian blue (AF-AB) stain using standard protocol. 4 cases of each OED and OSCC, wherein minor salivary glands was present were taken as control.
The nature of mucins was analysed by evaluating 5 randomly selected fields at 10 x magnifications. The assessment in OED was performed in the juxtaepithelial and deep connective tissue and in OSCC the assessment was performed in around and away from the tumour islands. Firstly, the pattern of the mucins was categorised as either focal or diffuse. Secondly, the predominant color exhibited by mucins was analysed. Thirdly, the intensity of staining was scored as 0 = absent, 1 = minimal, 2 = weak and 3 = bright.
3. Statistical analysis
Data were entered into the Statistical Package for Social Sciences (SPSS) version 21 for analysis. It was subjected to descriptive and inferential statistics to generate frequencies and percentages. For comparison of categorical variables between groups, the Chi-square test was used at 95% confidence interval.
4. Results and observations
Fig. 1, Fig. 2 represents the predominant color exhibited by the stromal mucin in PAS, PAS -AF and AF- AB of varying grades of OSCC and OED respectively.
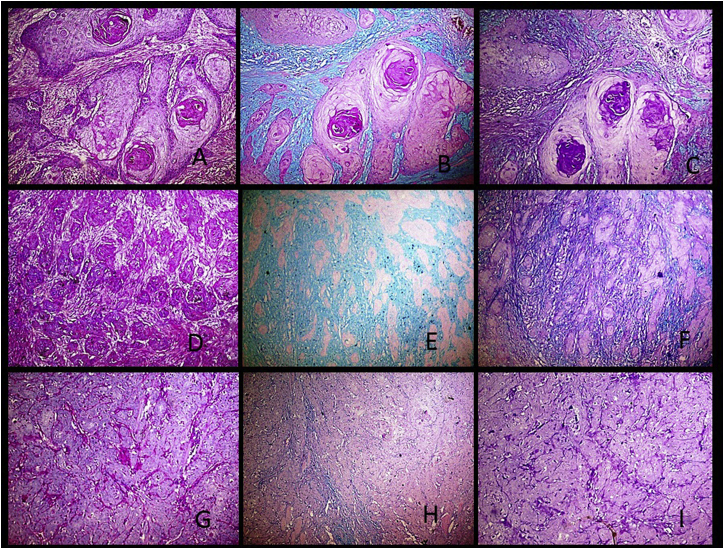
Fig. 1.
A-Photomicrograph of Well OSCC showing Weak PAS staining intensity (10X). B-Photomicrograph of Well OSCC showing bright Alcian blue staining intensity and minimal PAS stain (10X). C-Photomicrograph of Well OSCC showing weak stain intensity for sulphated mucins and weak staining for carboxylated mucins (10X). D-Photomicrograph of moderate OSCC showing weak intensity of PAS stain (10X). E-Photomicrograph of moderate OSCC showing weak intensity of Alcian blue stain and absence of the PAS stain (10X). F-Photomicrograph of moderate OSCC showing weak intensity of Alcian blue stain and weak intensity of the aldehyde fuschin stain (10X). G- Photomicrograph of poor OSCC showing bright intensity of PAS stain (10X). H-Photomicrograph of poor OSCC showing weak intensity of Alcian blue stain and bright intensity of the PAS stain (10X). I-Photomicrograph of poor OSCC showing bright staining intensity of the Aldehyde fuschin and minimal of Alcian blue stain (10X). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
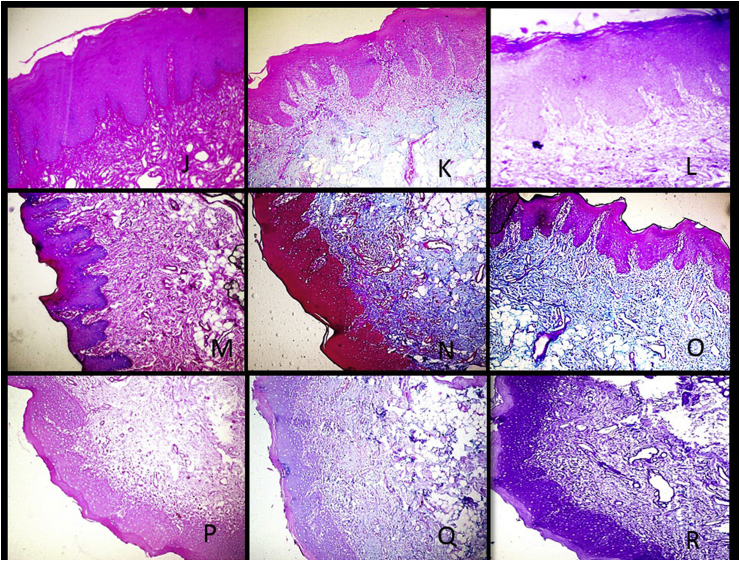
Fig. 2.
