Abstract
Study Objectives:
To assess whether gout is associated with a higher risk of obstructive sleep apnea (OSA) in older adults.
Methods:
We used the 5% United States Medicare beneficiary sample from 2006–2012 to assess whether gout was independently associated with new diagnosis of OSA in adults 65 years or older, adjusting for demographics, medical comorbidity (Charlson-Romano index) and hypertension, hyperlipidemia and coronary artery disease, and the use of medications for cardiovascular diseases or gout (allopurinol, febuxostat).
Results:
Based on 10,448,472 person-years of follow-up in a cohort of 1.74 million adults 65 years or older, the crude incidence rates of OSA were 14.3 per 1,000 person-years in people with gout and 3.9 per 1,000 person-years in people without gout. In multivariable-adjusted analyses, gout was associated with higher risk of a new diagnosis of OSA during the follow-up, hazard ratio was 2.07 (95% confidence interval [CI] 2.00, 2.15). In sensitivity analyses that substituted continuous Charlson-Romano score with a categorical variable or individual Charlson-Romano comorbidities plus hypertension, hyperlipidemia and coronary artery disease, the main finding was confirmed, hazard ratios were 2.11 (95% CI 2.03, 2.18) and 1.79 (95% CI 1.73, 1.85).
Conclusions:
The independent association of gout with a twofold higher risk of OSA in older adults indicates that common mechanisms may be shared by the two conditions. More studies are needed to investigate these mechanisms further.
Citation:
Singh JA, Cleveland JD. Gout and the risk of incident obstructive sleep apnea in adults 65 years or older: an observational study. J Clin Sleep Med. 2018;14(9):1521–1527.
Keywords: gout, obstructive sleep apnea, older adults, OSA, risk
BRIEF SUMMARY
Current Knowledge/Study Rationale: Both gout and obstructive sleep apnea (OSA) are associated with cardiovascular disease and the metabolic syndrome. Emerging data indicate that inflammation and/or oxidative stress may play a role in the pathogenesis of OSA. We investigated whether gout was associated with an increase in the risk of new/incident OSA in adults 65 years or older.
Study Impact: Gout was independently associated with a twofold higher risk of the development of OSA, after adjusting for demographics, comorbidity, and common cardiovascular and gout medications. This indicates that when it coexists with other OSA risk factors, a diagnosis of gout may trigger screening for OSA. Future studies should attempt to replicate this finding for this patient population and elucidate the mechanistic underpinnings of this association.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder caused by a partial or complete collapse of the upper airway due to the relaxation of the muscles controlling the soft palate and tongue.1 At least 25 million Americans have OSA.2 OSA affects the cardiovascular system.3 OSA is associated with an increased risk of coronary artery disease and heart failure4 and stroke5 in the elderly, in addition to its well-known association with hypertension.6,7 The annual economic burden of undiagnosed sleep apnea among United States adults is approximately $150 billion.8 OSA is also associated with reduced health-related quality of life.9 Thus, OSA constitutes a major public health burden.
Despite the advances in the knowledge related to OSA, most patients affected with OSA remain undiagnosed,10,11 a major barrier to optimal patient care.12 A delayed diagnosis and treatment can contribute to significant medical morbidity and increased health care-related costs.13 Continuous positive airway pressure (CPAP) is an effective treatment of OSA that improves OSA outcomes and reduces associated morbidity.14,15 Although causal associations need to be investigated for OSA in basic and translational studies, new knowledge from epidemiological studies that can identify risk associations can also be very helpful. Recognition of common diseases as novel potential risk factors/associations can trigger screening for and early and appropriate diagnosis of OSA, which can reduce morbidity and mortality associated with undiagnosed OSA, at least in these disease cohorts.
Gout, the most common inflammatory arthritis that affects 8.3 million Americans, is characterized by chronic inflammation and oxidative stress.16 A recent study found that sleep apnea was associated with 1.5 to 1.7 times higher odds of gout.17 Gout and sleep apnea are both associated with the metabolic syndrome, obesity, and associated cardiovascular comorbidi-ties.18,19 The comorbid disease burden and/or associated chronic inflammation and oxidative stress in gout could be a risk factor for the development of OSA. Our study objective was to assess whether a common arthritis such as gout was independently associated with an increased risk of the development of incident (new) sleep apnea, above and beyond that associated with comorbidity including cardiovascular conditions. If an association were to be detected, a diagnosis of gout would raise the clinical suspicion of OSA in patients who also have other OSA risk factors, which can then lead to appropriate and timely OSA diagnosis.
