Key Points
VAMP isoforms regulate the kinetics and extent of platelet granule exocytosis.
Manipulating platelet sensitive factor attachment protein receptors alters granule secretion, which affects the hemostatic balance.
Abstract
We genetically manipulated the major platelet vesicle-associated membrane proteins (VAMP2, VAMP3, and VAMP8) to create mice with varying degrees of disrupted platelet secretion. As previously shown, loss of VAMP8 reduced granule secretion, and this defect was exacerbated by further deletion of VAMP2 and VAMP3. VAMP2Δ3Δ8−/− platelets also had reduced VAMP7. Loss of VAMP2 and VAMP3 (VAMP2Δ3Δ) had a minimal impact on secretion when VAMP7 and VAMP8 were present. Integrin αIIbβ3 activation and aggregation were not affected, although spreading was reduced in VAMP2Δ3Δ8−/− platelets. Using these mice as tools, we asked how much secretion is needed for proper thrombosis and hemostasis in vivo. VAMP2Δ3Δ mice showed no deficiency, whereas VAMP8−/− mice had attenuated formation of occlusive thrombi upon FeCl3-induced arterial injury but no excessive bleeding upon tail transection. VAMP2Δ3Δ8−/− mice bled profusely and failed to form occlusive thrombi. Plasma-coagulation factors were normal in all of the strains, but phosphatidylserine exposure was reduced in VAMP2Δ3Δ and VAMP2Δ3Δ8−/− platelets. From our data, an ∼40% to 50% reduction in platelet secretion in vitro (dense and α granule) correlated with reduced occlusive thrombosis but no compromise in hemostasis. At a >50% reduction, thrombosis and hemostasis were defective in vivo. Our studies are the first systematic manipulation of platelet exocytic machinery to demonstrate a quantitative linkage between in vitro platelet secretion and hemostasis and thrombosis in vivo. The animals described will be invaluable tools for future investigations into how platelet secretion affects other vascular processes.
Visual Abstract
Introduction
Platelets are anucleate cell fragments that contribute to a myriad of processes: thrombosis, hemostasis, angiogenesis, wound healing, and immunity.1,2 Many of these functions are mediated by their releasate, which is generated through the exocytosis of cargo from the 3 types of granules: dense, α, and secretory lysosomes. The importance of these cargo is seen in dense and α-granulopathies, which cause a range of bleeding diatheses and other pathologies.3 Given its importance, controlling releasate generation offers potential therapeutic usefulness. In this article, we develop a collection of genetically altered mice as tools to define how exocytosis is needed for proper hemostasis.
Platelet granule exocytosis is facilitated by a family of membrane proteins called soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNAREs). Based on localization and residues at the center of their SNARE domains, SNAREs are classified as target (t/Q, Glu) SNAREs and vesicle (v/R, Arg) SNAREs (also known as vesicle-associated membrane proteins [VAMPs]).4 A complex of v- and t-SNAREs spans opposing bilayers to mediate membrane fusion and, thus, vesicle/granule cargo release.5 The t-SNAREs, SNAP23,6-8 syntaxin-11,9 and syntaxin-8,10 are important for platelet secretion. Proteomic studies report 6 v-SNAREs (VAMP2, VAMP3, VAMP4, VAMP5, VAMP7, and VAMP8); however, 4 of these v-SNAREs (VAMP2, VAMP3, VAMP7, and VAMP8) are the most abundant in human and mouse platelets.11-14 VAMP7 and VAMP8 play the most critical roles, whereas VAMP2 and VAMP3 have been considered secondary v-SNAREs.15,16 The importance of VAMP8 is underscored by studies correlating single nucleotide polymorphisms in VAMP8 with early-onset myocardial infarction.17,18 Additionally, a microRNA that regulates VAMP8 expression correlates with platelet hyperreactivity.19 These data imply that the levels of VAMP8 play a dominant, but not exclusive, role in controlling platelet secretion.
To determine how much platelet secretion is necessary to attain a hemostatic balance, we generated mouse strains in which we genetically manipulated the major platelet VAMPs. We generated platelet-specific V2Δ3Δ and V2Δ3Δ8−/− animals and characterized secretion from their platelets. The hemostatic profiles of these animals were compared with wild-type (WT) and V8−/− animals. We found that the loss of VAMP2 and VAMP3 had little effect on the kinetics or magnitude of secretion from platelet granules as long as VAMP7 and VAMP8 were present. However, V2Δ3Δ8−/− platelets showed significantly diminished granule secretion, and V2Δ3Δ8−/− animals bled profusely in tail-bleeding assays and failed to form occlusive thrombi. By comparing in vitro granule cargo release profiles and in vivo bleeding phenotypes, we distinguished the levels of platelet exocytosis required for normal hemostasis from those that are needed to sustain occlusive thrombus growth.
Methods
The methods are described in detail in supplemental Methods.
Mouse strains and genotyping
To delete VAMP2 and VAMP3, we crossed an RC::PFtox strain (generously provided by S. Dymecki, Harvard Medical School, Boston, MA20) with a PF4Cre strain (generously provided by R. Skoda, University Hospital, Basel, Switzerland21). The RC::PFtox strain has a genomic insertion encoding the catalytic subunit of the tetanus toxin endopeptidase, which can be expressed upon Cre-mediated excision of a STOP cassette. The STOP cassette consists of the 3′ portion of the yeast His3 gene, an SV40 polyadenylation sequence, and a false translation initiation codon followed by a 5′ splice donor site. The floxed STOP cassette is inserted between the promoter and tetanus toxin–coding sequences of a transgene, ensuring that few, if any, transcripts containing the coding region are generated. Tetanus toxin specifically cleaves only VAMP2 and VAMP3.22,23 This strategy was needed because VAMP2−/− mice are embryonically lethal,24 as are VAMP3/8−/− mice (S.W.W., unpublished observations). These RC::PFtox/PF4Cre mice (V2Δ3Δ) were further crossed with a global VAMP8−/− strain (V8−/−) to create an RC::PFtox/PF4Cre/VAMP8−/− strain (V2Δ3Δ8−/−). For genotyping RC::PFtox,20 the primers were forward primer 5′-GCCGATCACCATCAACAACTTC-3′ and reverse primer 5′-GCAGAGCTTCACCAGCAACG-3′ using the following conditions: 94°C for 4 minutes for 1 cycle, 94°C for 30 seconds, 58°C for 30 seconds, 72°C for 1 minute for 35 cycles, and, finally, 72°C for 5 minutes. The genotyping of V8−/− mice16 and PF4Cre21 was as described.
Study design and controls
The breeding schemes produced 3 lines with normal VAMP levels: RC::PFtox+/PF4Cre−, RC::PFtox−/PF4Cre+, and RC::PFtox−/PF4Cre−. These strains were considered WT controls because their VAMP levels were unchanged (data not shown). They were evaluated alongside the VAMP-deletion strains in the tail bleeding time and FeCl3-induced arterial injury assays. Bleeding times and occlusion times showed no significant differences among these 3 WT strains (supplemental Figure 5). The in vitro experiments described in this article were performed using pooled blood from ≥1 of 3 WT control strains.