J-Photomicrograph of mild dysplasia showing bright PAS staining intensity (10X). K-Photomicrograph of mild dysplasia showing weak Alcian blue staining intensity and weak PAS stain (10X). L-Photomicrograph of mid dysplasia showing minimal to weak stain for sulphated mucins and absent staining for carboxylated mucins (10X). M-Photomicrograph of moderate dysplasia showing bright intensity of PAS stain (10X). N-Photomicrograph of moderate dysplasia showing bright intensity of Alcian blue stain and weak intensity of the PAS stain (10X). O-Photomicrograph of moderate dysplasia showing bright intensity of Alcian blue stain and weak intensity of the aldehyde fuschin stain (10X). P-Photomicrograph of severe dysplasia showing weak intensity of PAS stain (10X). Q-Photomicrograph of severe dysplasia showing weak intensity of Alcian blue stain and minimal intensity of the PAS stain (10X). R-Photomicrograph of severe dysplasia showing weak staining intensity of the Aldehyde fuschin and absence of Alcian blue stain (10X). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
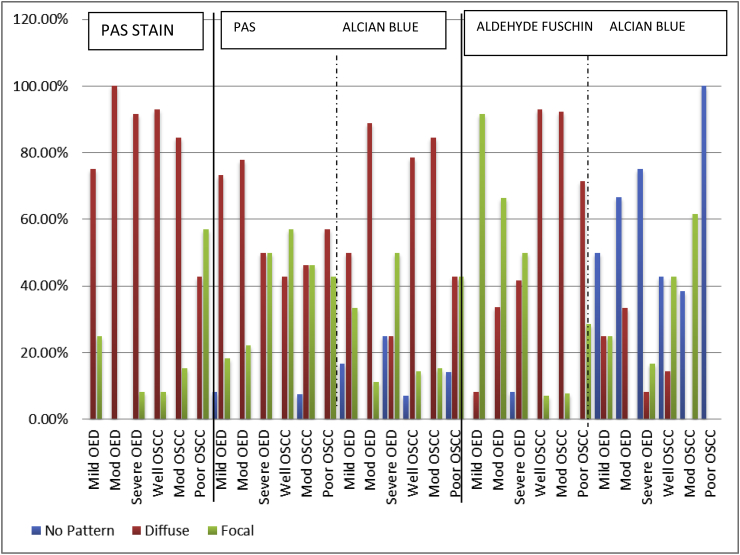
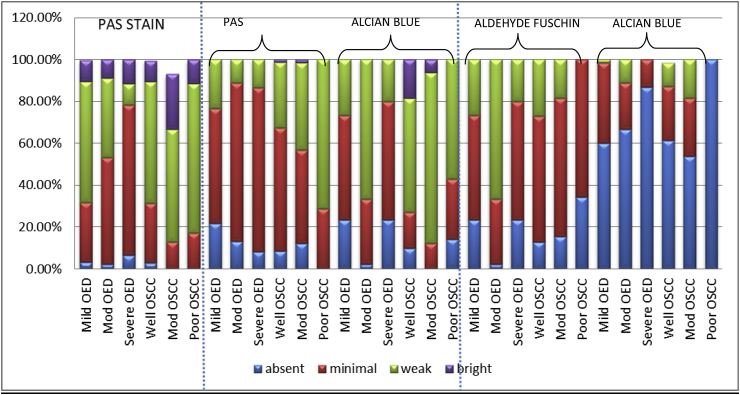
PAS STAIN- PAS staining demonstrate the glycoprotein of stroma which exhibit magenta color. In present study, predominantly diffuse staining pattern was observed in all grades of OED and OSCC except poor OSCC (Bar Diagram 1). On intergroup statistical analysis of OED and OSCC, difference in the distribution of patterns was found to be significantly significant. (p = 0.040) (Table 1). When PAS-intensity was compared it was seen that predominant juxtaepithelial intensity in mild to moderate to severe OED was weak, minimal and minimal respectively (Bar Diagram 2). On intra-group comparison of variable grades of OED, highly statistical significant difference was found between mild and severe OED (p = <0.001) and moderate and severe OED (p = 0.007) (Table 2). The overall intensity of glycoproteins around the tumour islands was weak in progressive grades of OSCC (Bar Diagram 2). On intra-group statistical analysis, statistical significant difference was found between well and moderate OSCC. (p = 0.047) (Table 2). When intergroup comparison of variable grades of OED and OSCC was carried out highly statistical significant difference was found between the PAS intensity. (p= <0.001) (Table 1).