METHODS
Data Source and Study Population
We used data from the 2006–2012 5% Medicare claims data, obtained from the Centers for Medicare and Medicaid Services (CMS). People were included in the study, if they were Medicare fee-for-service recipients (Part A, B), not currently enrolled in a Medicare Managed plan (Part C), and had a valid current United States mailing address. The 5% random-sample file is a standard Medicare analytic file available from the CMS data warehouse that is commonly used to address epidemio-logical and outcomes questions.20–22 The recommendation for studies based on analysis of claims data is to exclude Part C claims because CMS generally does not receive claims data for Medicare beneficiaries who enroll in Medicare Part C plans.23 The Institutional Review Board at the University of Alabama at Birmingham approved the study.
Variables of Interest, Outcome, Covariates, and Statistical Analyses
The diagnosis of gout was based on two claims at least 4 weeks apart during the study period 2006–2012, with an International Classification of Diseases, Ninth Revision, Common Modification (ICD-9-CM) code 274.xx, a validated approach with sensitivity of 90% and specificity of 100%.24
A new diagnosis of OSA was our outcome of interest and was based on the occurrence of two or more ICD-9-CM codes of 327.23 more than 4 weeks apart, which could only be after someone met the definition for a diagnosis of gout. This approach for administrative data had a positive predictive value of 75%, though specificity was 68% and sensitivity was 24%.25 Patients who had any claim for OSA in 2005 were considered to have baseline OSA and were excluded from the cohort.
We included potential confounders as covariates and adjusted for them in the analyses. Patient demographics, age, sex, and race were included, and data were obtained from the Medicare denominator file and the beneficiary summary file. The 2005 claims data were used to identify preexisting comorbidi-ties and calculate the baseline Charlson-Romano comorbidity score. Part D files were used to assess for medication use for cardiovascular disease, including statins, beta blockers, diuretics, and angiotensin-converting enzyme inhibitors, and gout (allopurinol, febuxostat) as time-varying covariates, as surrogates of disease and/or disease severity.
We calculated the crude incidence rates for OSA occurrence in patients with versus without gout at baseline. Characteristics of people with versus without OSA were compared using t test or chi-square test, as appropriate. A multivariable-adjusted Cox proportional hazard model assessed whether gout at baseline was associated with a new diagnosis of OSA during the follow-up, while adjusting for demographics, comorbidity, and common medications (model 1). Sensitivity analyses treated Charlson-Romano index in categories (model 2) or as individual comorbidities, and also adjusted for hypertension, hyper-lipidemia, and coronary artery disease (model 3).
RESULTS
Demographic and Clinical Characteristics
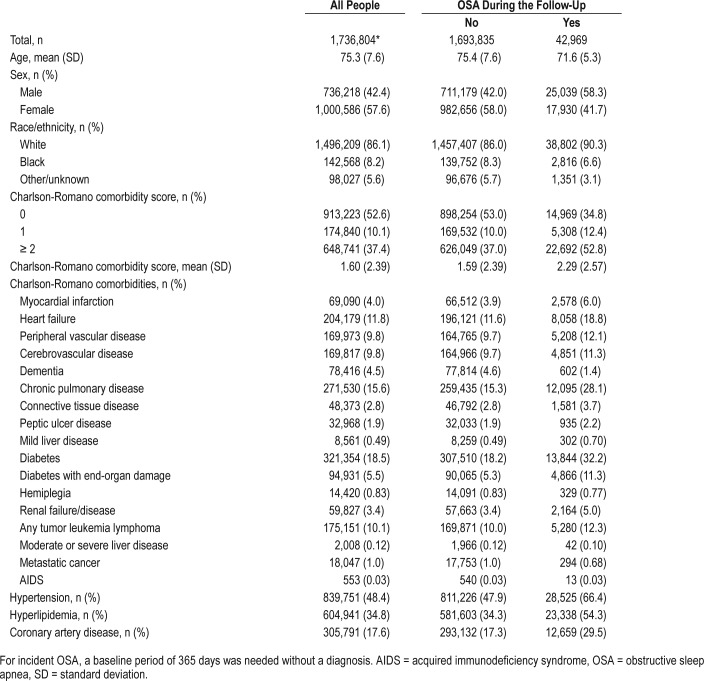
The study cohort consisted of 1.73 million people, of whom 1.64 million had a gout diagnosis and 0.09 million did not (Appendix 1 in the supplemental material). Compared to people without OSA, people with OSA were younger by 3 years, more likely to be male, White, and had higher medical comorbidity (Table 1). Compared to those without OSA, people with OSA were twice as likely to have myocardial infarction, heart failure, or peripheral vascular disease (Table 1). The mean (standard deviation) time from the diagnosis of gout to the new OSA diagnosis was 2.4 (1.7) years; and the median time was 2.1 years (interquartile range, 0.9 to 3.6 years).
