Highlights
-
•
First in vitro study performed in an X-ray SARRP for minibeam irradiations.
-
•
At equal mean dose, the same tumor control can be obtained with standard and minibeam irradiations.
-
•
This contradicts the established paradigms of the standard radiation therapy.
Keywords: Radiotherapy, Minibeam, MBRT, Clonogenic assay
Abstract
The goal is to compare, in vitro, the efficiency of minibeam radiotherapy (MBRT) and standard RT in inducing clonogenic cell death in glioma cell lines. With this aim, we report on the first in vitro study performed in an X-ray Small Animal Radiation Research Platform (SARRP) modified for minibeam irradiations. F98 rat and U87 human glioma cells were irradiated with either an array of minibeams (MB) or with conventional homogeneous beams (broad beam, BB). A specially designed multislit collimator was used to generate the minibeams with a width of a center-to-center distance of 1465 (±10) μm, and a PVDR value of 12.4 (±2.3) measured at 1 cm depth in a water phantom. Cells were either replated for clonogenic assay directly (immediate plating, IP) or 24 h after irradiation (delayed plating, DP) to assess the effect of potentially lethal damage repair (PLDR) on cell survival. Our hypothesis is that with MBRT, a similar level of clonogenic cell death can be reached compared to standard RT, when using equal mean radiation doses. To prove this, we performed dose escalations to determine the minimum integrated dose needed to reach a similar level of clonogenic cell death for both treatments. We show that this minimum dose can vary per cell line: in F98 cells a dose of 19 Gy was needed to obtain similar levels of clonogenic survival, whereas in U87 cells there was still a slightly increased survival with MB compared to BB 19 Gy treatment.
The results suggest also an impairment of DNA damage repair in F98 cells as there is no difference in clonogenic cell survival between immediately and delayed plated cells for each dose and irradiation mode. For U87 cells, a small IP-DP effect was observed in the case of BB irradiation up to a dose of 17 Gy. However, at 19 Gy BB, as well as for the complete dose range of MB irradiation, U87 cells did not show a difference in clonogenic survival between IP and DP. We therefore speculate that MBRT might influence PLDR. The current results show that X-ray MBRT is a promising method for treatment of gliomas: future preclinical and clinical studies should aim at reaching a minimum radiation (valley) dose for effective eradication of gliomas with increased sparing of normal tissues compared to standard RT.
1. Introduction
Distinct dose delivery methods, such as those in X-ray minibeam radiation therapy (MBRT), have shown the potential to overcome the main limitation in radiotherapy: the tolerances of normal tissues. MBRT is a technique originated at synchrotrons [1], [2], which uses arrays of thin (500–700 µm) parallel beams spaced by 1– 3 mm. It delivers a spatial dose fractionation that, in contrast to standard radiotherapy (RT), results in dose profiles consisting of a pattern of peaks and valleys. The peaks correspond to the beam paths, i.e. high doses, and the valleys are the areas between two consecutive beams that are filled in by the scattered radiation from the peaks, i.e. low doses [3]. The ratio between the peak and valley doses (peak-to-valley dose ratio, PVDR) is considered a relevant dosimetric parameter in terms of biological response [4]. A remarkable increase of rat brain resistance to irradiation has been observed after MBRT irradiations [4], [5], [6]: doses as high as 100 Gy in one fraction are still well-tolerated. In addition, a significant tumor growth delay in aggressive animal tumor models has been observed with MBRT [5], [6]. MBRT, therefore, is a promising irradiation technique, especially for highly radioresistant tumors such as gliomas, which still receive mostly palliative treatment. Unfortunately, the confinement of MBRT to synchrotrons has limited its worldwide exploration and comprehensive evaluations. Because of this, we have transferred the X-ray MBRT to a small animal irradiator [7], making in vitro and in vivo investigations of MBRT easily performable, with similar PVDR values to those at synchrotrons [3].
Our goal of the current study is to compare, in vitro, the efficiency of MBRT to induce clonogenic cell death in glioma cell lines, to investigate its use for further preclinical and clinical studies and comparison to standard radiotherapy. Our hypothesis is that, with MBRT, a similar level of clonogenic cell death can be reached compared to the standard radiotherapy, when equal average radiation doses are used. We have investigated whether a minimal radiation valley dose is needed. The clonogenic assay was used as this is the method of choice to determine cell reproductive death after treatment with ionizing radiation [8]. To the best of our knowledge, this is the first study to show that minibeam (MB) and standard (broadbeam, BB) radiotherapy can be equally effective in inducing clonogenic cell death in radioresistant glioma cell lines, when using high radiation doses. For F98 cells, the minimum integrated dose to reach similar clonogenic survival levels for BB and MB treatments was 19 Gy both for IP and DP. For U87 cells, this dose is expected to be higher, as the clonogenic survival after 19 Gy is still somewhat increased after MB compared to BB treatment. For DP, there was relatively little difference between BB and MB in induction of U87 clonogenic cell death for all doses studies. An IP-DP effect was absent in the case of MB treatment in both cell lines studied. Although the results of in vitro studies cannot replace in vivo works, they help designing better animal experiments and delimit to some extent the range of doses where the therapeutic window for a new radiation treatment could be expected.