Bar Diagrams 1.
Distribution of patterns of different stains in various grades of OED and OSCC.
Table 1.
Intergroup comparison of pattern in OED and OSCC using Chi- Square test.
| Parameter | PAS STAIN | ALCIAN BLUE – PAS STAIN |
ALDEHYDE FUSCHIN- ALCIAN BLUE |
||
|---|---|---|---|---|---|
| Neutral mucins | Acid mucins | Sulphated mucins | Carboxylated mucins | ||
| Pattern | 0.040* | 0.003* | <0.001* | <0.001* | <0.001* |
| Intensity | <0.001* | 0.012* | <0.001* | <0.001* | <0.001* |
Significant*.
Bar Diagram 2.
Intensity of various stains in different grades of OED and OSCC.
Table 2.
Intragroup comparison of intensity in all grades of OED and OSCC using Chi- Square test.
| PAS STAIN |
ALCIAN BLUE – PAS STAIN |
ALDEHYDE FUSCHIN- ALCIAN BLUE |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral mucins |
Acid mucins |
Sulphated mucins |
Carboxylated mucins |
|||||||
| JE/around the tumour islands | Deeper CT/away from the tumour islands | JE/around the tumour islands | Deeper CT/away from the tumour islands | JE/around the tumour island | Deeper CT/away from the tumour islands | JE/around the tumour islands | Deeper CT/away from the tumour islands | JE/around the tumour islands | Deeper CT/away from the tumour islands | |
| Mild OED vs Moderate OED | 0.12 | 0.066 | 0.09 | 0.03* | <0.001* | 0.22 | <0.001* | <0.001* | 0.042* | 0.0335* |
| Moderate OED vs Severe OED | 0.007* | 0.556 | 0.69 | 0.02* | <0.001* | 0.002* | <0.001* | 0.038* | 0.010* | 0.034* |
| Severe OED vs Mild OED | <0.001* | 0.166 | 0.022* | 0.95 | 0.057 | 0.004* | 0.663 | 0.002* | 0.004* | 0.001* |
| Well OSCC vs Moderate OSCC | 0.047* | 0.26 | 0.445 | 0.700 | 0.002* | 0.003* | 0.481 | 0.071 | 0.486 | 0.104 |
| Moderate OSCC vs Poor OSCC | 0.164 | 0.063 | 0.017* | 0.003* | 0.001* | <0.001* | 0.006* | 0.469 | <0.001* | <0.001* |
| Poor OSCC vs Well OSCC | 0.409 | 0.415 | 0.001* | 0.002* | 0.039* | 0.006* | 0.001* | 0.009* | <0.001* | 0.006 |
Significant*.
OED- Oral epithelial dysplasia and OSCC- Oral squamous carcinoma.