Results
Generation of platelet-specific V2Δ3Δ and V2Δ3Δ8−/− mouse models
To overcome embryonic lethality in V2−/− animals, megakaryocyte-specific V2Δ3Δ animals were generated by crossing PF4Cre recombinase mice with a mouse harboring a tetanus toxin gene downstream of a floxed STOP cassette. Tetanus toxin light chain cleaves VAMP2 and VAMP3.23,24 Megakaryocyte-specific V2Δ3Δ animals were crossed with global V8−/− animals to generate V2Δ3Δ8−/− animals. Animals from both novel strains, V2Δ3Δ and V2Δ3Δ8−/−, were born at expected Mendelian ratios and were healthy, fertile, and had no gross developmental or anatomical abnormalities. The average weight of littermate controls at 12 weeks (24.41 ± 2.96 g) was comparable to V2Δ3Δ (23.48 ± 2.97 g) and V2Δ3Δ8−/− (23.58 ± 1.90 g) mice. Hematological parameters, including red blood cell count, white blood cell count, platelet number, and mean platelet volume, were not statistically different from littermate controls (supplemental Figure 2), indicating normal hematopoiesis. There were no significant defects in serotonin uptake (dense-granule cargo), PF4 (α-granule cargo), or β-hexosaminidase (lysosomal cargo) levels in platelet lysates from these strains (data not shown). See supplemental Methods and supplemental Figure 5 for a discussion of WT controls.
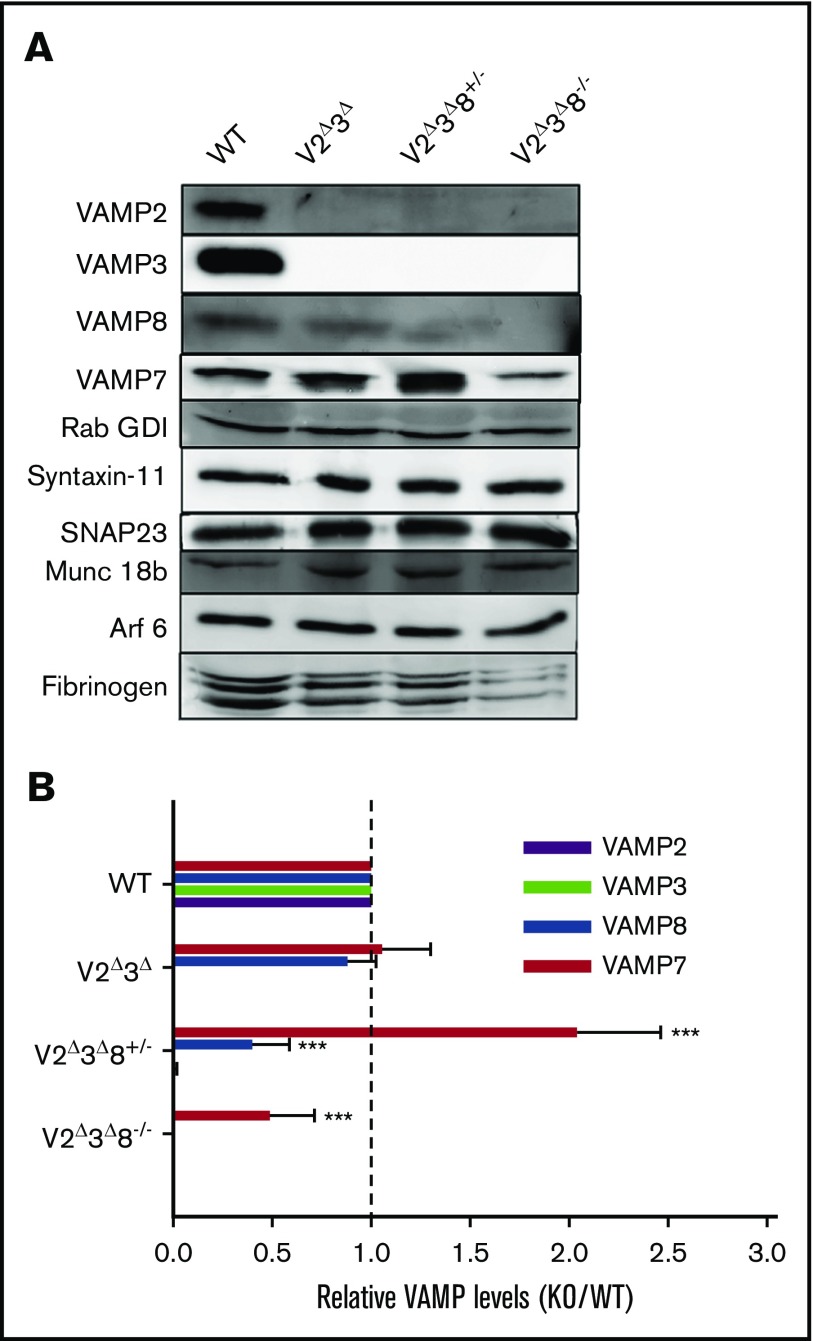
Semiquantitative western blotting, using Rab GDI as a loading control,25 confirmed the appropriate deletion or reduction of VAMP2, VAMP3, and VAMP8 in platelets from these strains (Figure 1). There was no change in expression of VAMP7 and VAMP8 in V2Δ3Δ platelets; consistent with our previous observations for VAMP2 and VAMP3.26 However, manipulating VAMP8 levels on the V2Δ3Δ background surprisingly affected VAMP7 levels. A partial decrease in VAMP8 (eg, V2Δ3Δ8+/− platelets) correlated with increased VAMP7, whereas complete loss of VAMP8 resulted in decreased VAMP7. These data imply coordinated control of VAMP levels in platelets or megakaryocytes and demonstrate that the V2Δ3Δ8−/− platelets are hypomorphic for VAMP7. Expression of the t-SNAREs syntaxin-11 and SNAP23, as well as the t-SNARE regulator Munc18b, were unaffected. Expectedly, loss of VAMP3 reduced fibrinogen levels in platelets,27 which was exacerbated in V2Δ3Δ8−/− platelets.
Figure 1.
Generation of V2Δ3Δand V2Δ3Δ8−/−animals. (A) A collage of representative western blots comparing protein levels among V2Δ3Δ, V2Δ3Δ8+/−, and V2Δ3Δ8−/− platelets. Washed platelets (5 × 107 platelets per lane) were prepared from WT, V2Δ3Δ, V2Δ3Δ8+/−, and V2Δ3Δ8−/− mice, and the indicated proteins were probed for. The blots for VAMP7, VAMP8, and Rab GDI were from the same membrane. (B) The VAMP isoforms in V2Δ3Δ, V2Δ3Δ8+/−, and V2Δ3Δ8−/− platelets were quantified using Rab GDI as a loading control and ImageQuant TL software (v 7.0). The data were plotted as the ratio of knockout/WT. The vertical dashed line indicates a ratio of 1. Data are representative of platelets collected from individual mice from each strain and ≥3 independent experiments (see supplemental Figure 1 for additional blots for VAMP7 and VAMP8). A Student t test was used to analyze the data. ***P < .001.
