Abstract
Objective
To determine the persistence of no evident disease activity (NEDA) in a population-based relapsing-remitting MS (RRMS) cohort.
Methods
All incident cases of RRMS in Olmsted County between 2000 and 2011 were identified using a medical records linkage system. Persistence of NEDA after RRMS diagnosis was determined by retrospective chart review. MRI activity, relapse, or Expanded Disability Status Scale (EDSS) worsening resulted in failure of NEDA.
Results
We identified 93 incident cases of RRMS including 82 individuals with sufficient follow-up to determine the persistence of NEDA. There were 44 individuals not on disease-modifying therapy (DMT), whereas 37 individuals were prescribed an injectable DMT and 1 received mitoxantrone during the interval over which NEDA was maintained. NEDA was maintained by 63% at 1 year, 38% at 2 years, 19% at 5 years, and 12% at 10 years according to routine care assessment. At 10 years, there was no difference in EDSS disability among patients who maintained NEDA vs those who failed NEDA at 1 year (p = 0.3).
Conclusions
NEDA infrequently persists beyond 2 years in a population-based cohort of newly diagnosed patients with RRMS.
“No evident disease activity” (NEDA) is a proposed indicator of disease activity free status in MS.1 The most common NEDA definition, NEDA-3, is a composite measure, which requires no relapses, no progression, and no MRI activity.1 NEDA may be a useful primary end point for clinical trials of disease-modifying therapy (DMT).2 NEDA has also been proposed as a goal in the clinical management of relapsing-remitting MS (RRMS), given the availability of DMT to suppress inflammatory disease activity.1 Cohort studies from MS clinics suggest that NEDA is not sustained over time in most patients despite DMT but may be limited by referral bias.3,4 We are not aware of previous population-based assessments of NEDA. We sought to characterize the persistence of NEDA among newly diagnosed patients with RRMS in a geographically defined region.
Methods
Standard protocol approvals, registrations, and patient consents
This study was approved by the institutional review boards at Mayo Clinic (08-007846) and Olmsted Medical Centre (060-OMC-12). All patients consented for the use of their medical records for research.
Patients
The Rochester Epidemiology Project medical records linkage system, a database of all medical practitioners in Olmsted County, was used to identify all cases of RRMS.5 Cases were identified by searching medical records from January 1, 2000, to December 31, 2011, as described previously.6
Definitions
NEDA was defined as freedom from (1) relapses, (2) progression, and (3) MRI activity.1,7 Relapses were new or worsening neurologic symptoms persisting ≥24 hours in the absence of fever/infection.5 Progression was defined as the Expanded Disability Status Scale (EDSS) score increase confirmed by 2 assessments separated by ≥ 6 months of ≥1.5 points for EDSS 0, ≥1 point for EDSS 1.0–5.0, and ≥0.5 if EDSS ≥5.5.1,7 MRI activity was any new or enlarging T2 lesion or gadolinium-enhancing lesion.1,7 Retrospective chart review was conducted to evaluate time to NEDA failure from RRMS diagnosis according to clinical evaluations and imaging performed during routine care. We evaluated the median number of clinic visits and MRI studies as a rate from RRMS diagnosis until NEDA failure. Patients without clinical and neuroimaging follow-up were excluded from further analysis because NEDA could not be determined. In the event of partial follow-up, patients were categorized as having failed NEDA if there was any component of NEDA failure, whereas patients with no activity with incomplete assessment (lacking clinical examination or MRI brain) were excluded.
Long-term functional status was determined by the EDSS at approximately 10 years after RRMS diagnosis. As assessments were performed during routine care, the EDSS score closest to 10 years was considered, provided it was between 8 and 12 years after RRMS diagnosis.
Statistical analysis
Univariate associations between baseline characteristics and DMT status were assessed using the Wilcoxon rank-sum test and χ2 test. Kaplan-Meier survival analysis was used to determine the probability of NEDA survival. The effect of DMT on maintenance of NEDA was evaluated using the log-rank test. The effect of NEDA at 1 year on the EDSS at 10 years was evaluated using the Kruskal-Wallis test. Univariate logistic regression was performed for persistence of NEDA at 1, 2, and 5 years. All tests were 2 sided, and p values <0.05 were considered statistically significant. Analysis was performed using SAS software version 9.4 (SAS Inc., Cay, NC).