ALCIAN BLUE- PAS STAIN (AB-PAS) - This combination helps to demonstrate the acid mucin via Alcian blue and neutral mucin via PAS. It was seen that the chief pattern of neutral mucins in OED was diffuse and in OSCC no chief pattern was seen (Bar Diagram 1). On intergroup statistical analysis, difference in patterns distribution was found to be statistically significant. (p = 0.003) (Table 1) Juxtaepithelially, the predominant intensity of neutral mucins in the varying grades of OED was minimal and in OSCC it was minimal, minimal to weak and weak respectively (Bar Diagram 2). On intra-group statistical analysis, significant difference was found between mild OED and severe OED (p = 0.022), moderate OSCC and poor OSCC (p = 0.017) and poor OSCC and well OSCC respectively (p = 0.001) (Table 2). On inter-group analysis, the difference in PAS intensity for the neutral mucins between OED and OSCC was found to be statistically significantly different. (p= <0.001) (Table 1).
For the acid mucins, diffuse pattern in varying grades of OED and OSCC was primarily observed (Bar Diagram 1). The intergroup statistical analysis of OED and OSCC, showed statistically significant difference (Table 1). The peak intensity in all grades of OED ranged between weak to absent intensity (Bar Diagram 2). Predominant weak intensity of acid mucins in all the progressive grades of OSCC was observed (Bar Diagram 2). Intra-group statistical evaluation revealed significant difference in mild OED and moderate OED (p=<0.001) and moderate and severe OED (p=<0.001) (Table 2). Significant difference was found between all variable grades of OSCC. (p = 0.003, <0.001, 0.006) (Table 2). On inter-group analysis of OED and OSCC, the intensity of acidic mucins was found to be statistically significantly different. (p= <0.001) (Table 1).
ALDEHYDE FUSCHIN – ALCIAN - BLUE (AF-AB) - To differentiate acid mucins in to carboxylated mucins and sulphated mucins AF-AB staining was done. For sulphated mucins it was seen that the prominent pattern of all grades of OED was focal and in OSCC it was diffuse (Bar Diagram 1). On intergroup statistical analysis of OED and OSCC, distribution of patterns for sulphated mucins was found to be statistically significant. (p=<0.001) (Table 1) When intensity was taken the highest intensity in progressive grades of OED and OSCC was minimal (Bar Diagram 2). Intra-group statistical evaluation showed significant difference between mild OED and moderate OED (p=<0.001) and moderate and severe OED (p=<0.001) (Table 2). Intra-group significant results was found to be significant between well and poor OSCC (p = 0.001) and moderate and poor OSCC (p = 0.006). On intergroup statistical analysis of OED and OSCC, the intensity of sulphated mucins in AF stain was found to be significant. (p=<0.001). (Table 1).
When carboxylated mucins intensity was taken it was seen that there was absence in all grades of OED and in OSCC absence to focal distribution (Bar Diagram 1). Intergroup comparison of OED and OSCC, the difference in patterns for carboxylated mucin was found to be significant. (p value = <0.001) (Table 1) The frequency of the absence of carboxylated mucins was noted to be increasing during the progressive grades of OED & OSCC. The maximum intensity attained was minimal by OED and OSCC with the complete absence in poor OSCC (Bar diagram 2). On intra-group comparison of among the various grades of OED and OSCC, statistical significant difference was found except well OSCC and moderate OSCC (Table 2). On intergroup statistical analysis, the difference in intensity of carboxylated mucins in AB stain was found to be significant. (p = 0.031) (Table 1).
