Abstract
Study Objectives:
To study the feasibility and accuracy of home sleep apnea testing (HSAT) in the diagnosis of obstructive sleep apnea (OSA) in a stroke rehabilitation unit.
Methods:
Stroke patients referred to a neurorehabilitation center underwent OSA screening by means of HSAT within the Home Polygraphic Recording with Telemedicine Monitoring for Diagnosis and Treatment of Sleep Apnea in Stroke, or HOPES study (ClinicalTrials.gov identifier: NCT02748681). Feasibility was determined by evaluating the acceptability of recording quality. Patients in whom moderate OSA was diagnosed subsequently underwent unattended polysomnography (PSG) confirmation. Accuracy was studied by comparing the respiratory event index (REI)/monitoring time (MT) of screening HSAT with the apnea-hypopnea index (AHI)/total sleep time (TST) obtained during subsequent PSG with Bland-Altman plots. The influence of PSG-evaluated wake time and arousals on OSA classification was studied by comparing the AHI and REI of the same night.
Results:
A total of 265 patients (58 ± 9 years, 70% male) were screened. A total of 92% of HSAT studies were performed with acceptable recording quality. In total, 33 patients (63 ± 5 years, 58% male) with moderate OSA (REI ≥ 15 to < 30 events/h) were included in the HSAT/PSG comparison. The Bland-Altman plot shows acceptable limits of agreement from −19.5 to +16.4, with a mean difference of −1.33. The REI detected in the PSG night demonstrated no significant differences to the AHI and a high correlation (r = .97; P < .001). The 95% confidence interval of the Bland-Altman plots varied from −7.61 to +4.80.
Conclusions:
These findings confirm a good feasibility and sufficient accuracy of HSAT attached in a stroke rehabilitation unit. Therefore, the authors suggest that American Academy of Sleep Medicine recommendations for HSAT should include stroke patients.
Citation:
Saletu MT, Kotzian ST, Schwarzinger A, Haider S, Spatt J, Saletu B. Home sleep apnea testing is a feasible and accurate method to diagnose obstructive sleep apnea in stroke patients during in-hospital rehabilitation. J Clin Sleep Med. 2018;14(9):1495–1501.
Keywords: home sleep apnea testing, obstructive sleep apnea, polysomnography, stroke
BRIEF SUMMARY
Current Knowledge/Study Rationale: In stroke patients, current American Academy of Sleep Medicine guidelines still recommend attended polysomnography (PSG) for the diagnosis of obstructive sleep apnea (OSA). During in-hospital stroke rehabilitation, home sleep apnea testing (HSAT) might be a viable alternative to facilitate the decision of whether or not to initiate continuous positive airway pressure therapy.
Study Impact: The current findings confirm a good feasibility and sufficient accuracy of HSAT attached in a stroke rehabilitation unit. Wake time periods and arousals not evaluated by HSAT showed no relevant influence in apnea-hypopnea index classification and night-to-night variability was acceptable. Therefore, HSAT is an alternative to PSG in stroke patients during in-hospital rehabilitation to diagnose OSA.
INTRODUCTION
The new recommendations concerning portable polygraphic monitoring recording devices termed home sleep apnea testing (HSAT) recently issued by the American Academy of Sleep Medicine (AASM) explicitly excluded stroke patients.1 HSAT is an alternative to attended in-laboratory polysomnography (PSG) in patients with a high pretest probability of moderate to severe obstructive sleep apnea (OSA) without comorbidities. According to the AASM criteria, with a type II portable monitor (PM) an unattended full PSG (≥ 7 channels) can be recorded, type-III monitors have four to seven channels, and type IV monitors have one to two channels, one of them being oximetry.2
Meta-analyses and our own HSAT during in-hospital stroke rehabilitation confirmed a prevalence of relevant sleep apnea (respiratory event index [REI] ≥ 15 events/h) in more than 50% of stroke patients.3,4 OSA is linked to poor functional status in the subacute phase5 and recurrent stroke in the long term.4 Although the prevalence of OSA in stroke patients is very high, sleep studies are not routinely performed in stroke rehabilitation units. Because of long wait times, disability, and immobility caused by stroke, access to stroke-experienced sleep laboratories is limited. Another problem is that sleep technicians are often not experienced in bed/wheelchair transfer and management of severely affected stroke patients.