Release from activated V2Δ3Δ8−/− platelets was slower and less extensive
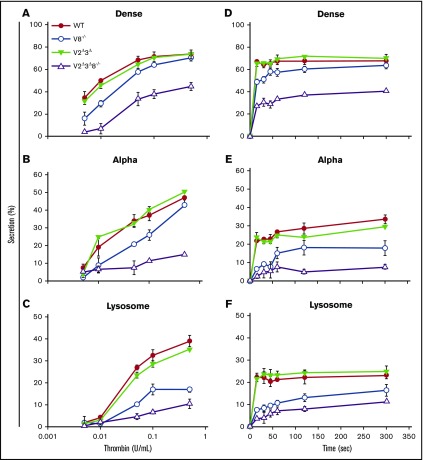
To measure how VAMP2, VAMP3, and VAMP8 influenced platelet granule secretion, we examined the agonist- and time-dependent release of cargo from all 3 platelet granules: dense, α, and lysosomes. The release from V8−/− platelets was reduced at lower thrombin concentration but reached comparable levels for dense and α-granule release from WT platelets as thrombin concentrations increased (Figure 2A-C). As previously seen,16 loss of VAMP8 had more influence on α-granule (∼40%-50% reduction) and lysosome (∼50% reduction) release than on dense granule release. This may be due, at least in part, to the defective release of secondary agonists from dense granules (adenosine triphosphate [ATP]/adenosine 5′-diphosphate [ADP], calcium, serotonin) that affects the release from α granules and lysosomes.28 Secretion of granule cargo from V2Δ3Δ platelets, in response to increasing thrombin concentrations, was essentially equal to that from WT platelets (Figure 2A-C) and underlined the ancillary roles of VAMP2 and VAMP3 in platelet secretion. V2Δ3Δ8−/− platelets showed an ∼50% decrease in dense granule secretion, an ∼80% to 90% reduction in α-granule secretion, and an ∼70% reduction in lysosomal secretion upon stimulation with 0.5 U/mL thrombin (Figure 2A-C), indicating that loss of VAMP2, VAMP3, and VAMP8 had a synergistic effect on secretion that was greater than the deletion of VAMP8 alone, perhaps due to the additional loss of VAMP7.
Figure 2.
Loss of VAMP2, VAMP3, and VAMP8 affects the kinetics and the extent of platelet secretion. [3H]-5-[HT] (serotonin)–labeled platelets from WT, V8−/−, V2Δ3Δ, and V2Δ3Δ8−/− mice were prepared as described in supplemental Methods. The release of [3H]-5-[HT] from dense granules (A,D), PF4 from α granules (B,E), and β-hexosaminidase from lysosomes (C,F) was measured, and percentage secretion was calculated as described in supplemental Methods. (A-C) For the thrombin dose-response experiment, platelets were stimulated for 2 minutes with the indicated concentrations of thrombin. (D-F) For the time-course experiments, platelets were stimulated with 0.05 U/mL thrombin for the indicated times Data are mean ± standard error of the mean of triplicate measurements and are representative of ≥3 independent experiments.
Time-course analysis (using 0.05 U/mL thrombin) showed that the loss of VAMP8 affected the rates of early (first 30 seconds) dense granule release but not its maximal extent16 (Figure 2D). Dense granule release rates in V2Δ3Δ platelets were not affected, whereas V2Δ3Δ8−/− platelets showed delayed dense granule release that reached only ∼50% of WT at 5 minutes (Figure 2D). Similar patterns were observed in α and lysosomal release (Figure 2E-F). These patterns are consistent with ancillary roles for VAMP2 and VAMP316 or, alternatively, the ∼50% reduction in VAMP7 levels noted in Figure 1. These data demonstrate that manipulation of the VAMPs in platelets alters release kinetics and extents. These data imply differential roles for these 3 VAMPs in platelet exocytosis and perhaps in some steps of granule cargo sorting or biogenesis (eg, our report of a role for VAMP3 in fibrinogen trafficking27).
V2Δ3Δ8−/− platelets showed normal integrin activation and modest platelet aggregation defects.
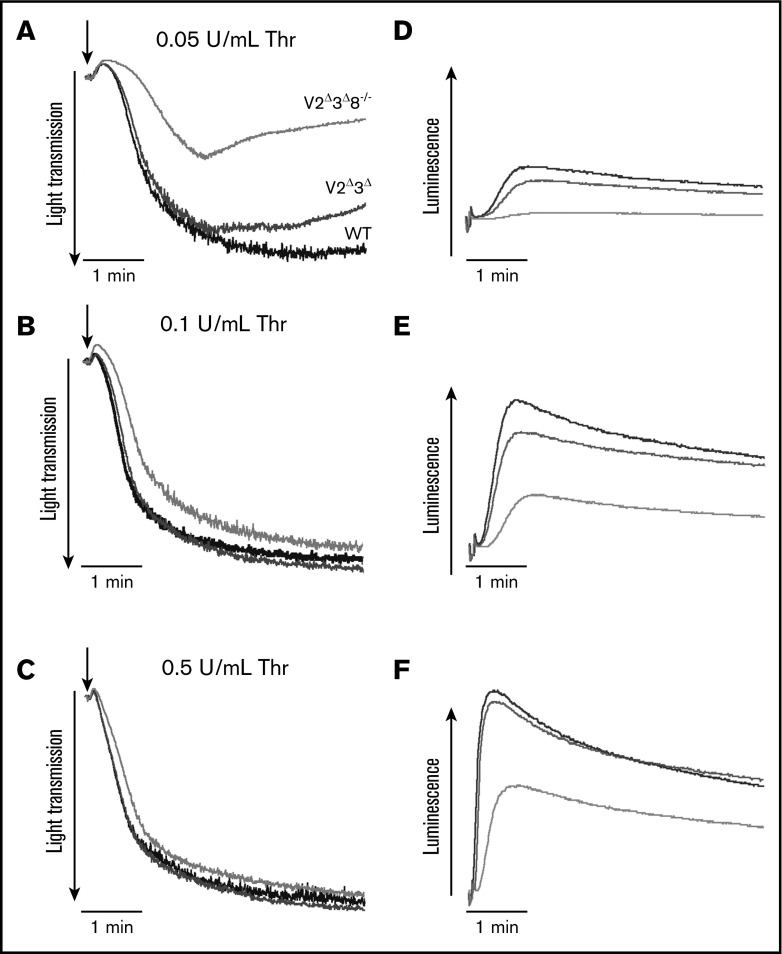
To confirm the phenotypes indicated by the secretion assays, we used lumi-aggregometry to analyze ATP/ADP release. Release in response to 0.05, 0.1, and 0.5 U/mL thrombin was minimally affected in V2Δ3Δ platelets but was less inhibited at the higher concentrations in V2Δ3Δ8−/− platelets (Figure 3D-F). Aggregation was not affected at the higher agonist doses (Figure 3A-C). Under our in vitro conditions (0.05 U/mL thrombin), there was no ATP secretion from, and limited aggregation of, V2Δ3Δ8−/− platelets. The ATP release at 0.1 and 0.5 U/mL thrombin was sufficient to support normal aggregation (Figure 3B-C).
Figure 3.