Data availability
Any data not published within this article will be made available in an anonymized fashion and shared if requested from any qualified investigator.
Results
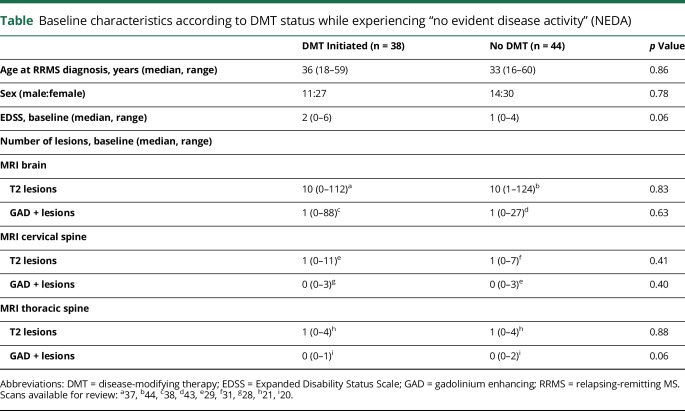
We identified 93 individuals who met the diagnostic criteria for RRMS. We excluded 11 individuals for inability to determine the persistence of NEDA because there was limited follow-up after RRMS diagnosis. Among the remaining patients (table), the median age at RRMS diagnosis was 34.5 years (range, 16–60 years) with female predominance (2.3:1). The median EDSS score was 1 (range, 0–6) at diagnosis. Before NEDA failure, 44 patients (54%) had not received any DMT, 37 received interferon-β or glatiramer acetate, and 1 patient was prescribed mitoxantrone. Among the 38 patients on DMT, 28 patients received DMT for ≥75% duration of NEDA. After RRMS diagnosis and before NEDA failure, the median number of clinic visits was 3 (interquartile range [IQR], 3–5), and the median number of MRIs undertaken was as follows: head, 2 (IQR, 2–3); cervical spine, 1 (IQR, 1–2); and thoracic spine, 1 (IQR, 0–1).
Table.
Baseline characteristics according to DMT status while experiencing “no evident disease activity” (NEDA)
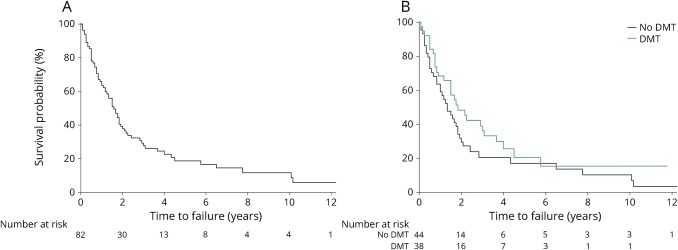
NEDA was maintained in 63% (95% confidence interval [CI] 52.0%–72.8%) at 1 year, 38% (27.3%–48.4%) at 2 years, 19% (10.3%–29.0%) at 5 years, and 12% (4.7%–22.0%) at 10 years (figure). Of 82 patients with RRMS at baseline, the number remaining at risk was 54 at 1 year, 30 at 2 years, 9 at 5 years, and 4 at 10 years. Kaplan-Meier analysis demonstrated that 50% of patients failed to maintain NEDA by 19 months (95% CI 14–23 months) (figure). Among those who failed to maintain NEDA, failure was due to 1 or more of MRI activity (52/68, 76%), relapse (39/68, 57%), or progression (9/68, 13%); 25 patients (37%) failed because of isolated MRI activity. DMT did not have an effect on sustained NEDA compared with no DMT (p = 0.2) (figure). In those who maintained NEDA, the median duration of follow-up was 41 months (range, 14–141 months).
Figure. NEDA survival.
Kaplan-Meier survival curves of (A) overall survival of NEDA in a population-based cohort of relapsing-remitting MS and (B) survival of NEDA with (green line) and without (black line) DMT. DMT = disease-modifying therapy; NEDA = no evident disease activity.