A recent retrospective study under real-life conditions confirmed that OSA is clinically underrecognized in patients with acute stroke and that continuous positive airway pressure (CPAP) therapy is underutilized.6 Nineteen percent of the sample was considered to have sleep apnea. Only 42 patients (22%) received treatment during their hospital stay.
A feasible way for quick access to OSA therapy is essential. Subjective evaluation with the Berlin questionnaire demonstrated low diagnostic utility in stroke rehabilitation. Thus, screening for sleep apnea should not only be based on clinical interviews.3 Sleep studies are needed for OSA diagnosis.
In-hospital HSAT in stroke patients might reduce wait times and make it possible for many patients to be tested for OSA by staff familiar with the disabilities and special needs of stroke patients. Clinical experience has shown PSG sleep studies to be difficult to perform during rehabilitation and also less reliable in stroke patients. Therefore, HSAT performed at the hospital bed by means of portable monitoring recording devices with at least six recording channels (type III sleep studies) might be a feasible and accurate method to diagnose OSA.
The most frequently used measurement in the classification of OSA severity is the apnea-hypopnea index (AHI). Despite its popularity as a primary measurement of the disorder, there are some limiting factors related to its application. There is a relatively high night-to-night variability of the AHI, which may distort results also with in-laboratory PSG.7 Comparing AHI scores of night 1 with the most variable value in any of four subsequent nights, the largest scattering was observed from −26.9 to +29.1 on a Bland-Altman plot.
There are also a number of concerns with HSAT. Compared with attended in-laboratory PSG, feasibility is limited, because it is frequently associated with sensor losses which lead to technically inadequate recordings in 5% to 30% of the cases.8,9 Concerning the accurate evaluation of OSA severity, sleep time cannot be assessed precisely and could therefore lead to underestimation of OSA.10 Moreover, microarousals are impossible to score without electroencephalography recordings, which can also lead to underestimation of OSA severity.
The aim of this investigation was to study the feasibility and accuracy of HSAT (type III sleep studies) as compared with nonattended PSG (type II sleep studies) in the evaluation of sleep apnea in a stroke rehabilitation unit in order to accelerate diagnosis and treatment of OSA in stroke patients.
METHODS
Study Design
The current investigation and data analysis is the diagnostic part of the HOPES (Home Polygraphic Recording with Tele-medicine Monitoring for Diagnosis and Treatment of Sleep Apnea in Stroke) study. The study protocol of this single-blind randomized controlled trial was published recently.11
During the HOPES project, all patients enrolled for neurological rehabilitation from April 18, 2016 to April 18, 2017 were offered HSAT studies for evaluation of OSA during their 4 to 8 weeks of in-hospital neurorehabilitation. In this study, confirmatory PSG with peripheral HSAT and central sensors was conducted on a different night a few days later, if screening with HSAT revealed an REI between 15 and 29 events/h. If HSAT showed an REI ≥ 30 events/h, the patient was directly referred to positive airway pressure therapy. For comparison of HSAT-REI versus PSG-AHI data from the same confirmatory PSG recording was used.
Inclusion and Exclusion Criteria
Subacute (> 1 month to < 1 year after stroke) adult stroke patients (19–70 years of age) referred to the Neurological Rehabilitation Centre Rosenhügel from acute care units of other hospitals or neurologists' offices were included in the study. Their diagnosis of a completed stroke was confirmed by a neurologist based on the history of a sudden onset of a neurological deficit lasting longer than 24 hours, the presence of a neurological deficit upon physical examination, and a brain lesion compatible with the neurological deficit on computed tomography or magnetic resonance imaging studies of the brain.
Exclusion criteria were as follows: patients with sopor/ coma, patients unable to understand the protocol because of severe cognitive impairments, previously diagnosed sleep apnea and established PAP therapy, central sleep apnea, chronic obstructive pulmonary disease higher than stage Gold III, cancer, chronic kidney disease higher than stage 4, coexisting causes of daytime sleepiness (eg, narcolepsy, night or rotating shift work; self-reported average sleep duration < 4 hours), a major psychiatric or any other acute medical condition, patients unable or unwilling to comply with the protocol.