2. Materials and methods
2.1. Cell line and culture conditions
This study was carried out with the F98 rat and the U87 human glioma cell line, which are often used in neuro-oncology experiments since these are considered good models for human gliobastoma multiforme [9]. F98 and U87 cells were grown in Dulbecco's Modified Eagle Medium (DMEM) plus GlutaMAX-I (Gibco by Life Technologies) supplemented with 10% fetal bovine serum (FBS OneShot, Gibco by Life Technologies) and Pen Strep antibiotics (penicillin 100 U/mL and streptomycin 100 µg/mL, Gibco by Life Technologies). Cells were maintained as monolayer cultures in tissue culture flasks and kept at 37 °C in an incubator with humidified air supplemented with 5% CO2.
2.2. X-ray irradiation setup and dosimetry
Both standard RT, i.e. broad beam (BB), and MBRT irradiations were performed using a Small Animal Radiation Research Platform (SARRP, Xstrahl Ltd., UK). The energy spectrum has an effective energy of 69 keV and the beam divergence is 20 ° [10]. Some modifications of the SARRP were carried out to make the system suitable for performing MBRT experiments [7]. Among others, a specially designed brass multislit collimator was used in this study setup. Fig. 1 shows the features of the divergent brass collimator considered to compensate the large divergence (20 ° reported for the beam divergence by the SARRP manufacturer) of the SARRP. The widths of the slits were progressively increased from the center towards the edges to homogenize the peak doses. As figure of merit, PVDR values and full width half at maximum (FWHM) similar to those obtained at the European synchrotron radiation facility (ESRF) were used [3]. Further details on modifications performed on the SARRP, to make it suitable for X-ray MBRT experiments, can be found elsewhere [7]. Our system provides an array of 690 (±20) μm-wide minibeams with a center-to-center distance of 1465 (±10) μm, measured at 1 cm depth in a water phantom. A PVDR value of 12.4 (±2.3) at 1 cm depth in a water phantom is obtained, similar to that at synchrotrons [3].
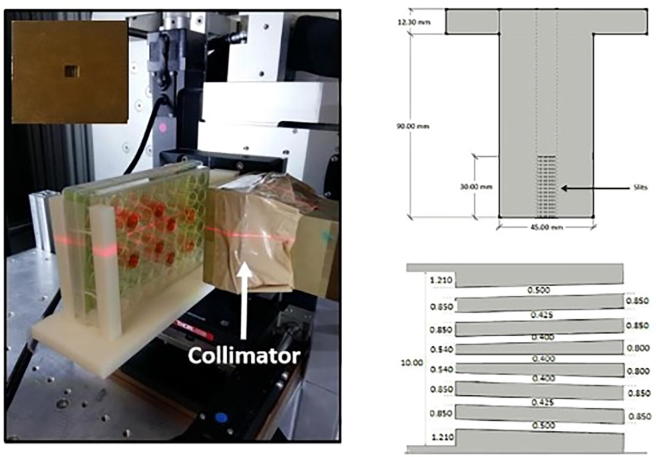
Fig. 1.
Left: picture of the experimental set-up inside the SARRP platform: 48-well plate containing the cells, brass collimator, and micromanipulator system. The micromanipulator is remotely guided by customized software. A front-view of the multi-slit MBRT collimator is shown in the inset on the upper left. This set-up provides a continuously variable micrometric range to position the 48-well plate (on a customized holder) in front of the irradiation field. Right: a cross section of the collimator is depicted on the right (upper row) and a zoom including the widths of the slits (white spaces) is shown on the right lower corner (dimensions in mm).
For radiation exposure, cells were transferred to 48-well plates that are completely filled with DMEM (see Fig. 1), so that they are located at 1.8 cm water equivalent depth. This was done to have similar valley doses like those delivered in previous in vivo experiments with rodents [7]. The area irradiated with the collimator so customized covers the surface where the cells are plating. These plates are positioned inside an in-house designed holder within a 3D-micromanipulator probe guided by remote control with a customized LabView software (see Fig. 1). The distance between the collimator and the top size of the well-plates is 3 cm. Similarly, a set of 48-well plates were irradiated with a BB X-ray configuration.
Gafchromic EBT3 films [11] were used for dose measurements and quality control of irradiations since they have been successfully used in MBRT dosimetry previously [12]. Films handling was performing taking into account the recommendations provided by Task Group 55 of the AAPM [13]. A flat bed red-greenblue (RGB) scanner (Epson Perfection V750-M Pro Scanner) was used for the readout at 1200 dpi resolution. We follow the methodology described by Devic et al. [14] and used the red channel. Films were previously calibrated in a configuration where they are placed perperdicularly to the beam direction interspersed amont 1 cm solid water-equivalent RW3 material phantom. The calibration curve was implemented in a home-made C++ code to convert the scanner readings into doses. Uncertainties in films dose meausrements were evaluated as described in Sorriaux et al. [15]. The overall uncertainty was 3.2%. To incorporate other possible sources of uncertainty (e.g. misplacing or misalignment of the films in the dishes), a conservative value of 4% was considered.