Among those with available EDSS scores at 10 years, there were 22 (59%) with NEDA at 1 year and 15 (41%) who had failed NEDA by 1 year. The median EDSS at 10 years was 1.0–1.5 among those with NEDA at 1 year compared with 2.0 among those who had failed NEDA at 1 year (p = 0.3). At final follow-up, 62 of 82 patients (76%) with RRMS had received DMT. Univariate logistic regression for NEDA status at 1, 2, and 5 years did not identify a consistent predictor of NEDA among age, sex, EDSS at diagnosis, and whether a DMT was initiated.
Discussion
In this population-based analysis of newly diagnosed patients with RRMS, the proportion of patients with NEDA was 63% at 1 year, 38% at 2 years, 19% at 5 years, and 12% at 10 years. In an MS clinic cohort with a mean disease duration of 6.6 years at study onset, NEDA was present among 46.0% at 1 year, 27.5% at 2 years, and 7.9% at 7 years.3 In another cohort with a mean disease duration of 5.3 years, only 9% had NEDA at 10 years.4 The rates of sustained NEDA between our study and clinic-based cohorts were similar, suggesting that those clinical cohort studies were likely not affected by referral bias, although differing frequency of DMT use across studies limits direct comparisons.
There was no difference in survival of NEDA stratified by whether a DMT, primarily first-tier injectable therapy, was initiated similar to previous studies,3,4 but these findings should be interpreted with caution. In this study, a selection bias toward treating more severe cases may contribute to the lack of difference in NEDA between treated and untreated individuals. Furthermore, NEDA failure early in the first year could have reflected the time taken for medications to take effect. However, the early separation in the Kaplan-Meier curves (figure) would suggest some possible early treatment effect of older-generation DMTs used in this study on sustaining NEDA with most eventually breaking through and failing NEDA in the long term. Patients maintain NEDA for longer with higher-potency therapies, as NEDA was demonstrated among 34% treated with natalizumab at 7 years8 and 60%–68% receiving autologous stem cell transplant at 5 years.9 We found that NEDA at 1 year was not associated with disability status measured by the EDSS at 10 years. This study may have been underpowered to detect minor differences, but a larger study demonstrated that NEDA at 2 years was not protective against EDSS progression at 10 years.10
Strengths of this study include that it is population based preventing referral bias. Follow-up was done as part of clinical care, so this is a real-world assessment of NEDA status according to current clinical practice.
Limitations of this study include variable interval of clinical and radiologic assessment. Persistence of NEDA is highly dependent on the frequency of MRIs undertaken, and because our study was retrospective, we could not control how often MRIs were undertaken, which can impact comparison to other studies. Nonetheless, our study gives a sense of NEDA in the real-world setting where interpatient variability in MRI frequency is typical. The effect of missing data from the 11 patients with insufficient follow-up to calculate NEDA status is unknown. We were unable to assess the effect of oral (dimethyl fumarate, fingolimod, and teriflunomide) or higher-potency (alemtuzumab, natalizumab, and ocrelizumab) therapy because no patient was prescribed these medications and most were not available during the study period. In our study and previous studies of NEDA, a floor effect may affect the assessment of the long-term impact of NEDA because most fail NEDA within the first 2 years.
Maintenance of NEDA beyond 2 years is uncommon among a population-based cohort of newly diagnosed RRMS patients treated with older-generation DMT and similar to what has been reported in clinic-based cohorts. Future studies including patients treated with higher-potency MS therapies are needed to assess the clinical utility of NEDA maintenance and its association with long-term disability.11
Acknowledgment
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the NIH under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Glossary
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- NEDA
no evident disease activity
- RRMS
relapsing-remitting MS
Author contributions
N.E. Parks: study design, data analysis/interpretation, and drafting of the manuscript. S.J. Pittock: data interpretation and drafting of the manuscript. J. Mandrekar: data analysis/interpretation. O.H. Kantarci, C.F. Lucchinetti, B.G. Weinshenker, B.M. Keegan, W.O. Tobin, J-M. Tillema, and M. Toledano: data interpretation and revision of the manuscript. E.P. Flanagan: study design, data analysis/interpretation, drafting of the manuscript, and supervision.
Study funding
This work was supported by the Mayo Clinic Center for Multiple Sclerosis and Autoimmune Neurology.