Measurements
Screening HSAT (type III monitoring) and PSG (type II monitoring) were both performed by neurologists and trained staff members using the SOMNOmedics Diagnostic Systems (SOMNOmedics GmbH, Germany). Hook-up of equipment for recordings was done in the patients' bedroom. Both measurements were conducted during the entire night (approximately 8.00 pm – 6.00 am) according to AASM standard criteria. Time in bed (TIB) was determined by the patients' sleep log.
HSAT recordings included oronasal air flow (using thermistor and nasal cannula), thoracic and abdominal effort, oxyhemoglobin saturation, electrocardiogram, and determination of the body position. PSG included the HSAT hook-up procedure and EEG sleep data (SOMNOmedics system) including 6 EEG channels (F3-A2, F4-A1, C3-A2, C4-A1, O1-A2, O2-A1) according to the 10/20 system, 2 electrooculogram channels (left/ right), submental electromyogram (EMG) and tibialis anterior EMG from both legs.
Scoring of REI and AHI
All data obtained were scored according to AASM criteria updated in 2012.12 Apnea was scored if there was a drop in the peak signal excursion by ≥ 90% of preevent baseline using an oronasal thermal sensor.12 Hypopnea was scored if the peak signal excursions dropped by ≥ 30% of preevent baseline, using nasal pressure for ≥ 10 seconds in association with either ≥ 3% arterial oxygen desaturation or an arousal. The diagnosis of a central sleep apnea syndrome as an exclusion criterion required the total number of central apneas and or/central hypopneas to be more than 50% of the total number of apneas and hypopneas.13 The REI used in HSAT was defined as the total number of hypopneas and apneas scored multiplied by 60 divided by monitoring time (MT). MT was defined as total recording time minus periods of artifacts and time the patient was awake, as determined by internal actigraphy (accelerometer, which detects movement during TIB and therefore can determine possible wake times), respiratory patterns, or sleep log. The AHI was defined as the sum of all apneas and hypopneas occurring per hour of total sleep time (TST) estimated with PSG. A technically adequate sleep study included a minimum of 4 hours of adequate oximetry and flow data, obtained during a recording attempt that encompassed the habitual sleep period. Sleep studies with fewer than 4 hours TIB or fewer than 4 hours of acceptable signal quality were repeated if patients agreed.
HSAT studies were evaluated by experienced sleep laboratory personnel. PSG tests were scored by an ESRS-certified sleep physician (MS).
For the intranight comparison of REI and AHI from PSG records, respiratory scoring and REI/MT evaluation was done before sleep and arousal scoring. The AHI/TST was determined after central scoring. Recording quality was rated as “good” if TIB was longer than 4 hours, with actigraphy indicating “not awake” and no noticed arousal time (as seen by activity, heart frequency, and body position) through all recorded channels. Recording quality was rated as “sufficient” if one sensor was lost (thermistor/nasal cannula or one effort) or signals were missing over some periods of time, but an evaluation of respiratory events was still possible.
User Statistics
Data exploration using descriptive statistical analysis and inferential statistics was performed. Baseline data are shown in frequencies or percentages, means, and standard deviation. In order to test the normal distribution, histograms and box plots were applied. For intranight comparison of REI and AHI, a nonparametric test, such as Wilcoxon or Mann-Whitney U test, was chosen. Spearman correlation and Pearson correlation were used.
To study feasibility we estimated the recording quality of all HSAT sleep studies performed and hypothesized a good feasibility in stroke patients during in-hospital rehabilitation, independent of stroke severity, if more than 90% of sleep studies showed acceptable recording quality.
Accuracy was evaluated by comparing the REI derived from HSAT with the AHI obtained during PSG performed several days later with Bland-Altman plots to graphically represent the observed differences of the two measures. We hypothesized a good validity if the difference was within the limits of night-tonight variability7 of the AHI.
To study the influence of sleep time evaluation and arousal detection on OSA severity we compared the REI and AHI obtained in the same PSG night and hypothesized a good validity if there was a high correlation (> .95) and acceptable limits of agreement in Bland-Altman plots.
SPSS Statistics for Windows, version 22 software (IBM Corp., Armonk, New York, United States) was used for all statistical analyses. All tests were two-sided and a value of P < .05 was considered statistically significant.