Table 1.
Baseline demographic and clinical characteristics of people with OSA versus people without OSA.
Based on 10,448,472 person-years of follow-up, the crude incidence rates of a new diagnosis of OSA was 14.3 per 1,000 person-years in those with no gout, 3.9 per 1,000 person-years in people without gout. Characteristics of individuals in whom incident OSA by gout status is developing or not developing is shown in Appendix 1.
Multivariable-Adjusted Analysis
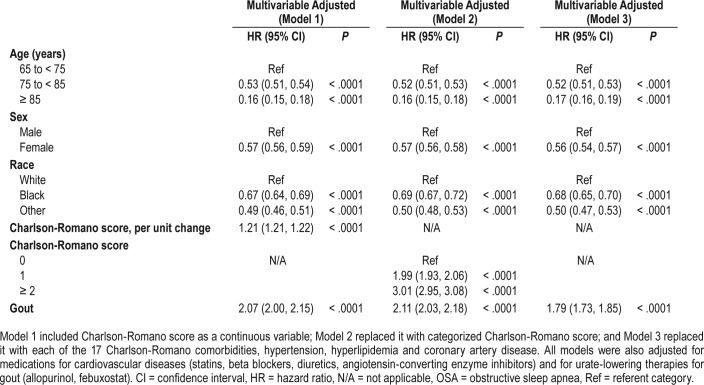
After adjusting for demographics, comorbidity, and common cardiovascular and gout medications, gout was associated with a new diagnosis of OSA during the follow-up period with a hazard ratio of 2.07 (95% confidence interval [CI] 2.00, 2.15) (Table 2). In sensitivity analyses that substituted continuous Charlson-Romano score with a categorical variable or individual Charlson-Romano comorbidities (and also adjusted for hypertension, hyperlipidemia, and coronary artery disease), the findings from the main regression model were confirmed, respective hazard ratios were 2.11 (95% CI 2.03, 2.18) and 1.79 (95% CI 1.73, 1.85) (Table 2).
Table 2.
Multivariable-adjusted association of gout and other risk factors with OSA.
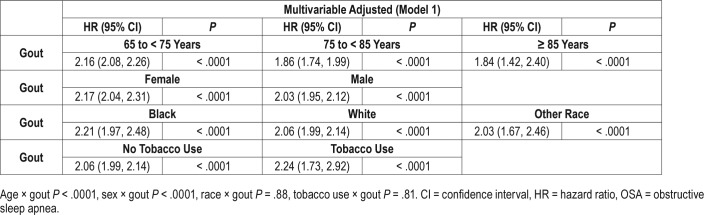
In subgroup analyses by age, sex, and race, we noted minor differences in hazard ratios, although interactions were significant for age × gout and sex × gout (Table 3). No significant differences in association of gout with OSA were noted by tobacco use.
Table 3.
Association of gout with OSA, in predefined subgroup analyses, by age, sex, race and tobacco use.
DISCUSSION
In elderly people 65 years of older, an existing diagnosis of gout was associated with a twofold higher risk of a new diagnosis of OSA. This association was independent of demographics, comorbidity including cardiovascular diseases and the components of metabolic syndrome, and the use of common medications. A practical use of this knowledge is that it can lead to clinical suspicion and further evaluation of OSA in people with gout. This can further lead to an early diagnosis of OSA and appropriate management with CPAP and other modalities that may improve outcomes in people with OSA.14,15 Because gout affects 4% of Americans and 63% of gout patients have metabolic syndrome16,19 (a potential risk factor for OSA), screening the 2.5% of Americans with gout and metabolic syndrome for OSA might improve its early detection and treatment. We observed a temporal relationship between the onset of gout and the subsequent onset of OSA, but this study only shows that gout and OSA have an association that would convey a risk of co-diagnosis. These findings should not be interpreted as causality, because our study was not designed to investigate causality.