Disclosure
N.E. Parks served on the scientific advisory boards of Biogen, EMD Serono, Roche, and Sanofi Genzyme; received travel funding and/or speaker honoraria from Biogen and Roche; and consulted for Biogen, EMD Serono, Roche, and Sanofi Genzyme. S.J. Pittock and Mayo Clinic have a financial interest in patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker. S.J. Pittock consulted for Alexion and MedImmune (fees paid to Mayo Clinic) and received research funding from Grifols, MedImmune, Alexion, AEA, and NIH. J. Mandrekar reports no disclosures. O.H. Kantarci received speaker honoraria paid to Mayo Clinic from Istanbul MS Days and Novartis; provided a grant review for The National Multiple Sclerosis Society; received research support from Biogen, Multiple Sclerosis Society, Mayo Foundation, and Hilton Foundation; his spouse served on the data safety monitoring board of the Takeda Global Research and Development Center, Pfizer, and Janssen Alzheimer Immunotherapy; and is funded by the NIH, Minnesota Partnership for Biotechnology, and Medical Genomics. C.F. Lucchinetti is a member of the NIH/NINDS CNBT Study Section; received travel funding from Biogen; was a guest editor of Brain Pathology; holds a patent on and receives royalties from aquaporin-4 associated antibodies for the diagnosis of neuromyelitis optica; receives royalties from Saunders Elsevier; consulted for Biogen and QuestCor; is a member of the Progressive MS Advisory Board for Genentech; and received research support from Novartis, Biogen, NIH, and National Multiple Sclerosis Society. B.G. Weinshenker served on the editorial boards of the Canadian Journal of Neurological Sciences, Turkish Journal of Neurology, and Neurology; holds a patent for and received royalties from NMO-IgG for diagnosis of neuromyelitis optica; consulted for Caladrius, Brainstorm Therapeutics, and Roivant; is an adjudication member of MedImmune and Alexion; and received research support from the Guthy-Jackson Charitable Foundation. B.M. Keegan served on the editorial board of Multiple Sclerosis; received royalties from Cambridge University; consulted for Novartis, Bristol-Myers Squibb, and Bionest; and received research support from Biogen. O. Tobin reports no disclosures; J-M Tillema served as an associate editor of the Journal of Child Neurology and received research support from the NCATS/NIH. M. Toledano reports no disclosures. E.P. Flanagan received research support from MedImmune. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NN.
References
- 1.Giovannoni G, Tomic D, Bright JR, et al. “No evident disease activity”: the use of combined assessments in the management of patients with multiple sclerosis. Mult Scler 2017;23:1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bevan CJ, Cree BAC. Disease activity free status: a new endpoint for a new era in multiple sclerosis clinical research? JAMA Neurol 2014;71:269–270. [DOI] [PubMed] [Google Scholar]
- 3.Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015;72:152–158. [DOI] [PubMed] [Google Scholar]
- 4.De Stefano N, Stromillo ML, Giorgio A, et al. Long-term assessment of no evidence of disease activity in relapsing-remitting MS. Neurology 2015;85:1722–1723. [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flanagan EP, Cabre P, Weinshenker BG, et al. Epidemiology of aquaporin-4 autoimmunity and neuromyelitis optica spectrum. Ann Neurol 2016;79:775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kappos L, De Stefano N, Freedman MS, et al. Inclusion of brain volume loss in a revised measure of “no evidence of disease activity” (NEDA-4) in relapsing-remitting multiple sclerosis. Mult Scler 2016;22:1297–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prosperini L, Fanelli F, Pozzilli C. Long-term assessment of no evidence of disease activity with natalizumab in relapsing multiple sclerosis. J Neurol Sci 2016;364:145–147. [DOI] [PubMed] [Google Scholar]
- 9.Sormani MP, Muraro PA, Saccardi R, et al. NEDA status in highly active MS can be more easily obtained with autologous hematopoietic stem cell transplantation than other drugs. Mult Scler 2017;23:201–204. [DOI] [PubMed] [Google Scholar]
- 10.Cree BA, Gourraud PA, Oksenberg JR, et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol 2016;80:499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parks NE, Flanagan EP, Lucchinetti CF, Wingerchuk DM. NEDA treatment target? No evident disease activity as an actionable outcome in practice. J Neurol Sci 2017;383:31–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any data not published within this article will be made available in an anonymized fashion and shared if requested from any qualified investigator.