The quality assurance of the irradiation was performed by means of the films attached to the well-plates during the irradiation. Table 1 summarizes the peak and valley doses and the integrated seamless doses delivered in MBRT and BB respectively. PVDR is 11.0 ± 0.7 at 1.8 cm depth in DMEM medium.
Table 1.
Dose values in MBRT and Standard RT.
| MBRT | Peak dose (Gy) | 5,8 | 23,1 | 46,1 | 54,3 |
| Valley dose (Gy) | 0,6 | 2,0 | 4,2 | 4,7 | |
| PVDR at 1.8 cm | 10,3 | 11,5 | 11,1 | 11,5 | |
| Average dose (Gy) | 4,4 | 8,6 | 17,1 | 19,3 | |
| Standard RT | Dose (Gy) | 4,5 | 9 | 17,2 | 19,9 |
Peak, valley, and average doses delivered with MBRT and integrated seamless doses delivered with standard (broad beam) irradiations on F98 cells at 1.8 cm depth. Standard error SE = ±4%. The MBRT average dose is the integrated dose assessed as the average of the central peak and valley doses.
The dose escalation study was performed with both X-ray MBRT and BB irradiation modes at dose rates of 2.6 and 4.6 Gy/min, respectively.
To compare the effects of MBRT with BB irradiation on the survival of glioma cells, clonogenic assays were conducted after immediate (IP) and delayed plating (DP) as described in Section 2.3.
2.3. Clonogenic assay of confluent cell cultures
Two days before X-ray exposure, 105 F98 or U87 cells per well were plated into 48-well plates to obtain a nearly confluent monolayer at the time of irradiation. Wells were filled up to 1.8 cm with fresh complete DMEM and covered with a PCR plate seal to prevent the leakage of medium and contamination in the vertical set-up. Cells were either replated directly (immediate plating, IP) or 24 h after irradiation (delayed plating, DP) in order to assess the effect of potentially lethal damage repair (PLDR) on cell survival. For immediate plating, cells were trypsinized and replated in appropriate dilutions in six-well culture plates (Costar). For delayed plating (DP), cells were kept for 24 h in a humidified 5% CO2 incubator at 37 °C to allow for the repair of potentially lethal damage (PLD) induced by the irradiation. After the incubation period, the cells were trypsinised and replated in appropriate dilutions. Seven to fifteen days later (F98 and U87 respectively), the colonies were fixated in fixative solution (formalin zinc, Sigma Aldrich) and stained with 0.05% crystal violet (Sigma Aldrich). Colonies of 50 cells or more were scored as originating from a single clonogenic cell. Average plating efficiencies of F98 cells (±S.E.M.) were 0.34 (±0.04)% for IP cells and 0.36 (±0.09)% for DP cells. Average plating efficiencies of U87 cells (±S.E.M.) were 0.14 (± 0.03)% for IP cells and 0.14 (±0.03)% for DP cells. All data represent the means of at least three experiments.
3. Results
3.1. Clonogenic survival
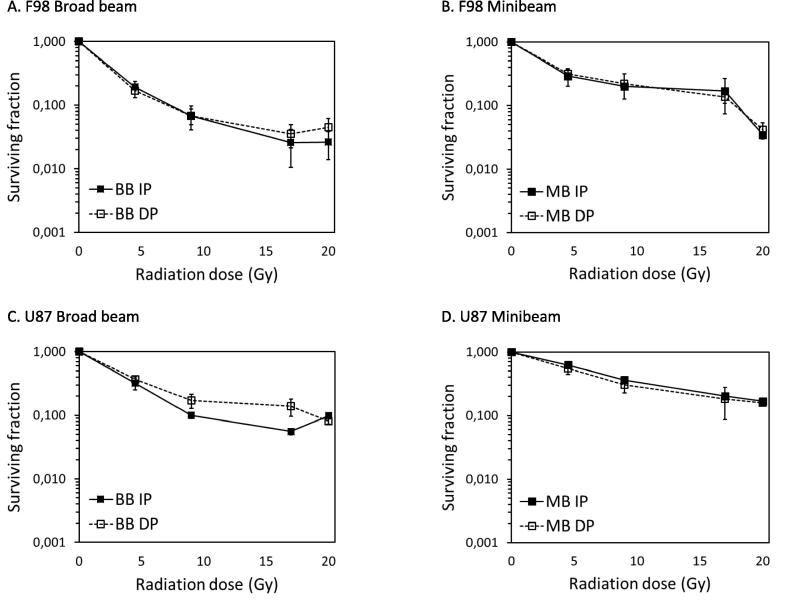
It has been shown that radiation doses higher than 20–25 Gy are needed to obtain long-term survivals in glioma-bearing rats using standard radiotherapy [16]. Therefore, we chose to escalate the doses as high as currently technically possible, which is 19 Gy, for our in vitro experiments. The survival curves for both MB and BB modes in F98 and U87 cells are shown in Fig. 2. When the same integrated dose is delivered, the BB irradiation yields a reduced clonogenic survival in comparison to that of MBRT for the lower doses, up to 19 Gy integrated dose. No significant differences in survival between BB and MB treatment are observed when an integrated dose of 19 Gy is used in F98 cells. This is the case for both IP and DP. These results indicate that for F98 cells, a minimum valley dose (i.e. 4.7 Gy, see Table 1) is needed to render the same clonogenic cell death after MB or BB treatment. For U87 cells, the radiation survival curves of BB and MB treatment were also converging at a dose of 19 Gy, but cell survival was still slightly higher after MB than after BB treatment. For DP, the difference in U87 clonogenic survival after MB or BB treatment was very few for all doses investigated.