Ethical Considerations
The study was approved by the Clinical Research Ethics Board of Vienna (EK-15-231-1115) and was performed in accordance with the relevant guidelines of the 1964 Declaration of Helsinki, as amended in Tokyo 1975, Venice 1983, Hong Kong 1989, and Somerset West 1996.14 Written informed consent for all examinations was obtained upon patients′ admission. The protocol was registered at ClinicalTrials.gov (identifier: NCT02748681).
RESULTS
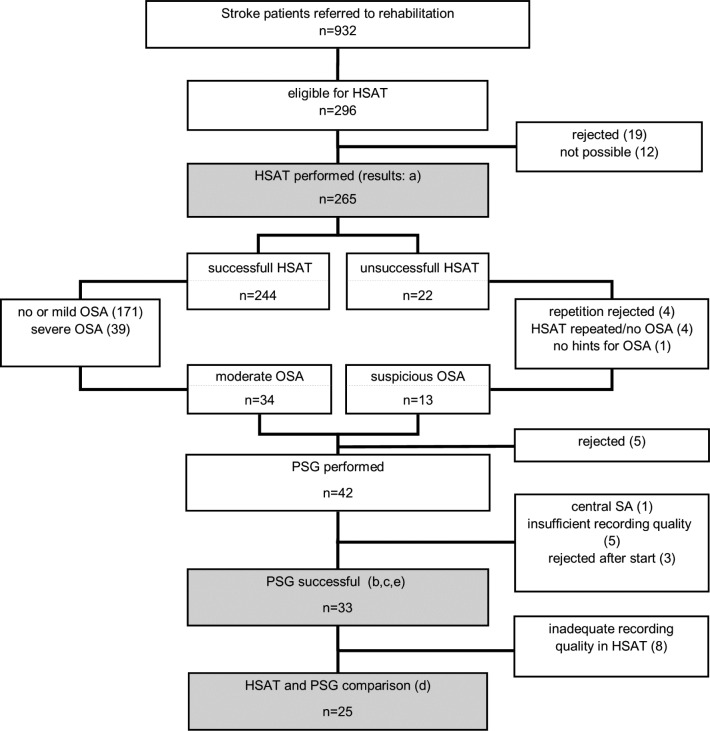
During the 1-year study period, 932 stroke patients were admitted to our rehabilitation center. After screening for exclusion criteria (Figure 1), 296 patients were offered an HSAT sleep study by their neurologist. 19 patients (6%) rejected screening. In 12 cases screening was not possible because of premature dropout from rehabilitation or illness during the stay and appointment failure. HSAT sleep studies were then performed in 265 patients (mean age 58 ± 9, male 70%).
Figure 1. Study flowchart.
HSAT = home sleep apnea test, OSA = obstructive sleep apnea, PSG = polysomnography, SA = sleep apnea.
Feasibility of In-Hospital HSAT Studies in Stroke Patients (n = 265)
Recordings were successful in 92% of all 265 patients, that is, data quality was sufficient for the evaluation of OSA. Recording quality was found to be good in 166 HSAT recordings (63%), in 77 (29%) it was sufficient. In 22 HSAT recordings (8%), recording quality was not sufficient to adequately assess sleep-disordered breathing because of missing flow data (9), missing oxygen data (7), sleep duration < 4 hours of TIB (4), or technical problems with the battery (2). After a test repetition, data quality was sufficient in four patients, but remained insufficient in three patients. All 34 patients with moderate OSA and 13 patients whose HSAT sleep studies had shown insufficient recording quality but indicated OSA were offered a PSG for confirmation of OSA diagnosis.
From the total of 47 patients, 5 with moderate OSA (11%) refused further investigations. Three patients (6%) did not tolerate EEG and surface EMG sensors during sleep studies and dropped out from the sleep studies and in 5 cases (11%) recording quality was insufficient. One patient showed central sleep apnea (> 50% central apneas). Finally, PSG data from 33 patients (70%) were included for further investigations (Table 1 and Table 2).
Table 1.
Patient characteristics (n = 33).

Table 2.