Depletion of VAMP2, VAMP3, and VAMP8 in platelets affects ATP/ADP release but not aggregation. Aggregation (A-C) and ATP/ADP release (D-F) were monitored simultaneously in a lumi-aggregometer. Washed platelets (4 × 108/mL) from WT (black traces), V2Δ3Δ (dark gray traces), and V2Δ3Δ8−/− (light gray traces) platelets were stimulated with 0.05 U/mL (A), 0.1 U/mL (B), or 0.5 U/mL (C) thrombin (Thr), and ATP release and aggregation were measured for 5 minutes.
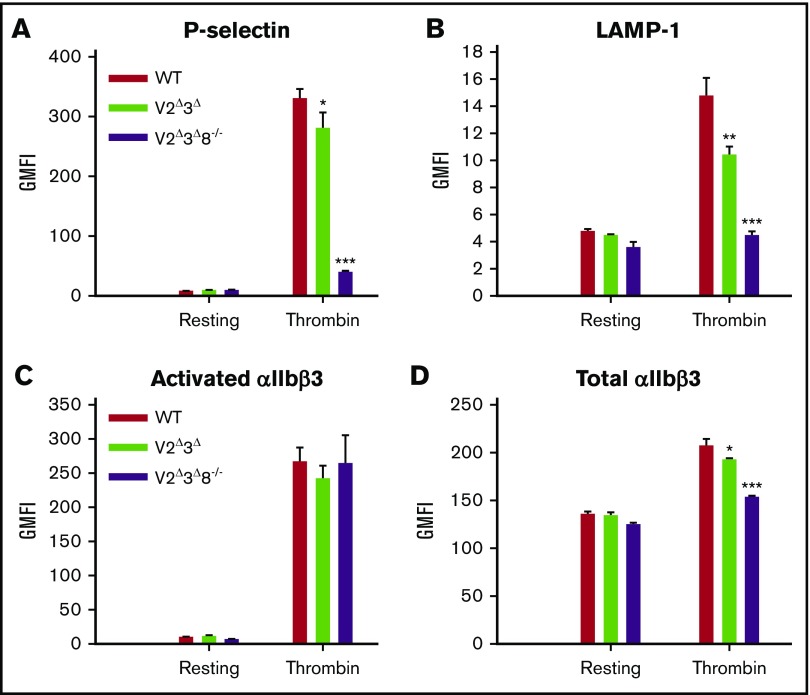
Surface exposure of P-selectin and LAMP-1 (α-granule and lysosome membrane markers, respectively) was analyzed by flow cytometry. Activation-dependent exposure of both granule membrane proteins was reduced in V2Δ3Δ8−/− platelets compared with WT platelets (Figure 4A-B). Total surface staining for αIIbβ3 integrin decreased by 10% to 15% in V2Δ3Δ platelets and by ∼25% in V2Δ3Δ8−/− platelets (Figure 4D). This may be due to an alteration of integrin trafficking to the plasma membrane (PM) and was not pursued further. In agreement with aggregometry data, there was no significant difference in activated integrin staining with phycoerythrin (PE)-conjugated JonA staining (Figure 4C). Thus, loss of VAMP2, VAMP3, and VAMP8 together, had minimal effects on αIIbβ3 integrin activation under the conditions tested.
Figure 4.
V2Δ3Δ8−/−platelets have lower P-selectin and LAMP-1 exposure and reduced integrin αIIbβ3 surface levels but normal activation. Washed platelets (5 × 107) from WT, V2Δ3Δ, and V2Δ3Δ8−/− mice were held resting or stimulated with thrombin (0.1 U/mL) for 2 minutes and then incubated with fluorescein isothiocyanate–conjugated anti–P-selectin (A), PE-conjugated LAMP-1 (B), PE-conjugated JonA (C), or fluorescein isothiocyanate–conjugated CD41/61 (D) antibodies for 20 minutes at room temperature. Fluorescence intensities were measured by flow cytometry. Shown are representative data and geometric mean fluorescence intensity (GMFI) (mean ± standard error of the mean) of ≥2 independent experiments. A Student t test was used to analyze the data. *P < .05, **P < .01, and ***P < .001.
Fusion pore dynamics were affected by the loss of platelet VAMPs.
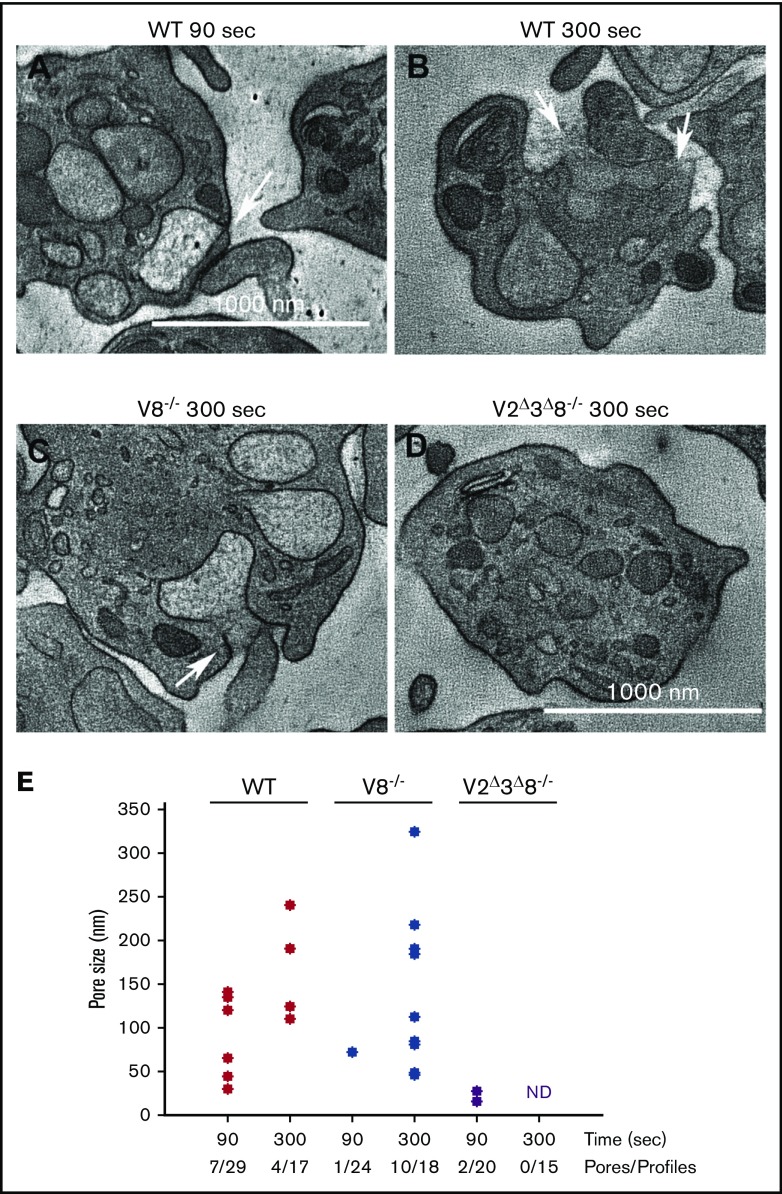
Because SNAREs mediate granule–PM fusion, we sought to determine how the loss of specific VAMPs affected fusion pore formation and/or dilation.29,30 Based on Figure 2, we selected 2 time points, 90 seconds (partial cargo release) and 300 seconds (maximal cargo release), and used electron microscopy (EM) tomography to measure fusion pore size and prevalence in platelets stimulated with thrombin. We focused on α granules because they are more abundant and readily identifiable. Fusion events were scored per platelet profile, and the widths of fusion pores were estimated across multiple z-steps in the EM tomographs (Figure 5). In the resting state, fusion pores were not observed (data not shown); however, upon stimulation, there was a time-dependent increase in prevalence and size, which culminated in granules becoming unidentifiable as they merged into the PM. For WT platelets, median pore diameter increased from 65 nm to 157 nm, whereas the mean was skewed slightly higher (90 seconds: 82.4 nm; 300 seconds: 166 nm; Figure 5A-B,E). This skew to larger pore size is further evidence that dilation is time dependent. In the V2Δ3Δ8−/− platelets, fusion pores were rare (2 per 35 profiles). Pores were only detected at the earliest time point, suggesting that the pores produced were unsustainable. Interestingly, the fusion pore prevalence and size distribution in the absence of VAMP8 showed a delay relative to WT. In V8−/− platelets, 1 fusion pore was identified in 24 profiles at 90 seconds, whereas 10 pores were identified in 18 profiles at 300 seconds. The median and mean values showed a similar skew to larger pores, as observed in WT platelets. The delayed pore formation in V8−/− platelets and the lack of sustained pore formation in V2Δ3Δ8−/− platelets are consistent with the decreased α-granule cargo release seen in Figure 2B,E.