Our study was motivated by a recent finding from a qualitative study of people with gout that revealed sleep disorders had a profound effect on health on quality of life of patients with gout and were common.26 A recent UK study found that sleep apnea was associated with 1.5–1.7 times higher odds of a new diagnosis of gout,17 indicating that sleep apnea may be a risk factor for gout. Both gout and sleep apnea are associated with the metabolic syndrome, obesity, and cardiovascular comorbidities,18,19 which could be potential explanations for the finding from this previous study and our study. Both the previous study and our study adjusted for several components of metabolic syndrome (hyperlipidemia, hypertension, diabetes) and cardiovascular disease. The presence of the association of gout and sleep apnea in both directions indicates that the association is independent of the factors adjusted in each of the analyses, including most components of the metabolic syndrome. It is important to note that we were unable to control for obesity (component of metabolic syndrome), due to the lack of body mass index data in Medicare claims, and a very low accuracy of diagnostic code for obesity in Medicare claims (< 2.5% prevalence), given a reported prevalence of obesity at 35% in adults 65 years or older almost a decade ago (2007–2010).27
So, what pathophysiologic process can potentially explain this association? Gout leads to acute and chronic systemic inflammation. Gout is characterized by the formation of mono-sodium urate crystals in joints, synovium, and other body tissues, which are phagocytosed by macrophages or monocytes. This leads to the disruption of lysosome and the activation of NALP3 inflammasome,28 which results in the formation of interleukin 1β and other proinflammatory cytokines29 and elevated C-reactive protein,30 that is, acute and chronic inflammation. Hyperuricemia and urate crystals, hallmarks of gout, are associated with oxidative stress.31–34 Inflammation and oxidative stress also play an important role in the pathogenesis of OSA. OSA was associated with elevated levels of tumor necrosis factor,35 interleukin-6, and C-reactive protein36 and markers of oxidative stress.37 Treatment with CPAP was associated with a reduction in levels of inflammatory markers,35,36 and increased endothelial nitric oxide synthase (NOS) and phosphorylation of endothelial NOS synthase expression and decreased nitrotyro-sine and inducible NOS expression (consistent with reduction of oxidative stress).37 Long-term effects of OSA-related hypoxemia, including those on the cardiovascular system, are mediated by inflammation, oxidative stress, and effects on vascular endothelium, at least partially.38 Endothelium-dependent flow-mediated dilation was reduced in OSA,39,40 and these changes reversed with CPAP treatment.39 Sleep apnea measures were associated with baseline diameter and the percentage of flow-mediated dilation.41 Serum levels of vascular endothelial growth factor, a hypoxia-sensitive glycoprotein stimulating neoangio-genesis, were elevated in severely hypoxic patients with OSA and were related to the degree of nocturnal oxygen desaturation.42 Thus, gout and OSA share two potential common mechanisms, inflammation and oxidative stress, which partially reverse with CPAP treatment in OSA. Whether one or both of these mechanisms can explain the increased risk of OSA associated with gout remains to be examined in future studies; such a mechanism could explain our study finding. However, residual confounding due to obesity, a component of metabolic syndrome we could not control in our analyses due to lack of data, might also at least partially explain our findings.
We noted that lower hazard of new OSA diagnosis in older age groups and non-White race in this cohort of people 65 years or older confirmed racial differences noted in the Cleveland Family Study.43 Although more than 90% of Americans have Medicare coverage that should minimize health care access differences by race, non-Whites have lower access to medical care44 (ie, supplementary private insurance) or may be less likely to seek care for their symptoms, leading to underdiagnosis, which can explain the lower OSA risk in non-Whites. Racial differences in socioeconomic status (ie, higher poverty rate), body mass index, and behavioral risk factors (smoking, overeating, lack of exercise, alcohol use, etc.),45 may also be related to the differences noted.
Many advances in our understanding of the pathophysiology and consequences of OSA have been made in the past two decades. Some risk factors (male sex, obesity, alcohol, smoking, menopause, etc.) for sleep apnea are known.12 Whether the increased risk associated with gout makes gout a risk factor for OSA is unclear at this time; more studies are likely needed before concluding that gout is a risk factor for OSA. Studies need to replicate these findings in other cohorts and also investigate the underlying mechanisms of this association. OSA is associated with an increased risk of coronary artery disease and hypertension,6,7 and cardiovascular events such as heart failure4 and stroke.5 A better understanding of the link between chronic inflammatory diseases such as gout and OSA can provide valuable insights into mechanisms of OSA and help the development of targeted interventions to reduce its risk. This indicates that targeting modifiable risk factors of OSA has the potential to positively affect the risk of incident OSA and the associated cardiovascular burden.
