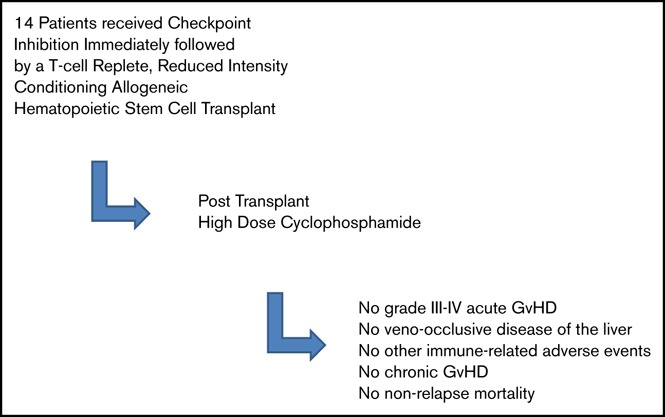
Key Points
Checkpoint inhibition use before alloBMT followed by PTCy is not associated with increased aGvHD or transplant-related mortality/morbidity.
Prior checkpoint inhibitor therapy should not be a contraindication to allogeneic transplantation, especially in the setting of PTCy.
Abstract
Published reports suggest that immune checkpoint inhibitors (ICIs) before allogeneic blood or marrow transplantation (alloBMT) may increase the incidence of graft-versus-host disease (GvHD), immune-related adverse events, and nonrelapse mortality (NRM); this led to the US Food and Drug Administration issuing a “Warning and Precaution” regarding the potential for life-threatening immune-mediated complications associated with alloBMT after nivolumab and pembrolizumab. We retrospectively reviewed the outcomes of 14 consecutive patients who received ICIs as their final salvage therapy before T-cell–replete alloBMT using reduced-intensity conditioning. All patients received posttransplant cyclophosphamide (PTCy), which significantly limits severe GvHD, even in the mismatched-donor setting. There was no grade 3-4 acute GvHD (aGvHD), and all 6 cases of grade 2 aGvHD readily resolved with immunosuppression. No patient experienced veno-occlusive disease of the liver, other immune-related adverse events, chronic GvHD, or NRM. There have been 2 relapses (15-month median follow-up), with 12 of 14 patients remaining alive, well, and progression-free. The only death was a result of disease relapse. Although more experience is needed, our data suggest that concerns over immunologic complications associated with ICIs should not preclude allogeneic bone marrow transplantation with PTCy as GvHD prophylaxis.
Visual Abstract
Introduction
Several preliminary reports suggest that toxicity, including veno-occlusive disease (VOD) of the liver (VOD), graft-versus-host disease (GvHD), and death, occur when immune checkpoint inhibitors (ICIs) are used before or after allogeneic blood or marrow transplantation (alloBMT).1-3 In a retrospective review of 39 lymphoma patients treated with PD-1 inhibition before T-cell–replete nonmyeloablative alloBMT, Merryman et al observed a higher than expected incidence of rapid and virulent acute GvHD (aGvHD).1 Moreover, the current nivolumab package insert cites 6 deaths in 17 patients who underwent alloBMT after nivolumab treatment: 5 from GvHD and 1 from VOD. Steroid-requiring febrile syndromes, without identified infectious causes, were also reported in 6 patients in the first 6 weeks after alloBMT.4 The etiology of these complications may be the persistence of PD-1 inhibition after alloBMT that may augment alloreactive T cells.5 The US Food and Drug Administration has since issued a “Warning and Precaution” regarding the potential for life-threatening immune-mediated complications associated with alloBMT after nivolumab and pembrolizumab. Moreover, they mandated a postmarketing study to further characterize the safety of alloBMT after ICIs as a condition of continued approval,6 and the Center for International Blood and Marrow Transplant Research is currently conducting a retrospective analysis of patients treated with ICIs before alloBMT to further evaluate safety.
Posttransplant cyclophosphamide (PTCy) for primary GvHD prophylaxis after alloBMT is associated with low rates of severe acute and chronic GvHD in patients receiving matched7,8 and partially mismatched9,10 grafts. Several registry analyses have shown that rates of GvHD are actually lower with haploidentical donors using PTCy than after matched unrelated and even matched sibling donors using conventional calcineurin inhibitor/methotrexate GvHD prophylaxis.9-13 Here, we report our experience with ICIs used as a planned bridge to alloBMT using PTCy. As in other settings, PTCy appears to lessen the risk for serious GvHD.