5. Discussion
The extracellular matrix (ECM) is a dense latticework of collagen and elastic fibers which is embedded in a viscoelastic ground substance and is composed of proteoglycans and glycoproteins. This matrix acts as a scaffold which isolates tissue compartments, mediates cell attachment and influences tissue architecture. Interactions between normal cells and the matrix may be altered in neoplasia and thus may influence tumor proliferation and invasion.8
Among the tumours of head and neck region, oral squamous cell carcinoma (OSCC) is one of the most common malignancies with poor prognosis. OED is characterized by the altered proliferation of squamous cells within the epithelium. This disturbance gradually progress and invades into the stroma to form carcinoma.2 Thus, traditionally OSCC's have two interdependent compartments - tumour epithelial cells and stroma. Numerous studies exist in the literature on tumour epithelial cells and even the stromal fibrous/cellular components have been well explored in OSCC and OED.4,5,9, 10, 11
Various immunohistochemistry and genetic studies have been done to evaluate the changes in the ECM of OED/OSCC.4,12, 13, 14, 15, 16, 17, 18 Mucin, one of the main ECM components seems to play a role in the process of tumour progression, invasion and metastasis and also in tumour cell survival and protection against the host immune response. Changes in the expression levels and glycosylation of mucins have been associated with several diseases including carcinomas.12
In the present study, the majority of fields of progressive grades of OED showed weak, minimal and minimal PAS intensity respectively and were diffusely distributed. Thus, it was seen that the glycoproteins content had gradually regressed from mild to severe OED juxtaepithelially. In OSCC, diffuse weak PAS positivity was observed in majority of the fields in all grades except in poor OSCC where focal PAS positivity was noted. In accordance to the present study, Fuentes et al. also observed a similar change in intensity of PAS between OED and OSCC.4 According to them, there was a progressive loss of PAS stain in OED and complete absence of PAS stain in OSCC, particularly in the areas of connective tissue invasion. However, the limitation of their study was that they did not evaluate the intensity of the PAS reactivity in all grades of OED and categorization of OSCC was lacking. George J et al. also observed the weak PAS staining in variable grades of OSCC around the tumour islands.5 However, neither had they mentioned about the distribution of the PAS reactivity nor OED was considered in their study.
Malignancies derived from simple epithelial tissues (carcinomas) frequently contain detectable glycoproteins. The reason for the weak reactivity of glycoproteins in stroma of OSCC could be the lysis of stromal components which facilitates dysplastic tumour cell migration.5 According to Pereria A et al., there is a change in stromal composition and either such changes is induced by tumor cells through the release of certain mediator or may be it is causing carcinogenesis.12 In the present study, few fields in all grades of OED and OSCC showed a bright PAS staining, juxtaepithelially and around the tumor islands respectively. In OED, the dysplasia is restricted up to the epithelium with no breach in the basement membrane (BM). Hence, the BM gives a bright staining which can be attributed to the BM components such as laminin, entactin and heparin sulphate. OSCC is characterized by nests/islands/strands/cords of malignant epithelial cells in the connective tissue. These tumour cells have a property of secreting the proteins which may have the similar composition as BM. Hence, the bright PAS staining selectively around the tumour islands in OSCC can be because of the reduplicated BM secreted by the tumour cells. The quantitative and qualitative change in the BM occurring during the carcinogenesis is already known, which is essentially important in tumour invasion and metastasis.12
Besides glycoproteins, PAS staining demonstrate the other structures such as glycogen, starch and other BM material present in the connective tissue. The PAS technique is particularly sensitive for the detection of neutral mucins as well as acid mucins that contain significant quantities of sialic acid.19
The target of the present study was to observe the changes in mucin which is one of the types of glycoproteins. So to differentiate between neutral mucins and acid mucins, AB-PAS technique was employed.
In our study it was seen that the intensity of the neutral mucins was increased gradually from OED to OSCC, even within the group the intensity was enhanced, juxtaepithelially and around the tumour islands. For acid mucins, there was no intensity which was predominant in mild OED. In moderate OED, predominantly weak staining and in severe OED absence of staining was observed, juxtaepithelially. In OSCC, around the tumour islands, the overall intensity was weak in all grades. The result of the present study was in accordance to the study of George J et al.5 According to them, the source of the neutral mucins could either be epithelial cells as they were present adjacent to the tumor islands or could be the stromal cells which produce them in response to the invading heterogenous tumor cell population, acting probably as a scaffold around the tumour cells. These neutral mucins have increased amount of hexose components or accumulation of uronic acid containing substances like hyaluronic acid, chondroitin sulphate and heparan sulphate.5
Since acid mucins are further of two types i.e., sulphated and carboxylated mucins, to differentiate these two, AF-AB technique was employed. There was a gradual increase in the number of fields with minimal intensity of sulphated mucins in varying grades of OED and OSCC. The carboxylated mucins was absent in majority of fields.
As the grade of OED and OSCC increases, the expression of carboxylated mucins decreases. There was addition of sulphated mucins, rendering a mixture of sulphated mucins and carboxylated mucins and in poor OSCC only expression of sulphated mucins was appreciated. This was supported by the findings of study conducted by George J et al. in OSCC.5
To the best of our knowledge, the literature of acidic mucin histochemistry in OED is not available. Limited studies have been documented where the extensive mucin changes in varying grades of OSCC.5,10,20 However, the reason for such transition in the acidic mucins has not been explained.