Figure 5.
The loss of VAMP8 delays fusion pore formation and dilation. Platelets from the indicated strains were isolated and fixed in resting or thrombin-stimulated states (0.1 U/mL thrombin for 90 or 300 seconds). EM tomographs were analyzed for fusion pore structures, and the diameters were measured in z-series. Representative EM images of WT platelets at 90 seconds (A) and at 300 seconds (B), V8−/− platelets at 300 seconds (C), and V2Δ3Δ8−/− platelets at 90 seconds (D). The arrows indicate fusion pores. (E) The distribution of fusion pore size in platelets at early (90 seconds) and late (300 seconds) times poststimulation for the indicated strains is graphed as a scatter plot. Mean pore sizes are as follows: (WT: 90 seconds, 82.4 nm; WT: 300 seconds, 166 nm; V8−/−: 90 seconds, 72 nm; V8−/−: 300 seconds, 160.8 nm; V2Δ3Δ8−/−: 90 seconds, 21 nm, V2Δ3Δ8−/−: 300 seconds, pores could not be detected). Pores detected per platelet profile are indicated. ND, not detected.
Role of platelet secretion in spreading
Previous studies reported a role for granule secretion in platelet spreading.15,31 Using platelets from the mutant mice, we addressed the significance of granule secretion to spreading (supplemental Figure 4). When plated on fibrinogen-coated coverslips, V8−/− platelets showed a slight reduction in spreading compared with WT platelets (supplemental Figure 4B). V2Δ3Δ platelets covered more area than WT platelets at 30 minutes, although the area covered was similar at later time points (supplemental Figure 4). This is most likely due to the loss of VAMP3, which we previously showed causes platelets to spread more rapidly.27 Spreading of V2Δ3Δ8−/− platelets was markedly slower and the area covered was significantly reduced compared with WT platelets (supplemental Figure 4A).
Effect of platelet secretion on thrombosis and hemostasis
Together, these data demonstrate that VAMP-deficient mice have distinct platelet exocytosis profiles. These mice were used to assess how exocytosis affects platelet function in vivo, using 2 assays: tail bleeding time and FeCl3-induced carotid injury (Figure 6A-B). Tail bleeding times were comparable for WT, V3−/−, V2Δ3Δ, and V8−/− mice. Even after reducing VAMP8, the tail bleeding times did not increase in V2Δ3Δ8+/− mice. However, tail bleeding was significantly increased in V2Δ3Δ8−/− mice (mean time, 571.74 ± 72.61 seconds) compared with WT mice (mean time, 250.07 ± 86.82 seconds). For most V2Δ3Δ8−/− mice, application of pressure was required to stop the bleeding. In the FeCl3-induced injury model, the strains responded differently. The average times to occlusion were comparable for WT (∼6.5 minutes), V8+/− (∼7 minutes), V2Δ3Δ (∼7 minutes), and V2Δ3Δ8+/− (∼6.5 minutes) mice. Occlusion times were more variable in V8−/− mice, and the average was significantly increased (∼21 minutes). This was even more true in V2Δ3Δ8−/− mice (∼28 minutes), which generally failed to form occlusive thrombi during our observation period of 30 minutes. Males and females showed no differences in bleeding or occlusion times (supplemental Figure 6). These data demonstrate that platelet exocytosis contributes differentially to thrombosis vs hemostasis, because normal thrombosis appears to require more platelet secretion.
Figure 6.
Hemostasis and thrombus formation are impaired in V2Δ3Δ8−/−animals. (A) Tail bleeding times were measured after tail transection of WT (n = 28), V3−/− (n = 38), V2Δ3Δ (n = 20), V8+/− (n = 26), V8−/− (n = 31), V2Δ3Δ8+/− (n = 41), and V2Δ3Δ8−/− (n = 34) mice. (B) Thrombus formation in the carotid artery was induced by topical application of 5% FeCl3, and blood flow was monitored in WT (n = 6), V8+/− (n = 6), V2Δ3Δ (n = 7), V2Δ3Δ8+/− (n = 9), V8−/− (n = 7), and V2Δ3Δ8−/− (n = 7) mice. For panels A and B, data are mean ± standard error of the mean. The P values (log-rank test) are indicated and ***P < .001. The red line indicates the mean, and the black line indicates the median. The box represents 25th-75th percentiles. Whiskers/error bars above and below the box indicate the 90th and 10th percentiles, respectively. Outliers are as shown in tail bleeding figure. (C) APTT and PT were measured in platelet-poor plasma prepared from WT (n = 7), V8−/− (n = 5), V2Δ3Δ (n = 8), V2Δ3Δ8+/− (n = 3), and V2Δ3Δ8−/− (n = 5) mice. (D) PS exposure on the surface of thrombin-stimulated platelets from WT, V8+/−, V8−/−, V2Δ3Δ, V2Δ3Δ8+/−, and V2Δ3Δ8−/− mice was measured by fluorescein isothiocyanate–lactadherin binding and flow cytometry. The data are representative of 2 independent assays. *P < .05, Student t test. (E) A representative western blot showing the levels of TMEM16F in the indicated strains. Rab GDI was used as a loading control.