Methods
After Institutional Review Board (IRB) approval (IRB00163803), we retrospectively reviewed the records of all alloBMT recipients between January 2006 and July 2017 to identify patients who received ICIs as a treatment to control disease prior to alloBMT with PTCy. Only patients who received ICIs as their last salvage therapy immediately before alloBMT were included. GvHD was assessed using the Center for International Blood and Marrow Transplant Research GvHD index.14 Immune-related adverse events were graded using Common Terminology Criteria for Adverse Events (version 4.0).
Fourteen consecutive patients were identified (Table 1). The indication for alloBMT was acute myeloid leukemia (AML)/myelodysplastic syndrome (n = 2), non-Hodgkin lymphoma (n = 2), and Hodgkin lymphoma (n = 10). All received reduced-intensity conditioning with fludarabine, cyclophosphamide, and total body irradiation and GvHD prophylaxis with PTCy, mycophenolate and tacrolimus or sirolimus as previously described.15-17 No changes in approach were made because of ICI therapy. Matched unrelated (n = 2), mismatched unrelated (n = 2), and related haploidentical (n = 10) donors were used. The median age at time of alloBMT was 35.6 years (range, 24.3-61.9). Bone marrow allografts were used for 12 patients, and peripheral blood grafts were used for 2 patients.
Table 1.
Baseline patient characteristics
| Number | |
|---|---|
| Age at time of alloBMT, y | 35.6 (range, 24.3-61.9) |
| Illness, n | |
| Hodgkin lymphoma | 10 |
| AML/myelodysplastic syndrome | 2 |
| Primary effusion lymphoma | 1 |
| Diffuse large B cell lymphoma | 1 |
| Stem cell donor, n | |
| Related haploidentical | 10 |
| Degree of mismatch | |
| 6 of 10 | 1 |
| 5 of 10 | 9 |
| Matched unrelated | 2 |
| Mismatched unrelated | 2 |
| Degree of mismatch | |
| 9 of 10 | 1 |
| 7 of 10 | 1 |
| Stem cell source, n | |
| Bone marrow | 12 |
| Peripheral blood | 2 |
| Checkpoint inhibitory history | |
| Checkpoint inhibitor(s) received, n | |
| Nivolumab | 8 |
| Ipilimumab | 3 |
| Pembrolizumab | 2 |
| Ipilimumab and nivolumab | 1 |
| No. of infusions, median | 6 (range, 3-24) |
| Median time between last checkpoint inhibitor infusion and stem cell infusion, d | 42 (range, 18-231) |
| Response to checkpoint inhibitor, n | |
| Complete response | 8 |
| Partial response | 6 |
All patients received ICIs as salvage therapy before a planned alloBMT; 8 patients received single-agent nivolumab, 3 patients received single-agent ipilimumab, 2 patients received single- agent pembrolizumab, and 1 patient received a combination of nivolumab and ipilimumab (Table 1). The patients received a median of 6 cycles (range, 3-24) of therapy. Eight patients achieved a complete response prior to alloBMT, and 6 patients received a partial response. The median interval from last ICI treatment to transplant was 42 days, (range, 18-212). The patient with the longest interval had alloBMT delayed awaiting resolution of ipilimumab-induced severe colitis and transaminitis.