Such demonstration of mucins with their changes in the histochemistry is already been well explored and established in foetus, salivary glands tumours, carcinomas of lungs, colon, GIT and their precursor lesions.21, 22, 23, 24, 25, 26
The present study also observed the alteration of mucin nature in the progressive grades of OED and OSCC. There is a definite morphologic variation in the mucin expression occurring during the evolution of carcinoma in the oral mucosa as observed in other mucosa of the body. Such transformation in the mucins may be induced by the tumour cells, stromal cells or tumour associated fibroblasts (TAFs) need to be further investigated. Also, further studies to establish its correlation with clinical details (TNM staging, follow up, habits & history) is mandatory to validate its diagnostic and prognostic purpose.6 Our study challenges the glycobiologists to delve deeper in elucidating the role of mucins in OED and OSCC that whether these adaptive changes produced by stroma is the consequence to precursor/malignant changes or these cause the neoplasia is still query. Also, it remains to be investigated whether all mucins genes carry carboxylated or sulphated oligosaccharide side chain.
Nevertheless, knowledge of mucins and its implications in oral precursor/cancer process as well as potential of using it as a scaffold for therapeutic modalities are an unsolved puzzle. Thus the present study and further research on mucin will prevail hereafter.
6. Conclusion
The present study could be the stepping stone in the exploration of mucinous modification in OED and OSCC. Although in GIT and other mucosa, the expression of altered mucin is a recognized factor, seldom research has been done in OED and OSCC.
Undoubtedly, molecular and recent technologies help us in better understanding of the tumour biology. However their unavailability, cost, extensive laboratory preparations etc. are the major hindrances. Despite the fact that, there is variation in staining pattern when stained in different batches at different intervals, these cost effective special stains are definitely better for diagnostic as well as prognostic purposes due to its ease of standardization.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
Nil.
Acknowledgement
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jobcr.2018.07.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Jayaraj G., Ramani P., Herald J., Kumar P., Anuja N. Inter-observer agreement in grading oral epithelial dysplasia – a systematic review. J Oral Maxillofac Surg Med Pathol. 2014;270:1–5. [Google Scholar]
- 2.Speight P.M. Update on oral epithelial dysplasia and progression to cancer. Head and Neck Pathol. 2007;1:61–66. doi: 10.1007/s12105-007-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright A., Shear M. Epithelial dysplasia immediately adjacent to oral squamous cell carcinoma. J Oral Pathol. 1985;14:559–564. doi: 10.1111/j.1600-0714.1985.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 4.Fuentes B., Duaso J., Droguett D., Castillo C., Donoso W., Rivera C. Progressive extracellular matrix disorganization in chemically induced murine oral squamous cell carcinoma. ISRN Pathol. 2012;359421:1–7. [Google Scholar]
- 5.George J., Narang R.S., Rao N.N. Stromal response in different histological grades of oral squamous cell carcinoma: a histochemical study. Indian J Dent Res. 2012;23:842–849. doi: 10.4103/0970-9290.111291. [DOI] [PubMed] [Google Scholar]
- 6.Astekar M., Metgud R., Sharma A., Soni A. Hidden keys in stroma: unlocking the tumor progression. J Oral Maxillofac Pathol. 2013;17:82–88. doi: 10.4103/0973-029X.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kufe D.W. Mucins in cancer: function, prognosis and therapy. Nat Rev Canc. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liotta L. Tumor invasion and metastases-role of the extracellular matrix. Canc Res. 1986;46:1–7. [PubMed] [Google Scholar]