An interesting negative finding was that age, sex, or race were not associated with any meaningful difference in the hazard ratios of OSA related to gout. In this sample of older adults, these biological variables do not seem to influence the association of gout with new-onset OSA.
Our findings should be interpreted considering study limitations. Milder forms of the metabolic syndrome without formal diagnoses may have been missed in our study, potentially leading to residual confounding. Disparities related to socioeconomic factors, health care access factors, body mass index, etc. may also have potentially confounded this association. However, we adjusted for hyperlipidemia, hypertension, and diabetes (components of metabolic syndrome), as well as other comorbidities including coronary heart disease, peripheral vascular disease, cerebrovascular disease, and heart failure as potential confounders in addition to demographics and medications, which likely decreased the possibility of confounding bias. We recognize nondifferential misclassification bias between the groups because of the underdiagnosis of OSA and very low sensitivity and low specificity of ICD-based algorithm (sensitivity 24%, specificity 68% in the previous validation study25) for OSA. This likely biased our estimate of association toward null because a larger proportion of patients were likely misclassified as nonpatients than false positives, making them conservative estimates and indicating that the true association may be even stronger. Some overdiagnosis of OSA based on the diagnostic code is also possible, when the clinician suspects OSA, but may not have confirmed the diagnosis; the extent of this is unclear and cannot be determined from data in our cohort study. Other determinants of obtaining a diagnosis of OSA and/or preceding diagnosis of gout may be due to a patient's age or sex affecting the likelihood that the health care provider would put forth the various diagnoses (confirmed or suspected) for the purpose of reimbursement for health care services. We considered using the ICD-9-CM code 780.53, which denotes “hypersomnia with sleep apnea, unspecified” (ie, is nonspecific for OSA), but decided not to include this code for our study, because compared to the specific ICD-9-CM code we used it had much lower specificity (38% [95% CI 36% to 40%]) for OSA in a recent validation study.46 The low specificity of using the code 780.53 (not the approach we used) may be related to the fact that it is nonspecific for OSA and that it might have been more widely used in earlier years when the diagnosis was suspected, but not routinely confirmed with a polysomnogram. In some cases, OSA diagnosis may be preexisting, but because of diagnostic delay, OSA may have been diagnosed later than gout. In unadjusted analyses, people with OSA had lower prevalence of dementia and meta-static cancer, which might be related to lower likelihood of a successful sleep study in people with these conditions. People with several comorbidities (chronic pulmonary disease, diabetes, diabetes with end-organ damage, heart failure, etc.) had higher unadjusted incidence of OSA, which might indicate surveillance bias, that is, higher likelihood of (1) clinical suspicion of OSA and/or (2) screening for OSA and/or (3) pursuing a clinical diagnosis of OSA, often by performing a sleep study in the presence of these comorbidities compared to those without each comorbidity.
Diagnostic suspicion bias would arise if physicians referred people with gout to sleep studies more frequently than those without gout. To our knowledge, no estimates of this are available in the literature. Although this is possible in small numbers of patients, the association of gout and OSA is not well established and therefore this might at best explain the noted association only partially. The lack of laboratory tests in the Medicare data limited us from conducting analyses to assess the mediators of the association of gout with sleep apnea in our study. Medicare claims do not provide results of tests, such as the sleep study; therefore, correlation with sleep study confirmed OSA or the severity of apnea-hypopnea index (AHI) could not be done. We did not search for a procedure code for sleep study, a limitation of our study; we chose not to do this, because completion of a sleep study would depend on the patient's insurance coverage, the availability of a sleep study center in close proximity to the patient's residence, and patient preference. The results (positive versus negative) of the sleep study are not available in the claims data. Future research should address important mechanistic questions including the assessment of hyperuricemia, oxidative stress, or other inflammatory pathways as the underlying mechanisms for this association. Findings should only be generalized to the population from which they are derived, that is, adults 65 years or older enrolled in fee-for-service Medicare,47 and not all adults. Study strengths were that we used a representative sample, the number of events was large, and the findings were robust in sensitivity analyses.