Results
All patients had full hematologic recovery after alloBMT. The median day to an absolute neutrophil count of 0.5 × 109/L was 18 days (range 14-36), and the median day to platelet transfusion independence was 18.5 days (range 13-39). By 28 days posttransplant, 12 patients achieved donor T-cell engraftment: 8 were ≥95% and 4 were 5% to 95% donor. One of the 4 latter patients lost all evidence of donor chimerism by day 110. The 2 primary and 2 secondary graft failures all had full host hematologic recovery. At a median follow-up of 10 months (range, 6-13), 7 patients continue to exhibit full donor chimerism, and 4 patients have stable mixed T-cell chimerism (72% [range, 15-91%] donor-derived CD3+ cells). No patient experienced VOD of the liver or grade 3-4 aGvHD. Six patients developed grade 2 GvHD and responded to steroids (n = 2) or to steroids combined with tacrolimus/sirolimus (n = 4). Five of the 6 cases of GvHD occurred in patients with full donor engraftment. One patient experienced grade 2 cutaneous aGvHD confirmed by skin biopsy, despite partial T-cell engraftment. Her symptoms resolved entirely with steroids. She remains a partial chimera without evidence of lymphoma. One of the 6 cases of GvHD occurred in a patient who received a peripheral blood product. Of the 6 aGvHD patients, 4 patients received single-agent nivolumab, 1 patient received pembrolizumab, and 1 patient received ipilimumab.
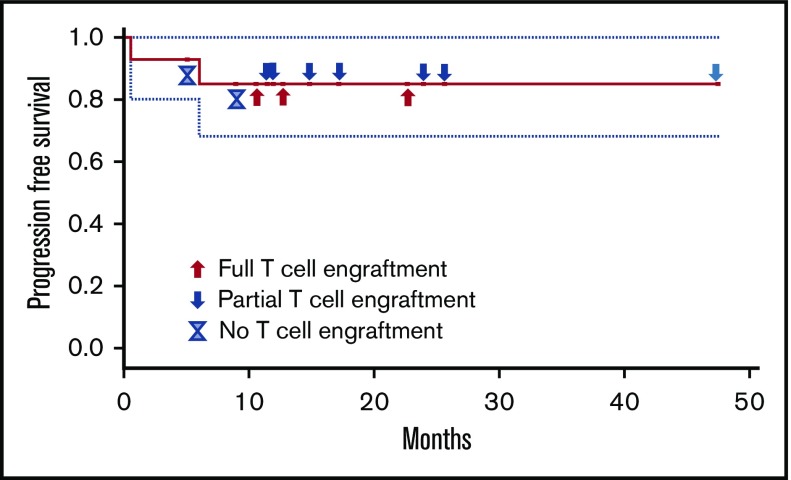
There were no other immune-related adverse events, there were no cases of grade ≥3 fevers before hematopoietic engraftment, and no chronic GvHD has been seen. At an estimated median follow-up of 12.7 months, 12 patients remain without disease progression (Figure 1). Two patients had disease progression (1 patient with primary effusion lymphoma and 1 patient with AML), with 1 patient eventually dying from the malignancy, the only death to this point in the 14 patients. The AML patient relapsed in the setting of having achieved full donor T-cell engraftment, whereas the lymphoma patient never achieved donor engraftment.
Figure 1.
Progression-free survival and T-cell engraftment status.
Discussion
We studied a group of patients who underwent alloBMT with PTCy after bridge-to-transplant therapy with ICIs. There were no nonrelapse deaths and no severe GvHD. Merryman et al have similarly reported on immune complications following alloBMT.1 They report that the incidence of grade 4 aGvHD appeared higher than in previous studies and that 3 patients died with a particularly rapid and virulent form of aGvHD. The absence of severe GvHD in our report may reflect the uniform use of PTCy in our patients, the predominance of bone marrow rather than peripheral blood grafts in our patients, or other factors yet to be defined. However, we should note that the absence of severe GvHD in our series does not reflect better HLA matching, because the great majority of our donors (86%) were only partially matched. Transient mild (grade 2) GvHD occurred in 6 patients, consistent with what has been reported in larger series of patients receiving PTCy.15 It is also perhaps worth noting a report regarding the use of ICI after alloBMT that included 5 patients who were received PTCy, and none developed GvHD.18
In this series, 2 patients (nivolumab/ipilimumab and pembrolizumab) did not attain donor engraftment, and 1 patient (nivolumab/ipilimumab) showed only transient donor engraftment. This higher than expected rate of nonengraftment may be attributable to ICI use; however, larger studies are needed to determine the effect of ICI on primary graft failure.