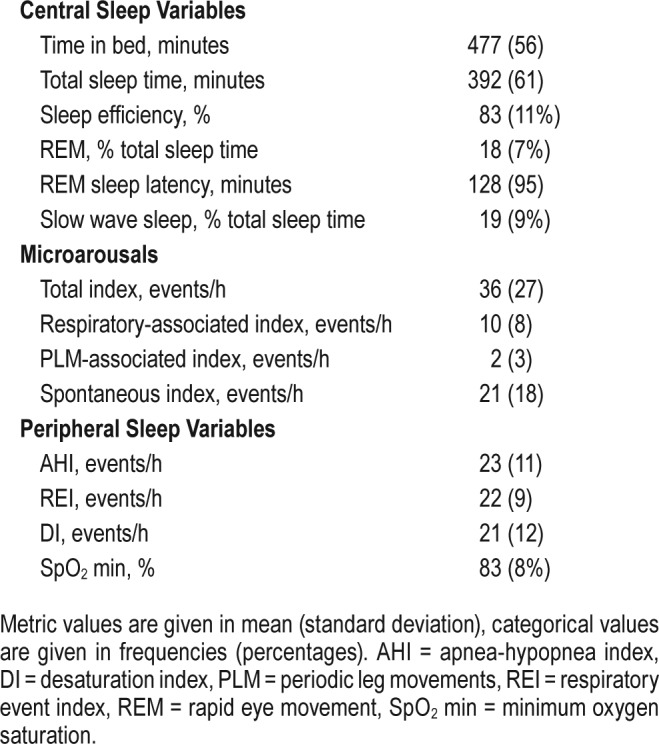
Sleep variables.
Night-To-Night Variability: Accuracy of HSAT/REI Compared With PSG/AHI From a Different Night Several Days Later
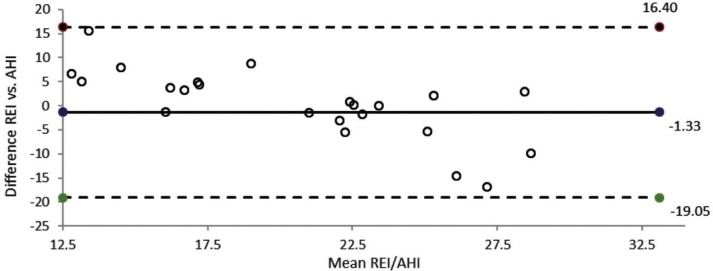
Data from 25 patients were eligible for comparison of HSAT and PSG. Eight sleep studies were excluded because of insufficient recording quality either in HSAT or PSG studies. Time between the two measurements was 6 days (range: 2–14 days). The mean REI was 21 ± 3 events/h, compared with an AHI of 23 ± 11 events/h. The mean AHI and REI showed no significant difference. Sensitivity for the AHI was 72%.
OSA severity classification was changed in 43% of cases; 26% were reclassified as mild and 17% as severe OSA. A change of > 10 events/h was found in 21% of cases (> 15 events/h in 17%, 10–15 events/h in 4%) and 5–10 events/h in 35%. The differences between the REI and the AHI are shown in Figure 2.
Figure 2. Bland-Altman plot: HSAT-derived REI and PSG-derived AHI in different nights.
The solid horizontal line represents the mean difference, and the dotted lines represent the 95% limits of agreement between the two measurements. The mean difference between the REI and the AHI is −1.33 units. AHI = apnea-hypopnea index, HSAT = home sleep apnea test, PSG = polysomnography, REI = respiratory event index.
Accuracy of the REI/MT and the AHI/TST Obtained in the Same Night to Estimate the Influence of Sleep Time and Microarousal Evaluation By Means of EEG on OSA Classification
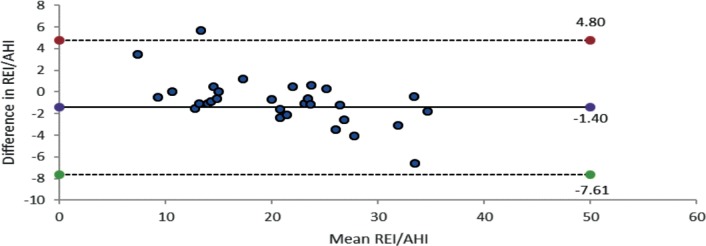
Data from 33 patients were eligible for intranight REI/MT versus AHI/TST comparison. The mean REI was 22 ± 9, the mean AHI was 23 ± 11, with no significant difference. The Pearson correlation coefficient showed a high correlation coefficient of r = .97 (P < .001). Sensitivity for the AHI was 94%. A change of OSA classification was found in two cases: REI 14.5 versus AHI 15.1; REI 16.2 versus AHI 10.5. The arousal index associated with respiratory events was 10 events/h sleep time. Most detected arousals were spontaneous.