Fig. 2.
Radiation survival curves of glioma cell lines after treatment with broad beam (BB) or minibeam (MB) therapy. A: F98 rat glioma cells replated immediately (immediate plating, IP) after BB or MB irradiation. B: F98 rat glioma cells replated after a recovery period of 24 h (delayed plating, DP) to allow PLDR after BB or MB irradiation. C: U87 human glioma cells replated immediately (immediate plating, IP) after BB or MB irradiation. D: U87 human glioma cells replated after a recovery period of 24 h (delayed plating, DP) to allow PLDR after BB or MB irradiation. Near confluent cultures were irradiated using the BB or MB configuration. Means ± SEM are shown for at least three separate experiments.
Fig. 3 shows the same radiation survival curves from Fig. 2, replotted to show the effect of immediate and delayed plating in F98 and U87 cells. The graphs show that F98 glioma cells do not exhibit PLDR, as there is no difference between IP and DP after both BB and MB treatment. For U87, a slight IP-DP effect and therefore PLDR is observed up to a dose of 19 Gy but only in case of BB treatment. However, no IP-DP effect is observed for U87, as in F98 cells, after treatment with MBRT.
Fig. 3.
Radiation survival curves of Fig. 2. replotted to show the effect of immediate plating (IP) and delayed plating (DP) after treatment with broad beam (BB) or minibeam (MB) radiotherapy. A: F98 rat glioma cells replated IP or DP after BB irradiation. B: F98 rat glioma cells replated IP or DP after MB irradiation. C: U87 human glioma cells replated IP or DP after BB irradiation. D: U87 human glioma cells replated IP or DP after MB irradiation. Near confluent cultures were irradiated using the BB or MB configuration. Means ± SEM are shown for at least three separate experiments.
4. Discussion
Gliomas are highly aggressive tumors of the central nervous system and, despite numerous pre-clinical and clinical studies, they are still almost always incurable. Due to their strong resistance to chemo- and radiotherapy, these tumors either show no treatment response at all or they often relapse after an initial response. Their formation of a tumor cell network in the brain and their capacity to regrow after damage induced by radiotherapy [17] is thought to be an important factor in limiting the success of conventional radiotherapy. Conventional standard RT fractions for glioma treatment consist of 1.8–2 Gy delivered over 5–6 weeks up to a total dose of 45–60 Gy [18] and most in vitro studies use doses up to 10 Gy only. In order to eradicate as many tumor cells as possible and preventing their regrowth, the aim should be to deliver a radiation dose as high as tolerable by the surrounding healthy brain tissue. With MBRT [1], [2] a much higher dose can be delivered to the tumor in one single (temporal) fraction, due to a spatial fractionation of the dose. Previously, we have shown that doses as high as 100 Gy of X-rays administered to the (whole) healthy brain by MBRT are well tolerated in rats, in contrast to similar doses of conventional X-ray therapy, which lead to a high morbidity and mortality [19]. To further investigate the potential of MBRT for glioma treatment in a clinical setting, we investigated its efficiency to induce clonogenic cell death in two different glioma cell lines and compared this to standard RT. The endpoint of clonogenic survival was chosen as this is considered the method of choice to determine cell reproductive death after treatment with ionizing radiation [8]. For this first assessment, an in vitro glioma model (F98 rat and U87 human glioma cell lines) was used to rule out the interference of other factors, such as neovascularization or immune modulation, with the response of tumor cells to X-ray MBRT and standard RT irradiations. The radiation dose was escalated stepwise to find out whether a minimal (threshold) valley dose is needed to obtain a similar induction of clonogenic cell death with the two irradiation modes. We went up to a dose of 19 Gy, which is still technically feasible with the current experimental setup and which would be clinically relevant for MBRT. Such a high dose has rarely been investigated according to the literature.