Because several factors control hemostasis, we examined the coagulation cascades and the degree of phosphatidylserine (PS) exposure in our strains. Prothrombin time (PT) and activated partial thromboplastin time (APTT) assays of plasma showed no differences among the strains, suggesting no defects in the coagulation system of these animals (Figure 6C). Although the loss of VAMP8 did not affect PS exposure, loss of VAMP2/3 did (∼30% reduction), as measured by lactadherin binding. Further deletion of VAMP8 (ie, V2Δ3Δ8−/−) exacerbated this defect to an ∼50% decrease in PS exposure in response to thrombin (Figure 6D). Although not significant in cases of V2Δ3Δ and V2Δ3Δ8+/−, the P values (.15 and .14, respectively) indicate a trend toward significance. These data reveal a connection between VAMP isoforms and PS exposure, perhaps through platelet granule secretion and\or intracellular trafficking in platelets. To gain insight into this effect, we probed for transmembrane protein 16F (TMEM16F), a phospholipid scramblase important for PS exposure.32-34 Its levels did not differ significantly in any of the strains tested (Figure 6E).
Discussion
In this article, we describe a collection of genetically engineered mice in which the platelet-secretory machinery is altered to yield differential effects on platelet exocytosis, thrombosis, and hemostasis (Figure 7A). We have measured platelet secretion and defined how the 4 major platelet VAMPs mediate the release of cargo from the 3 classes of granules. Using these mice, we have estimated the level of secretion needed for hemostasis in tail bleeding and occlusive thrombosis in a FeCl3-injury model. Our studies represent the first attempt at defining the extent to which platelet secretion is needed for thrombosis and hemostasis in vivo.
Figure 7.
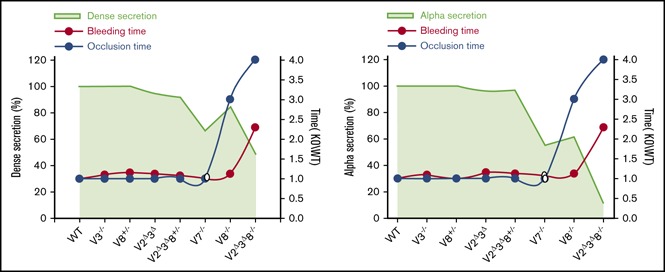
Correlating VAMP-mediated exocytosis to platelet function. VAMP levels, secretion, and function are graphically correlated using our data (closed circles) and that from Koseoglu et al15 (open circles). (A) Calculated VAMP levels remaining in each of the strains tested, relative to WT, were based on the data in Figure 1B and from Graham et al.12 (B) The VAMP levels in each strain are represented by green shading. The red line indicates α-granule secretion, and the blue line indicates dense granule secretion. Secretion levels are from Figure 2 (0.05 U/mL thrombin for 2 minutes). Secretion from dense and α granules of V7−/− platelets (open symbols) were estimated based on ATP release and P-selectin exposure, respectively.15 All values are normalized to WT. Dense granule release (C) and α-granule release (D) are as in panel B and are plotted as green shading. The red line indicates tail bleeding times, and the blue line indicates the occlusive thrombosis times in the FeCl3-injury model. Data are normalized to WT. These data were taken from other figures in this article in which variations were indicated; therefore, error indicators were omitted from these graphs to improve figure clarity.
Role of VAMPs in platelet secretion
In previous reports,15,16 single deletion of VAMP7 or VAMP8 blocks cargo release, but not completely, suggesting that compensatory effects or alternative membrane-fusion pathways could occur. Using recombinant VAMPs as standards and quantitative western blotting, Graham et al showed that VAMP2 and VAMP8 are most abundant in mouse platelets, whereas VAMP3, VAMP7, and VAMP8 are abundant in human platelets.12 This was validated by mass spectrometry.11,13 Using the semiquantitative western blotting data in Figure 1, we can estimate the remaining relative VAMP levels in our mice (Figure 7A) and, thus, correlate their levels to platelet secretion. Discordance of the curves in Figure 7B demonstrate that total VAMP levels, in aggregate, do not correlate with platelet secretion. This rebuts promiscuous VAMP functions, which might be predicted based on the heterogeneity of VAMP interactions observed in some systems.35-37 Instead, our data confirm major contributions for VAMP7 and VAMP8.15,16
Although the loss of VAMP2/3 had minimal effect on its own, it exacerbated the effects of VAMP8 loss, perhaps due to effects on VAMP7. V2Δ3Δ8−/− platelets had significant secretion defects at all agonist levels, and V2Δ3Δ8−/− mice had profound bleeding and occlusion defects in vivo. Although this suggests some contributions from VAMP2 and VAMP3, the nature of their role is unclear. VAMP3 mediates integrin trafficking and endocytosis of granule cargo in platelets.27 VAMP8 is also needed for endocytic trafficking in some cells.38 Possibly, VAMP3 and VAMP8 function in overlapping pathways; thus, the double deletion of VAMP3 and VAMP8 affects the trafficking and/or localization of VAMP7, accounting for its reduction in V2Δ3Δ8−/− platelets (Figure 1; supplemental Figure 1A). Consistently, we have been unable to create a global VAMP3/8 deletion strain due to embryonic lethality (S.W.W., unpublished observations). VAMP2’s role is unclear because its levels in human platelets are low.11,12,14,16 In practical terms, our V2Δ3Δ8−/− mice are hypomorphic for VAMP7 (Figure 1), which, given the importance of VAMP7 and VAMP8, explains the severity of the secretion and hemostatic defects measured (Figure 6). Fortuitously, we have created a strain with a greater reduction in secretion than either of the single VAMP deletions. Future attempts to create VAMP7/8−/− mice are underway to fully address this point.
To understand how VAMPs affect the kinetics of platelet granule secretion, we conducted detailed EM tomographic studies and examined fusion pore formation and subsequent dilation. Such pores are the most likely route of granule cargo release, and our data demonstrate that VAMPs are required for fusion pore formation and expansion (Figure 5). Few pores were detected in V2Δ3Δ8−/− platelets. The delayed pore formation in V8−/− platelets confirms the importance of VAMP8 for initial membrane fusion and parallels the delayed secretion noted in Figure 2. The remaining VAMP7 is not sufficient for rapid fusion pore formation and/or expansion. These data imply that VAMP8 and VAMP7 contribute to normal fusion pore formation/dilation. Consistently, reconstituted in vitro assays show that alterations in VAMP levels affect fusion pore dynamics and stability.39 Such alterations clearly affect granule cargo release (Figure 2).