In conclusion, we found that gout was associated with a twofold increased risk of a new diagnosis of OSA in adults 65 year or older. This association was independent of the patient demographics (including sex and race), cardiovascular disease, components of metabolic syndrome (except obesity), and other comorbidities. Sensitivity analyses confirmed the robustness of these findings. Future studies should explore the underlying mechanisms of this association, and also examine if this association is also present in younger individuals.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This material is the result of work supported by research funds from the Division of Rheumatology at the University of Alabama at Birmingham and the resources and use of facilities at the Birmingham VA Medical Center, Birmingham, Alabama, United States. The funding body did not play any role in design, collection, analysis, and interpretation of data. JAS has received research grants from Takeda and Savient pharmaceuticals and consultant fees from Savient, Takeda, Regeneron, Merz, Iroko, Bioiberica, Crealta/Horizon and Allergan pharmaceuticals, WebMD, UBM LLC, Medscape, Fidia pharmaceuticals and the American College of Rheumatology. JAS serves as the principal investigator for an investigator-initiated study funded by Horizon pharmaceuticals through a grant to DINORA, Inc., a 501 (c)(3) entity. JAS is a member of the executive of OMERACT, an organization that develops outcome measures in rheumatology and receives arms-length funding from 36 companies; a member of the American College of Rheumatology's (ACR) Annual Meeting Planning Committee (AMPC); Chair of the ACR Meet-the-Professor, Workshop and Study Group Subcommittee; and a member of the Veterans Affairs Rheumatology Field Advisory Committee. JAS is the editor and the Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis. JDC reports no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Dr. Jeffrey Curtis of the UAB Division of Rheumatology, who permitted us to re-use the 5% Medicare data. We thank the patients at the University of Alabama gout clinic for asking us to question whether gout is associated with other comorbidities, which prompted us to ask this question.
ABBREVIATIONS
- CMS
Centers for Medicare and Medicaid Services
- CPAP
continuous positive airway pressure
- ICD
International Classification of Diseases
- NOS
nitric oxide synthase
- OSA
obstructive sleep apnea
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Rising prevalence of sleep apnea in U.S. threatens public health. [Accessed February 24, 2018]; https://aasm.org/rising-prevalence-of-sleep-apnea-in-u-s-threatens-public-health/. American Academy of Sleep Medicine. [Google Scholar]
- 3.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association council for high blood pressure research professional education committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on sleep disorders research (National Institutes of Health) Circulation. 2008;118(10):1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips CL, Cistulli PA. Obstructive sleep apnea and hypertension: epidemiology, mechanisms and treatment effects. Minerva Med. 2006;97(4):299–312. [PubMed] [Google Scholar]
- 7.Wolk R, Shamsuzzaman AS, Somers VK. Obesity, sleep apnea, and hypertension. Hypertension. 2003;42(6):1067–1074. doi: 10.1161/01.HYP.0000101686.98973.A3. [DOI] [PubMed] [Google Scholar]
- 8.Frost & Sullivan; American Academy of Sleep Medicine. Hidden health crisis costing America billions: underdiagnosing and undertreating obstructive sleep apnea draining healthcare system. [Accessed March 2018]; http://aasm.org/wp-content/uploads/2017/10/sleep-apnea-economic-crisis.pdf. Published August 8, 2016. [Google Scholar]
- 9.Lacasse Y, Godbout C, Series F. Health-related quality of life in obstructive sleep apnoea. Eur Respir J. 2002;19(3):499–503. doi: 10.1183/09031936.02.00216902. [DOI] [PubMed] [Google Scholar]
- 10.Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 11.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 12.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banno K, Manfreda J, Walld R, Delaive K, Kryger MH. Healthcare utilization in women with obstructive sleep apnea syndrome 2 years after diagnosis and treatment. Sleep. 2006;29(10):1307–1311. doi: 10.1093/sleep/29.10.1307. [DOI] [PubMed] [Google Scholar]
- 14.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 15.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology. 2010;49(7):1229–1238. doi: 10.1093/rheumatology/keq037. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Peloquin CE, Dubreuil M, et al. Sleep apnea and the risk of incident gout: a population-based, body mass index-matched cohort study. Arthritis Rheumatol. 2015;67(12):3298–3302. doi: 10.1002/art.39330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5(2):207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 19.Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the third National Health and Nutrition Examination Survey. Arthritis Rheum. 2007;57(1):109–115. doi: 10.1002/art.22466. [DOI] [PubMed] [Google Scholar]