Here, we show that F98 and U87 cell survival fractions differ as a function of integrated dose and irradiation mode. When low doses are employed (<19 Gy), a homogenous standard RT proves to be more effective in terms of inducing clonogenic cell death. However, MBRT results in a similar survival fraction as standard RT (broad beam) when using an integrated dose of 19 Gy in F98 rat glioma cells. This could indicate that when a minimum valley dose (4.7 Gy) is attained in this cell line, the same integrated dose would lead to a similar fraction of clonogenic cell death for both irradiation modes. Therefore, from an integrated dose of 19 Gy, the spatial distribution of the dose does not affect its ability to induce clonogenic cell death in F98 cells. This suggests that the valley dose delimits the clonogenic cell survival, which must be taken into account when translating MBRT to a clinical situation. That this minimum valley dose can be different for different cell lines show our results with the human U87 glioma cell line. In the case of IP, the radiation survival curves converge at a dose of 19 Gy, but survival is still slightly higher for MB than for BB treated cells. It is probable that with an increased dose, the differences in cell survival between MB and BB would be diminished. In the case of DP U87 cells, the difference in clonogenic survival between BB and MB is little if any for all dose points investigated. We therefore speculate that MBRT might affect PLDR in this cell line. Interestingly, both cell lines showed an absence of an IP-DP effect when treated with MBRT. For F98 cells, we suggest that this could be due to the absence of PLDR in this cell line as this is also observed for BB treatment. U87 cells, however, do show a slight PLDR in the case of BB treatment up to 19 Gy. Although our findings are contradicting the dogma of existing PLDR capacity in most mammalian cells, cell lines without PLDR have been reported previously [20]. The mutant p53 status of the F98 cell line [21] together with its very low expression level [22], might be an explanation for the absence of PLDR as PLD expression has been suggested to be primarily dependent on p53 [23], [24]. In addition to impaired p53 function, they exhibit mutant p16/CDKn2y/INK4 and a negative BRCA1 localization after X-rays [25], which could further contribute to the absence of PLDR in these cells. This same study showed a higher fraction of unrepaired DNA double strand breaks persisting at 24 h after X-irradiation than two other rodent glioma cell lines, further confirming their incapacity to fully repair DNA damage. Furthermore, glioma cells are known to be highly resistant to ionizing irradiation as their interconnections have been found to regrow after radiation destruction [19]. To the best of our knowledge, we show for the first time the absence of PLDR in the F98 rat glioma cell line. In contrast to F98 cells, the U87 cell line does not exhibit mutant p53 but do repair radiation-induced DNA damage more slowly than normal fibroblasts [26]. As a consequence, they accumulate more residual DNA damage than fibroblasts, probably due to a loss of function in some aspects of DNA repair [26]. This could explain that we observe an IP-DP effect only up to a dose of 19 Gy, in which case the limited DNA repair capacity of U87 cells may be depleted. Therefore, we speculate that MBRT might affect PLDR in glioma and maybe also in other cancer cell lines. The difference in DNA repair capacity between tumor cells and healthy tissue cells might furthermore contribute to the sparing effect of normal tissues by MBRT while tumor cells are killed. More research, however, is needed to investigate the effect of MBRT on repair of DNA damage in tumor and healthy tissue cells.
In a previous work [7] using the same irradiation configuration (696 (±20) μm-wide beams and 1465 (±16) μm ctc at 1 cm-depth) we have already demonstrated that normal rat brain is able to withstand doses as high 20 Gy in one fraction (whole brain irradiation, excepting the olfactory bulb). This is in contrast with the severe toxicity observed with the same integrad dose in standard RT irradiations [7].
Moreover, the range of doses in MBRT fits with what is used in some current radiosurgery treatments, which might facilitate a possible clinical transfer as its tolerance in patients has already been shown [27]. With radiosurgery, the central doses delivered to the target volume in glioblastoma multiforme patients can vary between 30 and 40 Gy with a marginal dose of 15–20 Gy, after initial external beam radiotherapy [27].
Recently, another minibeam collimator setup has shown its utility for preclinical in vitro and in vivo studies [28]. The conclusion was that MBRT results in clonogenic cell death and leads to a more effective tumor control in mice than conventional radiotherapy. However, a direct comparison of the level of clonogenic cell survival between MBRT and BB was not possible. The used setup for the in vivo part of the study was whole-body irradiation with lead-shielding with exposure of only the target volume. With our current MBRT setup, however, imaging-controlled precise irradiation of the target volume is possible, which makes the possibilities for preclinical research endless.
A method for spatial fractionation of radiotherapy similar to MBRT, but with narrower beam sizes, is microbeam radiation therapy. Previous works investigating microbeam radiation therapy suggested that thick beams are more favorable for tumor control [29], but may affect healthy tissue tolerance.