A previous analysis40 suggests that VAMP8 is preferred for compound granule–granule fusion, whereas VAMP7 mediates PM–granule (primary) fusion. However, these discrete roles should not be considered mutually exclusive nor absolute. Loss of VAMP8 (V8−/−) delayed primary fusion of α granules, and loss of VAMP8 and reduction of VAMP7 (V2Δ3Δ8−/−) almost eliminated it (Figure 5). The partial release defects seen in platelets from the single-deletion mouse strains15,16 (Figure 2) argue that VAMP7 and VAMP8 are used for primary fusion, which is required for measurable cargo release. VAMP8’s abundance relative to that of VAMP7 makes it a logical choice to mediate compound fusion, because more of those events are likely to be required; however, further analysis of V7−/− platelets will be needed to directly address this point. It should be noted that using primary and compound fusion would differentially affect cargo release rates and extents and perhaps account for the heterogeneity of α-granule cargo release noted previously.41
Platelet secretion and thrombosis
Thrombi appear stratified with highly activated platelets forming a central core and less activated platelets at the periphery (ie, the shell).42-45 We posit that the low-agonist doses in our assays resemble platelet secretion in the shell, whereas the high-agonist doses resemble what occurs in the core of a thrombus, proximal to the tissue damage (Figure 2A-C). At either low- or high-agonist doses, loss of VAMP2/3 had no significant effect on secretion and no effect on our in vivo assays of thrombosis and hemostasis. Loss of VAMP8 alone had more effect on α-granule release than on dense granule release. V8−/− animals showed delayed thrombosis, with smaller thrombi in the laser-injury model.12 Consistently, there was also an effect on FeCl3-induced occlusion, but there was no overt defect on tail bleeding (Figure 6A-B). Thus, that level of secretion is sufficient to stop bleeding but is insufficient for extensive thrombus growth. Single deletion of VAMP7 caused no thrombosis or bleeding defects.15 Deletion of VAMP2, VAMP3, and VAMP8 together caused a profound effect on release from dense and α granules at all agonist levels. The defect was sufficient for a bleeding diathesis and an FeCl3-induced occlusion defect (Figure 6A-B). It should be noted that release from these platelets was not completely abolished; however, what little is released (presumably via VAMP7-mediated fusion) was insufficient for normal hemostasis or thrombosis.
Analysis of our data (Figure 7) demonstrates several aspects of how secretion may affect hemostasis and thrombosis. First, lysosomal release did not appear to be required. It was profoundly defective in several of our strains (Figure 2), but that, on its own, failed to correlate with any hemostatic defects. Second, defects in dense granule release had a greater impact on bleeding than did defects in α-granule release (Figure 7C-D). This is consistent with the phenotypes of patients (and mouse models) with dense and α granulopathies. Patients with Hermansky–Pudlak syndrome or Chédiak–Higashi syndrome, in general, have more severe bleeding than do patients with gray platelet syndrome.46 Third, bleeding was most profound when the platelets were less able to secrete at the highest agonist doses. Milder release defects with low-dose thrombin did not correlate with bleeding but did affect thrombus growth16 (Figure 7C-D). Taken together, our data underline the importance of rapid and maximal release from dense granules and indicate a threshold for effective hemostasis. Our data demonstrate that thrombosis is more sensitive to manipulations of platelet secretion than is hemostasis.
VAMP deficiency and coagulation
Given the bleeding phenotypes (Figure 6A-B) and the fact that coagulation factors are secreted proteins, it was important to measure coagulation in our mice. There were no differences in PT or APTT, indicating no defects in extrinsic or intrinsic pathways, respectively (Figure 6C). Exposure of PS on platelet surfaces drives the formation and localization of prothrombinase complexes.47 Surprisingly, platelets from 3 of our mouse strains showed some defect in lactadherin binding. V2Δ3Δ8−/− platelets had the most robust impairment (∼50% decrease), which could explain their hemostasis defects (Figure 6A-B). However, platelets from V2Δ3Δ and V2Δ3Δ8+/− mice had lower lactadherin binding (∼30% decrease); however, neither had secretion (Figure 2; supplemental Figure 3) or hemostatic (Figures 6 and 7) defects. This surprising finding yields insights into another facet of how platelet secretion could affect hemostasis and suggests a threshold for PS exposure requirements. Nbeal2−/− animals also have a PS exposure defect consistent with α granules being important for PS exposure.48 It is unclear whether the loss of dense granule release also contributes to this deficit, because the secondary agonists in dense granules (ie, ADP) could be needed to induce PS exposure.28 Alternatively, given VAMPs’ roles in intracellular trafficking (ie, proposed effect on VAMP7), sorting and/or targeting of the enzymes needed for PS exposure could have been influenced during platelet biogenesis. However, TMEM16F, which is a Ca2+-dependent phospholipid scramblase important for PS exposure,32-34 was unchanged in our strains (Figure 6E). Thus, further investigations are necessary to address these various questions.
Significance
This is the first study that defines the contributions of platelet granule secretion to hemostasis and thrombosis. By altering the types and amounts of VAMPs, we created a set of mouse strains with distinct platelet-secretion profiles that were used to define the threshold of secretion needed for occlusive thrombosis without bleeding. About 50% to 60% of dense and α-granule secretion is needed for this crucial balance. This insight is invaluable in developing novel antithrombotic strategies and better managing current antithrombotic regimens. Furthermore, the animals described in this study will be vital tools to address how platelet secretion affects other physiological and pathophysiological processes.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Qingjun Wang, Jeremy Wood, and members of the Whiteheart Laboratory, Subershan Wigna-Kumar, Harry Chanzu, Laura Tichacek, Josh Lykins, Shravani Prakhya, and Patrick Thompson, for their careful perusal of this manuscript. They are very grateful for the efforts of Ming Zhang in managing our mouse colony. The authors also thank Greg Bauman and Jennifer Strange (University of Kentucky Flow Cytometry Core Facility), as well as the Department of Laboratory Animal Resources for technical assistance.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (HL119393 [B.S.]; HL56652 and HL138179 [S.W.W.]), American Heart Association Grant-in-Aid AHA16GRNT27620001, a Veterans Affairs Merit Award (S.W.W.), and American Heart Association predoctoral fellowship 15PRE25550020 (S.J.).
Authorship
Contribution: S.J. and S.W.W. designed and performed the experiments, analyzed data, and wrote the manuscript; M.B., A.K., and J.Z. assisted with some of the platelet-secretion and spreading experiments and the biochemical analyses; and I.D.P. and B.S. performed the electron microscopy and image analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sidney W. Whiteheart, Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, 741 S Limestone, B271 BBSRB, Lexington, KY 40536; e-mail: whitehe@uky.edu.