Bland-Altman limits of agreement between the REI and the AHI derived from the same night showed a mean difference between the REI and the AHI of −1.40 units (Figure 3). Bland-Altman plot 95% limits of agreement between the REI and AHI were +4.8 to −7.61. The tendency for the REI underestimating the AHI is −1.4.
Figure 3. Bland-Altman plot: HSAT-derived REI and PSG-derived AHI intranight.
AHI = apnea-hypopnea index, HSAT = home sleep apnea test, PSG = polysomnography, REI = respiratory event index.
DISCUSSION
In the current investigation, 92% of HSAT studies were performed with acceptable recording quality. Night-to-night variability showed differences of > 10 events/h between the REI and the AHI in 21% of sleep studies. The REI and the AHI detected in the same PSG night demonstrated no significant differences.
Feasibility of Screening HSAT in Stroke Rehabilitation
The use of HSAT provides numerous advantages, including increased health care accessibility, shorter waiting times, earlier treatment initiation, and a potential cost reduction.15,16 Attended in-laboratory PSG is considered the “gold standard,” but as patients sleep in an artificial (and sometimes unpleasant) environment, which may result in increased sleep fragmentation, HSAT might reflect OSA severity even more accurately. In the current study, only 19 (6%) rejected HSAT screening. The most frequently mentioned reason for the rejection was that patients refused the nasal cannula, which reminded them of their stay at the acute care hospital.
Irrespective of these advantages, unmonitored HSAT is often associated with sensor losses and inadequate recordings requiring test repetition.16,17 In our study, 92% of HSAT sleep studies in stroke patients were performed with acceptable recording quality, independent of stroke severity.
Our low dropout rate may be due to the fact that at the hospital a careful hookup was performed by physicians on call or educated technicians. A nurse on the ward was always there to assist in case of patient immobility or trunk instability. In conventional out-of-center testing, HSAT is performed by the patients themselves. After being given a tutorial on how to use the equipment, patients take the overnight monitor to their home. In patients severely affected by the stroke, who suffer from limb/ trunk weakness or instability, self-attachment is not possible. In contrast to nurses and physicians at rehabilitation units, sleep laboratory staff often are not experienced in bed/wheelchair transfer and management of severely affected stroke patients. One of the shortcomings of the current study is that results cannot be generalized to classic HSAT performed at home.
The good feasibility of HSAT was confirmed by a recent prospective observational study (ApneaLink Plus) including inpatients and outpatients who had suffered a mild stroke or TIA.16 In a total of 82 patients (80.4%), 4 or more hours of sleep data that could be analyzed were obtained. The authors concluded that HSAT facilitates rapid diagnosis and management of OSA in both the outpatient and the inpatient setting.
As a further shortcoming, neither HSAT nor our unattended PSG setting without video recording can detect comorbid non-respiratory sleep disorders, such as REM parasomnia or sleep-related movement disorder. This requires online monitoring within a specialized sleep laboratory.
Accuracy of the REI Is Within the Night-to-Night Variability of the AHI
A limitation in the estimation of OSA severity is the variability in the determination of the AHI, which can distort results, also in attended PSG studies, where test repetition may yield different results.7 In regard to OSA severity, in 50% of the patients the classification changed from the first to the subsequent nights. Sixty-five percent presented a variation in the AHI of ≥ 10 events/h TST.7 In the current study, mean REI and AHI did not differ and the REI showed a night-to-night difference of > 10 in only 21% of sleep recordings. However, a limitation of the current study and our diagnostic in-hospital rehabilitation process might be that only patients with moderate sleep apnea in HSAT underwent a confirmation PSG. No follow-up PSG was performed in patients with negative HSAT or mild OSA. Thus, the presence of OSA could be missed in a percentage of these patients. However, in the real stroke environment it is unclear whether patients and doctors would agree to repeat a negative screening if there were no clinical symptoms.