A recent study [25] compares microbeam RT with conventional RT in the induction of clonogenic survival, DNA double strand breaks and chromosomal aberrations. Their finding that microbeam RT results in an enhanced clonogenic survival compared to conventional RT at low radiation doses (up to 4 Gy) is in agreement with our current findings. Furthermore, they find less chromosomal aberrations after microbeam RT than after conventional RT and induction of DNA double strand breaks in the peak areas. These results point to a tissue-sparing effect of microbeam treatment compared with conventional treatment. However, the effects of high radiation doses (above 4 Gy) were not assessed. Due to the different experimental setup, i.e. cell lines, dose rates and beam energies, a direct comparison of these results with our current findings needs to be considered with extreme care. Nevertheless, these reported in vitro findings show the same trend as we have observed in our study with low radiation doses, namely that spatially fractionated radiotherapy can effectively induce cell reproductive death in tumor cells. Other studies using a similar experimental set-up [30], [31], [32], [33] reported similar results in the low dose range as our current findings. The observation that the shape of our MBRT curves is similar to the ones observed in the context of microbeam radiation therapy [34] further underpins the validity of our results. An advantage of X-ray MBRT over microbeam XRT, however, is that MBRT uses doses 10 times lower than those needed in microbeam radiation therapy to reach good tumor control without substantial healthy tissue damage. It is important to note that spatial fractionation radiotherapy, like minibeam and microbeam treatments, take use of the mixture of cells receiving high (peak) and low (valley) radiation doses in order to spare healthy tissues. As a result, also the tumor cells receive these different doses but as our current results show, this still results in significant induction of clonogenic cell death. Since the cells located in the peak areas of the irradiation field receive extremely high doses (see Table 1), their survival most probably cannot be modeled using the linear quadratic model as it is expected to have reached a plateau in the amount of remaining DNA double strand breaks. The cells located in the valley areas, however, receive only about 10% of the peak dose and it is expected that clonogenic cell death induction in this area can be described with the linear quadratic model. The total cell survival is therefore a mixture of the colonies arising from the peak and from the valley areas.
An important observation in our study, furthermore, is that our radiation survival curves are flattening with an increase in the dose. This is true for both cell lines studied. The absence of a shoulder in the radiation survival curve of cell lines has been reported in the literature before [24], [35] and could, together with the diminished IP-DP effect, indicate a deficiency in the DNA damage repair capacity of the cells.
Indeed, a transfection with DNA-Pk of one cell line without a shoulder in its radiation survival curve resulted in an introduction of a shoulder [35]. However, doses as high as 19 Gy have rarely been investigated. On study by Knedlitschek et al. [36] observed surviving F98 cell spheroids after irradiation with 15 Gy. In this publication, the F98 radiation survival curve shows an almost absence of a shoulder in the low-dose range with an α value of 0.16 and β value of 0.007 for spheroids and 0.11 and 0.019 for monolayers, respectively. The flattening shape of our survival curves might be caused by a residual number of radiation resistant S-phase cells and an impaired DNA damage repair capacity. In in vivo experiments, it is known that at least 25 Gy is needed to obtain long term survivals in glioma-bearing rats [16]. The residual surviving clonogenic cells after irradiation with 19 Gy in vitro are therefore not implausible and the flattening radiation survival curve might indicate the existence of a threshold dose needed to achieve a glioma cure or at least control. This points to the extremely high radiation resistance of these cells, even in vitro. It would be interesting to explore this phenomenon further and to enhance the eradication of glioma cells, for example to escalate the radiation to even higher doses or to combine with different therapies such as newly emerging chemo-, nanoparticle- or immune therapies. As we have recently shown [37], minibeam irradiations of healthy rat brains with integrated doses as high as 25 Gy are feasible and well tolerated compared to conventional irradiation and have therefore the potential for combination with adjuvant therapies. For this reason, future studies aim at escalating the dose and investigating its effect on clonogenic survival, tumor control and healthy tissue tolerance.
5. Conclusion and limitations
We conclude that MBRT has the potential to improve the therapeutic index for gliomas. Due to its previously proven sparing of healthy tissues, it allows the use of exceptionally high radiation doses, which we show here to result in a significant amount of clonogenic cell death in F98 rat and U87 human glioma cell lines. A minimum threshold valley dose is needed in order to reach similar levels of clonogenic cell death with MBRT compared to standard RT. This threshold dose can vary for different cell lines, as a higher dose is needed for U87 than for F98 cells. We speculate that MBRT might affect PLDR in glioma cell lines, as the IP-DP effect is abolished after MBRT but not after standard RT in PLDR proficient U87 cells. However, this needs to be further investigated in different cell lines in vitro an in vivo. Furthermore, a flattening of the radiation survival curves for both cell lines suggest that a residual amount of clonogenic cells survives, even after such high radiation doses as 19 Gy, suggesting that the aim should be at reaching even higher radiation doses or a combination with adjuvant therapies. The current results allow us to address the forthcoming in vivo experiments with the goal of elucidating this therapeutic gain in glioma bearing rats and help to design better animal experiments in X-ray MBRT regarding the 3Rs ethical standards [38]. Future studies will focus on delivering a radiation dose of at least 19 Gy for gliomas and investigate the effects of MBRT on tumor control and healthy tissue damage, as well as exploring underlying mechanisms such as potential effects on DNA damage repair.
Conflict of interest
All the authors declare that there is not conflict of interest in the research developed in the submitted manuscript.