We find, as have other investigators, that selected patients with refractory hematologic malignancies can be effectively salvaged with ICIs.19 However, even in Hodgkin lymphoma, in which the best responses are seen, most responding patients will eventually relapse,19 leading many groups to use ICIs as a bridge to alloBMT. Our results do not provide any evidence for prohibitive immune toxicities in the posttransplant period for patients treated with ICIs and alloBMT with bone marrow and PTCy, and the excellent outcomes, especially in Hodgkin lymphoma, may even suggest that their use is advantageous in such patients.
Acknowledgment
This work was supported by the National Institutes of Health, National Cancer Institute (grants P01 CA015396 [R.J.J.] and P30 CA006973).
Authorship
Contribution: L.K.S. participated in data collection, the IRB submission, and drafting the manuscript; K.R.C. participated in concept design and manuscript writing and review; N.D.W.-J., I.G., L.J.S., P.I., E.J.F., and M.L. each participated in direct patient care, data entry, and review; R.F.A. participated in concept design, data entry, and review; R.J.J. is the corresponding author; and D.E.G. participated in IRB submission, data compilation, concept design, and authorship.
Conflict-of-interest disclosure: R.F.A. is a member of the data safety monitoring board for a checkpoint trial sponsored by Bristol-Myers Squibb. N.D.W.-J. serves on the advisory board for JUNO, ADC Therapeutics, and Janssen Pharmaceuticals. K.R.C. has received pharmaceutical funding to study checkpoint inhibition following reduced-intensity conditioning haploidentical transplantation to treat sarcomas. I.G. has received research funding from Merck. The remaining authors declare no competing financial interests.
Correspondence: Richard J. Jones, Johns Hopkins Oncology Center, Bunting-Blaustein CRB, Room 244, 1650 Orleans St, Baltimore, MD 21231; e-mail: rjjones@jhmi.edu.
References
- 1.Merryman RW, Kim HT, Zinzani PL, et al. Safety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphoma. Blood. 2017;129(10):1380-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soiffer RJ, Davids MS, Chen YB. Tyrosine kinase inhibitors and immune checkpoint blockade in allogeneic hematopoietic cell transplantation. Blood. 2018;131(10):1073-1080. [DOI] [PubMed] [Google Scholar]
- 3.Herbaux C, Merryman R, Devine S, et al. Recommendations for managing PD-1 blockade in the context of allogeneic HCT in Hodgkin lymphoma: taming a necessary evil. Blood. 2018;132(1):9-16. [DOI] [PubMed] [Google Scholar]
- 4.Opdivo prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125554s024lbl.pdf. Accessed 17 March 2017.
- 5.Saha A, Aoyama K, Taylor PA, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122(17):3062-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasamon YL, de Claro RA, Wang Y, Shen YL, Farrell AT, Pazdur R. FDA approval summary: nivolumab for the treatment of relapsed or progressive classical Hodgkin lymphoma. Oncologist. 2017;22(5):585-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moiseev IS, Pirogova OV, Alyanski AL, et al. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transplant. 2016;22(6):1037-1042. [DOI] [PubMed] [Google Scholar]
- 8.Carnevale-Schianca F, Caravelli D, Gallo S, et al. Post-transplant cyclophosphamide and tacrolimus-mycophenolate mofetil combination prevents graft-versus-host disease in allogeneic peripheral blood hematopoietic cell transplantation from HLA-matched donors. Biol Blood Marrow Transplant. 2017;23(3):459-466. [DOI] [PubMed] [Google Scholar]
- 9.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126(8):1033-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood. 2016;127(7):938-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh N, Karmali R, Rocha V, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched sibling donors: a Center for International Blood and Marrow Transplant Research analysis. J Clin Oncol. 2016;34(26):3141-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez C, Gayoso J, Canals C, et al. ; Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: a registry study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol. 2017;35(30):3425-3432. [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 15.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasamon YL, Ambinder RF, Fuchs EJ, et al. Prospective study of nonmyeloablative, HLA-mismatched unrelated BMT with high-dose posttransplantation cyclophosphamide. Blood Adv. 2017;1(4):288-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmariah H, Kasamon YL, Zahurak M, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide using non-first-degree related donors. Biol Blood Marrow Transplant. 2018;24(5):1099-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haverkos BM, Abbott D, Hamadani M, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130(2):221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]