We used the International Classification of Sleep Disorders, Third Edition definition of sleep apnea, which can be based on an HSAT study. In the clinical context, sleep apnea cannot be overestimated by HSAT, if artifacts are eliminated and respiratory events are carefully scored visually. The REI index should be congruent with the desaturation index.
To our clinical experience, a severe sleep apnea diagnosed by HSAT will be confirmed by PSG.
The AHI Is Hardly Influenced By Sleep Time Assessed By Means of the EEG and Arousal Scoring
Another concern in connection with HSAT is that OSA severity might be underestimated because of limitations in sleep time assessment and arousal scoring.10,17 The utilization of sleep logs did not reduce the risk of underestimation.10 In the current study, intranight comparison of the AHI/TST and the REI/MT demonstrated no significant differences and a high correlation. Only two AHI classification diagnoses were changed after PSG confirmation. Thus, wake-time periods not estimated by HSAT did not alter the AHI classification in an intranight comparison with PSG. Moreover, time- and personnel-consuming arousal detection did not influence results, as the arousal index associated with respiratory disturbance was lower than the REI.
In otherwise healthy patients, a total of 25% of respiratory events in OSA are terminated without a clear arousal.18,19 Arousals are thought to predispose to further respiratory events by promoting hyperventilation, hypocapnia, and upper airway dilator muscle hypotonia. In our sample of stroke patients, only 50% of respiratory events were terminated by an arousal. The arousal threshold seems to be increased and may be influenced by comorbidity and centrally acting sedative drugs (antidepressants, anticonvulsants, antispasticity drugs).
Current guidelines do not recommend HSAT in stroke because of a lack of data demonstrating efficacy and reliability of portable devices.1
A recent study compared a portable single-channel acoustic device for REI quantification with conventional PSG.20 With the portable device, in the laboratory overall AHI accuracy was 87.0%. Calculating the AHI with recording time rather than sleep time as the denominator had only a marginal effect on diagnostic accuracy for OSA.
After a highly positive HSAT screening with an REI of > 20 events/h based on MT, the recommended OSA diagnosis by means of PSG and subsequent CPAP treatment decision based on an AHI ≥ 15 events/h based on TST can sometimes be questionable in clinical routine. The AHI might reach a borderline value of, for instance, 14.4 events/h, in which case no therapy would be initiated, especially as stroke patients often do not complain about sleepiness.3
Type of Medical Comorbidity Is Relevant in Guidelines: Time Is Brain
In patients with high comorbidity, current guidelines do not recommend HSAT.1 However, patients requiring a capnography—potential respiratory muscle weakness due to neuromuscular condition, hypoventilation when awake or suspicion of sleep-related hypoventilation and chronic opioid medication— are listed in the same group as patients with a history of stroke or severe insomnia. This recommendation is based on the limited data available. In our opinion, there has to be a distinction between comorbidities. Nonobstructive sleep-disordered breathing is not common in stroke patients. Also according to the data shown, 70% of respiratory events are obstructive.3 “Time is brain” in stroke medicine and severely affected stroke patients need quick access to therapy.
In the current study we showed that our diagnostic procedure with HSAT, performed during in-hospital services, is a feasible and accurate method to assess OSA in this patient group. In our opinion there is no need for PSG sleep studies. Subsequent to in-hospital OSA diagnosis, CPAP treatment could start within the rehabilitation process. In this setting, experts in stroke rehabilitation could provide the best possible treatment in this patient group and therefore improve CPAP adherence. Therefore, further therapeutic comparative outcome studies (in-hospital rehabilitation management versus traditional sleep center management) are needed. HSAT recommendations for stroke patients should differ from other comorbid disorders.
DISCLOSURE STATEMENT
Work for this study was performed at Neurologisches Rehabilitationszentrum Rosenhügel, Vienna (NRZ). All authors have seen and approved the manuscript. This project was funded in its entirety with funds from the ‘NRZ research account’ from NRZ Rosenhügel Vienna. The authors report no conflicts of interest. The current investigation and data analysis is the diagnostic part of the HOPES study—Home Polygraphic Recording with Telemedicine Monitoring for Diagnosis and Treatment of Sleep Apnea in Stroke. The study protocol of this single-blind randomized controlled trial was recently published (BMJ Open 2018) and listed in ClinicalTrials.gov (Identifier: NCT02748681).