Acknowledgements
The authors thank Dr. F. Pouzoulet for scientific discussions, Dr. O. Seksek and Mme E. Gontran for sharing laboratory equipment, Dr. R. Delorme and Mme H. Soleimanzad for helping us with the set-up transport, and Dr. S. Heinrich and Mme S. Bonnet-Boissinot for carrying out the irradiations and their kind technical support. J. Bergs gratefully acknowledges funding from the German Research Foundation (GRK2260, BIOQIC). This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 745109.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ctro.2018.07.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Dilmanian F.A., Zhong Z., Bacarian T., Benveniste H., Romanelli P., Wang R. Interlaced x-ray microplanar beams: a radiosurgery approach with clinical potential. Proc Natl Acad Sci USA. 2006;103:9709–9714. doi: 10.1073/pnas.0603567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prezado Y., Renier M., Bravin A. A new method of creating minibeam patterns for synchrotron radiation therapy: a feasibility study. J Synchrotron Radiat. 2009 Jul;16(Pt 4):582–586. doi: 10.1107/S0909049509012503. [DOI] [PubMed] [Google Scholar]
- 3.Prezado Y., Martinez-Rovira I., Thengumpallil S., Deman P. Dosimetry protocol for the preclinical trials in white-beam minibeam radiation therapy. Med Phys. 2011;38:5012–5020. doi: 10.1118/1.3608908. [DOI] [PubMed] [Google Scholar]
- 4.Dilmanian F.A., Button T.M., Le Duc G., Zhong N., Peña L.A., Smith J.A. Response of rat intracranial 9L gliosarcoma to microbeam radiation therapy. Neuro Oncol. 2002;4:26–38. doi: 10.1215/15228517-4-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prezado Y., Sarun S., Gil S., Deman P., Bouchet A., Le Duc G. Increase of lifespan for glioma-bearing rats by using minibeam radiation therapy. J. Synchrotron Radiat. 2012;19:60–65. doi: 10.1107/S0909049511047042. [DOI] [PubMed] [Google Scholar]
- 6.Deman P., Vautrin M., Edouard M., Stupar V., Bobyck L., Farion R. Monochromatic minibeams radiotherapy: From healthy tissue-sparing effect studies toward first experimental glioma bearing rats therapy. Int J Radiat Oncol Biol Phys. 2012 Mar 15;82(4):e693–e702. doi: 10.1016/j.ijrobp.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Prezado Y., Dos Santos M., Gonzalez-Infantes W., Jouvion G., Guardiola C., Heinrich S. Transfer of Minibeam Radiation Therapy into a cost-effective equipment. Sci Rep 7. 2017 doi: 10.1038/s41598-017-17543-3. Article number: 7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franken N., Rodermond H.M., Stap J., Haveman J., Bree C. Clonogenic assay of cells in vitro. Nat Prot. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 9.Barth R.F., Kaur B. Rat brain tumor models in experimental neuro-oncology: the C6, 9L, T9, RG2, F98, BT4C, RT-2 and CNS-1 gliomas. J Neurooncol. 2009 Sep;94(3):299–312. doi: 10.1007/s11060-009-9875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delpon G., Frelin-Labalme A.M., Heinrich S., Beaudouin V., Noblet C., Begue M. Radiothérapie guidée par l’image pour les petits animaux : une nouvelle ère pour les études précliniques. Cancer/Radiothérapie. February 2016;20(1) doi: 10.1016/j.canrad.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Gafchromicc EBT3 dosimetry media. <http://www.ashland.com>; 2016.
- 12.Prezado Y., Martínez-Rovira I., Sánchez M. Scatter factors assessment in microbeam radiation therapy. Med Phys. 2012;39:1234–1238. doi: 10.1118/1.3681274. [DOI] [PubMed] [Google Scholar]
- 13.Niroomand-Rad A., Blackwell C.R., Coursey B.M., Gall K.P., Galvin J.M., McLaughlin W.L. Radiochromic film dosimetry: recommendations of AAPM radiation therapy committee task group 55. Med Phys. 1998;25:2093–2115. doi: 10.1118/1.598407. [DOI] [PubMed] [Google Scholar]
- 14.Devic S., Seuntjens J., Sham E., Podgorsak E.B., Schmidtlein C.R., Kirov A.S. Precise radiochromic film dosimetry using a flat-bed document scanner. Med Phys. 2005 Jul;32(7):2245–2253. doi: 10.1118/1.1929253. [DOI] [PubMed] [Google Scholar]
- 15.Sorriaux J., Kacperek A., Rossomme S., Lee J.A., Bertrand D., Vynckier S. Evaluation of Gafchromic R EBT3 films characteristics in therapy photon, electron and proton beams. Phys Med. 2013;29:599–606. doi: 10.1016/j.ejmp.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Adam J.F., Joubert A., Biston M.C., Charvet A.M., Peoc'h M., Le Bas J.F. Int J Radiat Oncol Biol Phys. 2006 Feb 1;64(2):603–611. doi: 10.1016/j.ijrobp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Osswald M., Jung E., Sahm F., Solecki G., Venkataramani V., Blaes J. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015 Dec 3;528(7580):93–98. doi: 10.1038/nature16071. [DOI] [PubMed] [Google Scholar]