References
- 1.Smyth SS, McEver RP, Weyrich AS, et al. ; 2009 Platelet Colloquium Participants. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7(11):1759-1766. [DOI] [PubMed] [Google Scholar]
- 2.Tesfamariam B. Involvement of platelets in tumor cell metastasis. Pharmacol Ther. 2016;157:112-119. [DOI] [PubMed] [Google Scholar]
- 3.Nurden A, Nurden P. Advances in our understanding of the molecular basis of disorders of platelet function. J Thromb Haemost. 2011;9(suppl 1):76-91. [DOI] [PubMed] [Google Scholar]
- 4.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95(26):15781-15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631-643. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Bernstein AM, Lemons PP, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 in dense core granule release. Blood. 2000;95(3):921-929. [PubMed] [Google Scholar]
- 7.Chen D, Lemons PP, Schraw T, Whiteheart SW. Molecular mechanisms of platelet exocytosis: role of SNAP-23 and syntaxin 2 and 4 in lysosome release. Blood. 2000;96(5):1782-1788. [PubMed] [Google Scholar]
- 8.Lemons PP, Chen D, Whiteheart SW. Molecular mechanisms of platelet exocytosis: requirements for alpha-granule release. Biochem Biophys Res Commun. 2000;267(3):875-880. [DOI] [PubMed] [Google Scholar]
- 9.Ye S, Karim ZA, Al Hawas R, Pessin JE, Filipovich AH, Whiteheart SW. Syntaxin-11, but not syntaxin-2 or syntaxin-4, is required for platelet secretion. Blood. 2012;120(12):2484-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golebiewska EM, Harper MT, Williams CM, et al. Syntaxin 8 regulates platelet dense granule secretion, aggregation, and thrombus stability. J Biol Chem. 2015;290(3):1536-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkhart JM, Schumbrutzki C, Wortelkamp S, Sickmann A, Zahedi RP. Systematic and quantitative comparison of digest efficiency and specificity reveals the impact of trypsin quality on MS-based proteomics. J Proteomics. 2012;75(4):1454-1462. [DOI] [PubMed] [Google Scholar]
- 12.Graham GJ, Ren Q, Dilks JR, Blair P, Whiteheart SW, Flaumenhaft R. Endobrevin/VAMP-8-dependent dense granule release mediates thrombus formation in vivo. Blood. 2009;114(5):1083-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeiler M, Moser M, Mann M. Copy number analysis of the murine platelet proteome spanning the complete abundance range. Mol Cell Proteomics. 2014;13(12):3435-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi S, Whiteheart SW. The nuts and bolts of the platelet release reaction. Platelets. 2017;28(2):129-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koseoglu S, Peters CG, Fitch-Tewfik JL, et al. VAMP-7 links granule exocytosis to actin reorganization during platelet activation. Blood. 2015;126(5):651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren Q, Barber HK, Crawford GL, et al. Endobrevin/VAMP-8 is the primary v-SNARE for the platelet release reaction. Mol Biol Cell. 2007;18(1):24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiffman D, O’Meara ES, Bare LA, et al. Association of gene variants with incident myocardial infarction in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2008;28(1):173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiffman D, Rowland CM, Louie JZ, et al. Gene variants of VAMP8 and HNRPUL1 are associated with early-onset myocardial infarction. Arterioscler Thromb Vasc Biol. 2006;26(7):1613-1618. [DOI] [PubMed] [Google Scholar]
- 19.Kondkar AA, Bray MS, Leal SM, et al. VAMP8/endobrevin is overexpressed in hyperreactive human platelets: suggested role for platelet microRNA. J Thromb Haemost. 2010;8(2):369-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63(3):305-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109(4):1503-1506. [DOI] [PubMed] [Google Scholar]
- 22.Schiavo G, Benfenati F, Poulain B, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359(6398):832-835. [DOI] [PubMed] [Google Scholar]
- 23.McMahon HT, Ushkaryov YA, Edelmann L, et al. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364(6435):346-349. [DOI] [PubMed] [Google Scholar]
- 24.Schoch S, Deák F, Königstorfer A, et al. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294(5544):1117-1122. [DOI] [PubMed] [Google Scholar]
- 25.Choi W, Karim ZA, Whiteheart SW. Protein expression in platelets from six species that differ in their open canalicular system. Platelets. 2010;21(3):167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schraw TD, Rutledge TW, Crawford GL, et al. Granule stores from cellubrevin/VAMP-3 null mouse platelets exhibit normal stimulus-induced release. Blood. 2003;102(5):1716-1722. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee M, Joshi S, Zhang J, et al. Cellubrevin/vesicle-associated membrane protein-3-mediated endocytosis and trafficking regulate platelet functions. Blood. 2017;130(26):2872-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper MT, van den Bosch MT, Hers I, Poole AW. Platelet dense granule secretion defects may obscure α-granule secretion mechanisms: evidence from Munc13-4-deficient platelets. Blood. 2015;125(19):3034-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science. 2002;297(5585):1349-1352. [DOI] [PubMed] [Google Scholar]
- 30.Koseoglu S, Dilks JR, Peters CG, et al. Dynamin-related protein-1 controls fusion pore dynamics during platelet granule exocytosis. Arterioscler Thromb Vasc Biol. 2013;33(3):481-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters CG, Michelson AD, Flaumenhaft R. Granule exocytosis is required for platelet spreading: differential sorting of α-granules expressing VAMP-7. Blood. 2012;120(1):199-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang H, Kim A, David T, et al. TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell. 2012;151(1):111-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujii T, Sakata A, Nishimura S, Eto K, Nagata S. TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc Natl Acad Sci USA. 2015;112(41):12800-12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baig AA, Haining EJ, Geuss E, et al. TMEM16F-mediated platelet membrane phospholipid scrambling is critical for hemostasis and thrombosis but not thromboinflammation in mice-brief report. Arterioscler Thromb Vasc Biol. 2016;36(11):2152-2157. [DOI] [PubMed] [Google Scholar]
- 35.Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R. Mixed and non-cognate SNARE complexes. Characterization of assembly and biophysical properties. J Biol Chem. 1999;274(22):15440-15446. [DOI] [PubMed] [Google Scholar]
- 36.Tsui MM, Banfield DK. Yeast Golgi SNARE interactions are promiscuous. J Cell Sci. 2000;113(Pt 1):145-152. [DOI] [PubMed] [Google Scholar]
- 37.Yang B, Gonzalez L Jr, Prekeris R, Steegmaier M, Advani RJ, Scheller RH. SNARE interactions are not selective. Implications for membrane fusion specificity. J Biol Chem. 1999;274(9):5649-5653. [DOI] [PubMed] [Google Scholar]
- 38.Antonin W, Holroyd C, Tikkanen R, Höning S, Jahn R. The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol Biol Cell. 2000;11(10):3289-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao H, Das D, Courtney NA, et al. Dynamics and number of trans-SNARE complexes determine nascent fusion pore properties. Nature. 2018;554(7691):260-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckly A, Rinckel JY, Proamer F, et al. Respective contributions of single and compound granule fusion to secretion by activated platelets. Blood. 2016;128(21):2538-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonnalagadda D, Izu LT, Whiteheart SW. Platelet secretion is kinetically heterogeneous in an agonist-responsive manner. Blood. 2012;120(26):5209-5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsh JD, Stalker TJ, Voronov R, et al. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood. 2014;124(11):1808-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomaiuolo M, Stalker TJ, Welsh JD, Diamond SL, Sinno T, Brass LF. A systems approach to hemostasis: 2. Computational analysis of molecular transport in the thrombus microenvironment. Blood. 2014;124(11):1816-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stalker TJ, Welsh JD, Tomaiuolo M, et al. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood. 2014;124(11):1824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welsh JD, Poventud-Fuentes I, Sampietro S, Diamond SL, Stalker TJ, Brass LF. Hierarchical organization of the hemostatic response to penetrating injuries in the mouse macrovasculature. J Thromb Haemost. 2017;15(3):526-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rendu F, Brohard-Bohn B. The platelet release reaction: granules’ constituents, secretion and functions. Platelets. 2001;12(5):261-273. [DOI] [PubMed] [Google Scholar]
- 47.Swieringa F, Kuijpers MJ, Lamers MM, van der Meijden PE, Heemskerk JW. Rate-limiting roles of the tenase complex of factors VIII and IX in platelet procoagulant activity and formation of platelet-fibrin thrombi under flow. Haematologica. 2015;100(6):748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deppermann C, Cherpokova D, Nurden P, et al. Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice. J Clin Invest. 2013;69210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.