- 9.Sharma R., Rehani S., Mehendiratta M. Architectural analysis of Picrosirius Red stained collagen in oral epithelial dysplasia and oral squamous cell carcinoma using Polarization Microscopy. J Clin Diagn Res. 2015 Dec;9(12) doi: 10.7860/JCDR/2015/13476.6872. EC13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kardam P., Mehendiratta M., Rehani S., Kumra M., Sahay K., Jain K. Stromal fibers in oral squamous cell carcinoma: a possible new prognostic indicator? J Oral Maxillofac Pathol. 2016;20:405–412. doi: 10.4103/0973-029X.190913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal U., Rai H., Jain A. Morphological and ultrastructural characteristics of extracellular matrix changes in oral squamous cell carcinoma. Indian J Dent Res. 2011;22:16–21. doi: 10.4103/0970-9290.79968. [DOI] [PubMed] [Google Scholar]
- 12.Pereira A., Veras S., Silveira E. The role of matrix extracellular proteins and metalloproteinases in head and neck carcinomas: an updated review. Rev Bras Otorrinolaringol. 2005;71:81–86. doi: 10.1016/S1808-8694(15)31289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nitta T., Sugihara K., Tsuyama S., Murata F. Immunohistochemical Study of MUC1 mucin in premalignant oral lesions and oral squamous cell carcinoma. Cancer. 2000;88:244–248. doi: 10.1002/(sici)1097-0142(20000115)88:2<245::aid-cncr1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Dziemiańczyk D., Grabowska S.Z., Balicki R. Evaluation of secretory mucin concentration of patients with squamous cell carcinoma oral cavity. Ann Acad Med Biol. 2005;50:334–339. [PubMed] [Google Scholar]
- 15.Rabassa M.E., Croce M.E., Pereyra A., Segal-Eiras A. MUC1 expression and anti-MUC1 serum immune response in head and neck squamous cell carcinoma (HNSCC): a multivariate analysis. BMC Canc. 2006;6:253–266. doi: 10.1186/1471-2407-6-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada T., Wakamatsu T., Miyahara M. MUC4: a novel prognostic factor of oral squamous cell carcinoma. Int J Canc. 2012;130:1768–1776. doi: 10.1002/ijc.26187. [DOI] [PubMed] [Google Scholar]
- 17.Narashiman S., Narasimhan M., Venkatraman G. Expression of Mucin 4 in leukoplakia and oral squamous cell carcinoma: an immunohistochemical study. J Oral Maxillofac Pathol. 2014;18:25–32. doi: 10.4103/0973-029X.131887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabassa M.E., Croce M.E., Pereyra A., Segal-Eiras A. MUC1 expression and anti-MUC1 serum immune response in head and neck squamous cell carcinoma (HNSCC): a multivariate analysis. BMC Canc. 2006;6:253–266. doi: 10.1186/1471-2407-6-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Why pick PAS for histology? | Bite size Bio http:/itesizebio.com/13413/why-pick-pas-for-histology//p.
- 20.Naag S., Adi R.P. Histochemical study of salivary mucins in normal and neoplastic salivary Glands. J Clin Diagn Res. 2010;4:3450–3458. [Google Scholar]
- 21.Ionila M., Margaritescu C.L., Pirici D., Mogoanta S.S. Mucinous adenocarcinomas of the colon – a histochemical study. Rom J Morphol Embryol. 2011;52:783–790. [PubMed] [Google Scholar]
- 22.Mahomed F. Recent advances in mucin immunohistochemistry in salivary gland tumors and head and neck squamous cell carcinoma. Oral Oncol. 2011;47:797–803. doi: 10.1016/j.oraloncology.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Ali U., Nagi A.H., Naseem N., Ullah E. Mucin histochemistry in tumours of colon, ovaries and lung. J Cytol Histol. 2012;3:163–167. [Google Scholar]
- 24.Awad E., Mohamed E., Raheem A. Demonstration of mucins in gastrointestinal tract carcinoma lesions in sudanese patients. Int J Pure Appl Sci Technol. 2014;21:28–31. [Google Scholar]
- 25.Lam K.Y., Loke S.L., Ma L.T. Histochemistry of mucin secreting components in mucoepidermoid and adenosquamous carcinoma of the oesophagus. J Clin Pathol. 1993;46:1011–1015. doi: 10.1136/jcp.46.11.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganesh I.M., Subramani D., Halagowder D. Mucin glycoarray in gastric and gallbladder epithelia. J Carcinog. 2007;6:1–4. doi: 10.1186/1477-3163-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.