- 18.Khan L., Soliman H., Sahgal A., Perry J., Xu W., Tsao M.N. External beam radiation dose escalation for high grade glioma (Protocol) Cochrane Database Syst Rev. 2015;(1) [Google Scholar]
- 19.Prezado Y., Deman P., Varlet P., Jouvion G., Gil S., Le Clec H.C. Tolerance to dose escalation in minibeam radiation therapy applied to normal rat brain: long-term clinical. Radiol Histopathol Anal Radiat Res. 2015 Sep;184(3):314–321. doi: 10.1667/RR14018.1. [DOI] [PubMed] [Google Scholar]
- 20.van Oorschot Oncol Rep. 2013;19(6):2175–2189. doi: 10.3892/or.2013.2364. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Palomo Cristian et al. Phys Med: Eur J Med Phys 31(6); 584–595. [DOI] [PubMed]
- 22.Senatus P.B., Li Y., Mandigo C., Nichols G., Moise G. Restoration of p53 function for selective Fas-mediated apoptosis in human and rat glioma cells in vitro and in vivo by a p53 COOH-terminal peptide. Mol Cancer Ther. 2006;5(1):20–28. doi: 10.1158/1535-7163.MCT-05-0181. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz J.L., Jordan R., Kaufmann W.K., Rasey J., Russel K.J., Weichselbaum R.R. Evidence for the expression of radiation-induced potentially lethal damage being a p53-dependent process. Int J Radiat Biol. 2000;76:1037–1043. doi: 10.1080/09553000050111505. [DOI] [PubMed] [Google Scholar]
- 24.Van Oorschot B., Oei A.L., Nuijens A.C. Cell Mol Biol Lett. 2014;19:37. doi: 10.2478/s11658-013-0113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burger K., Ilicic K., Dierolf M., Günther B., Walsh D.W.M., Schmid E. PLoS One. 2017 Oct 19;12:10. doi: 10.1371/journal.pone.0186005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Short S.C., Martindale C., Bourne S., Brand G., Woodcok M., Johnston P. DNA repair after irradiation in glioma cells and normal human astrocytes. Neuro Oncol. 2007 Oct;9(4):404–411. doi: 10.1215/15228517-2007-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B., Zhao P., Zhang Y. J Neurooncol. 2018;139:185. doi: 10.1007/s11060-018-2859-8. [DOI] [PubMed] [Google Scholar]
- 28.Bazyar S., Inscoe C.R., O’Brian E.T., Zhou O., Lee Y.Z. Phys Med Biol. 2017;62:8924–8942. doi: 10.1088/1361-6560/aa926b. [DOI] [PubMed] [Google Scholar]
- 29.Serduc R., Bouchet A., Brauer-Krisch E., Laissue J.A., Spiga J., Sarun S. Synchrotron microbeam radiation therapy for rat brain tumor palliation-influence of the microbeam width at constant valley dose. Phys Med Biol. 2009;54(21):6711–6724. doi: 10.1088/0031-9155/54/21/017. [DOI] [PubMed] [Google Scholar]
- 30.Bayart E., Pouzoulet F., Calmels L., Dadoun J., Allot F., Plagnard J. Enhancement of IUdR radiosensitization by low-energy photons results from increased and persistent DNA damage. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0168395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bencokova Z., Pauron L., Devic C., Joubert A., Gastaldo J., Massart C. Molecular and cellular response of the most extensively used rodent glioma models to radiation and/or cisplatin. J Neurooncol. 2008 Jan;86(1):13–21. doi: 10.1007/s11060-007-9433-0. Epub 2007 Jul 5. [DOI] [PubMed] [Google Scholar]
- 32.Taupin F., Flaender M., Delorme R., Brochard T., Mayol J.F., Arnaud J. Gadolinium nanoparticles and contrast agent as radiation sensitizers. Phys Med Biol. 2015 Jun 7;60(11):4449–4464. doi: 10.1088/0031-9155/60/11/4449. [DOI] [PubMed] [Google Scholar]
- 33.Charest G., Paquette B., Fortin D., Mathieu D., Sanche L. Concomitant treatment of F98 glioma cells with new liposomal platinum compounds and ionizing radiation. J Neurooncol. 2010 Apr;97(2):187–193. doi: 10.1007/s11060-009-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibahim M.J., Crosbie J.C., Yang Y., Zaitseva M., Stevenson A.W., Rogers P.A.W. An evaluation of dose equivalence between synchrotron microbeam radiation therapy and conventional broadbeam radiation using clonogenic and cell impedance assays. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerweck L.E., Vijayappa S., Kurimasa A., Ogawa K., Chen D.J. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006 Sep 1;66(17):8352–8355. doi: 10.1158/0008-5472.CAN-06-0533. [DOI] [PubMed] [Google Scholar]
- 36.Knedlitschek G., Anderer U., Weibezahn K.F., Dertinger H. Radioresistance of rat glioma cell lines cultured as multicellular spheroids. correlation with electrical cell-to-cell-coupling. Strahlenther Onkol. 1990 Feb;166(2):164–167. [PubMed] [Google Scholar]
- 37.Prezado Y., Jouvion G., Hardy D., Patriarca A., Nauraye C., Bergs J. Sci Rep 7. 2017 doi: 10.1038/s41598-017-14786-y. Article number 14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell WMS, Burch RL. The Principles of Humane Experimental Technique, Methuen, London; 1959.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.