ACKNOWLEDGMENTS
The authors are grateful to Elisabeth Grätzhofer for editorial assistance.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- EEG
electroencephalogram
- HSAT
home sleep apnea testing
- MT
monitoring time
- OSA
obstructive sleep apnea
- PLM
periodic leg movements
- PSG
polysomnography
- REI
respiratory event index
- TST
total sleep time
REFERENCES
- 1.Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(3):479–504. doi: 10.5664/jcsm.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 3.Kotzian ST, Stanek JK, Pinter MM, Grossmann W, Saletu MT. Subjective evaluation of sleep apnea is not sufficient in stroke rehabilitation. Top Stroke Rehabil. 2012;19(1):45–53. doi: 10.1310/tsr1901-45. [DOI] [PubMed] [Google Scholar]
- 4.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6(2):131–137. [PMC free article] [PubMed] [Google Scholar]
- 5.Yan-fang S, Yu-ping W. Sleep-disordered breathing: impact on functional outcome of ischemic stroke patients. Sleep Med. 2009;10(7):717–719. doi: 10.1016/j.sleep.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Festic N, Alejos D, Bansal V, et al. Sleep apnea in patients hospitalized with acute ischemic stroke: underrecognition and associated clinical outcomes. J Clin Sleep Med. 2018;14(1):75–80. doi: 10.5664/jcsm.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittencourt LR, Suchecki D, Tufik S, et al. The variability of the apnoeahypopnoea index. J Sleep Res. 2001;10(3):245–251. doi: 10.1046/j.1365-2869.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 8.Safadi A, Etzioni T, Fliss D, Pillar G, Shapira C. The effect of the transition to home monitoring for the diagnosis of OSAS on test availability, waiting time, patients' satisfaction, and outcome in a large health provider system. Sleep Disord. 2014;2014:418246. doi: 10.1155/2014/418246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masa JF, Corral J, Pereira R, et al. Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax. 2011;66(7):567–573. doi: 10.1136/thx.2010.152272. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi MT, Goparaju B. Potential underestimation of sleep apnea severity by at-home kits: rescoring in-laboratory polysomnography without sleep staging. J Clin Sleep Med. 2017;13(4):551–555. doi: 10.5664/jcsm.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotzian ST, Schwarzinger A, Haider S, Saletu B, Spatt J, Saletu MT. Home polygraphic recording with telemedicine monitoring for diagnosis and treatment of sleep apnoea in stroke (HOPES Study): study protocol for a single-blind, randomised controlled trial. BMJ Open. 2018;8(1):e018847. doi: 10.1136/bmjopen-2017-018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 14.Dale O, Salo M. The Helsinki Declaration, research guidelines and regulations: present and future editorial aspects. Acta Anaesthesiol Scand. 1996;40(7):771–772. doi: 10.1111/j.1399-6576.1996.tb04530.x. [DOI] [PubMed] [Google Scholar]
- 15.Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007;3(7):706–712. [PMC free article] [PubMed] [Google Scholar]
- 16.Boulos MI, Elias S, Wan A, et al. Unattended hospital and home sleep apnea testing following cerebrovascular events. J Stroke Cerebrovasc Dis. 2017;26(1):143–149. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Golpe R, Jimenez A, Carpizo R. Home sleep studies in the assessment of sleep apnea/hypopnea syndrome. Chest. 2002;122(4):1156–1161. doi: 10.1378/chest.122.4.1156. [DOI] [PubMed] [Google Scholar]
- 18.Jordan AS, Eckert DJ, Wellman A, Trinder JA, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184(10):1183–1191. doi: 10.1164/rccm.201106-0975OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas RJ. Arousals in sleep-disordered breathing: patterns and implications. Sleep. 2003;26(8):1042–1047. doi: 10.1093/sleep/26.8.1042. [DOI] [PubMed] [Google Scholar]
- 20.Ryan CM, Wilton K, Bradley TD, Alshaer H. In-hospital diagnosis of sleep apnea in stroke patients using a portable acoustic device. Sleep Breath. 2017;21(2):453–460. doi: 10.1007/s11325-016-1438-5. [DOI] [PubMed] [Google Scholar]