- 20.Tseng VL, Yu F, Lum F, Coleman AL. Risk of fractures following cataract surgery in Medicare beneficiaries. JAMA. 2012;308(5):493–501. doi: 10.1001/jama.2012.9014. [DOI] [PubMed] [Google Scholar]
- 21.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303(9):849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez I, Zhang Y. Risk of bleeding with dabigatran in 2010-2011 Medicare data. JAMA Intern Med. 2015;175(7):1245–1247. doi: 10.1001/jamainternmed.2015.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics, Office of Analysis and Epidemiology. Analytic Issues in Using the Medicare Enrollment and Claims Data Linked to NCHS Surveys. [Accessed April 16, 2018]; http://www.cdc.gov/nchs/data/datalinkage/cms_medicare_analytic_issues_final.pdf. [Google Scholar]
- 24.Singh JA, Hodges JS, Toscano JP, Asch SM. Quality of care for gout in the US needs improvement. Arthritis Rheum. 2007;57(5):822–829. doi: 10.1002/art.22767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laratta CR, Tsai WH, Wick J, Pendharkar SR, Johannson KA, Ronksley PE. Validity of administrative data for identification of obstructive sleep apnea. J Sleep Res. 2017;26(2):132–138. doi: 10.1111/jsr.12465. [DOI] [PubMed] [Google Scholar]
- 26.Singh JA. Any sleep is a dream far away: a nominal group study assessing how gout affects sleep. Rheumatology. 2018 Feb 23; doi: 10.1093/rheumatology/kex535. . [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Fakhouri TH, Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity among older adults in the United States, 2007-2010. NCHS Data Brief. 2012;(106):1–8. [PubMed] [Google Scholar]
- 28.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 29.Dalbeth N, Haskard DO. Mechanisms of inflammation in gout. Rheumatology. 2005;44(9):1090–1096. doi: 10.1093/rheumatology/keh640. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 31.Acharya C, Sharma AK, Kantharia ND. Involvement of oxidative stress in patients of gout and antioxidant effect of allopurinol. Int J Med Sci Public Health. 2015;4(2):168–172. [Google Scholar]
- 32.Glantzounis GK, Tsimoyiannis EC, Kappas AM, Galaris DA. Uric acid and oxidative stress. Curr Pharm Des. 2005;11(32):4145–4151. doi: 10.2174/138161205774913255. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology. 2010;49(7):1229–1238. doi: 10.1093/rheumatology/keq037. [DOI] [PubMed] [Google Scholar]
- 34.Zamudio-Cuevas Y, Martinez-Flores K, Fernandez-Torres J, et al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res Ther. 2016;18(1):117. doi: 10.1186/s13075-016-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112(17):2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 36.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107(8):1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 37.Jelic S, Padeletti M, Kawut SM, et al. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117(17):2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams A, Scharf SM. Obstructive sleep apnea, cardiovascular disease, and inflammation--is NF-kappaB the key? Sleep Breath. 2007;11(2):69–76. doi: 10.1007/s11325-007-0106-1. [DOI] [PubMed] [Google Scholar]
- 39.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169(3):348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 40.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102(21):2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 41.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169(3):354–360. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 42.Schulz R, Hummel C, Heinemann S, Seeger W, Grimminger F. Serum levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea and severe nighttime hypoxia. Am J Respir Crit Care Med. 2002;165(1):67–70. doi: 10.1164/ajrccm.165.1.2101062. [DOI] [PubMed] [Google Scholar]
- 43.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155(1):186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 44.Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57(Suppl 1):108–145. doi: 10.1177/1077558700057001S06. [DOI] [PubMed] [Google Scholar]
- 45.National Research Council. Understanding Racial and Ethnic Differences in Health in Late Life: A Research Agenda. Washington, DC: The National Academies Press; 2004. [PubMed] [Google Scholar]
- 46.McIsaac DI, Gershon A, Wijeysundera D, Bryson GL, Badner N, van Walraven C. Identifying obstructive sleep apnea in administrative data: a study of diagnostic accuracy. Anesthesiology. 2015;123(2):253–263. doi: 10.1097/ALN.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 47.Identifying Medicare Managed Care Beneficiaries from the Master Beneficiary Summary or Denominator Files. [Accessed April 16, 2018]; https://www.resdac.org/resconnect/articles/114. Research Data Assistance Center (ResDAC) website. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.