Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is associated with increased cardiovascular risk. The effect of OSA treatment with continuous positive airway pressure (CPAP) on the cardiovascular response to a stressor is unknown. We sought to determine the effect of CPAP therapy on heart rate variability (HRV) and arterial stiffness, at baseline, in response to, and recovery from a physiological stressor, Angiotensin II (AngII), in humans with OSA.
Methods:
Twenty-five incident healthy subjects (32% female; 49 ± 2 years) with moderate-severe OSA and nocturnal hypoxia were studied in high-salt balance, a state of maximal renin-angiotensin system (RAS) suppression, before CPAP, and after 4 weeks of effective CPAP therapy (usage > 4 h/night) in a second identical study day. HRV was calculated by spectral power and time domain analysis. Aortic augmentation index (AIx) and carotid-femoral pulse-wave velocity (PWVcf) were measured by applanation tonometry. HRV and arterial stiffness were measured at baseline and in response to AngII challenge (3 ng/ kg/min·30 minutes, 6 ng/kg/min·30 minutes, recovery·30 minutes). The primary outcome was the association between CPAP treatment and HRV and arterial stiffness responses to, and recovery from, AngII challenge. In an exploratory analysis subjects were stratified by sex.
Results:
CPAP corrected OSA and nocturnal hypoxemia. CPAP treatment was associated with increased sensitivity and delayed recovery from AngII (Δln HF [high frequency; recovery: −0.09 ± 0.19 versus −0.59 ± 0.17 ms2, P = .042; ΔrMSSD [root mean successive differences; recovery: −0.4 ± 2.0 versus −7.2 ± 1.9 ms, P = .001], ΔpNN50 [percentage of normal waves differing ≥ 50 ms compared to the preceding wave; AngII: 1.3 ± 2.3 versus −3.0 ± 2.4%, P = .043; recovery: −0.4 ± 1.4 versus −6.0 ± 1.9%, P = .001], all values pre-CPAP versus post-CPAP treatment). No differences were observed by sex. There was increased AIx sensitivity to AngII after CPAP among men (8.2 ± 1.7 versus 11.9 ± 2.2%, P = .046), but not women (11.4 ± 1.5 versus 11.6 ± 2.1%, P = .4). No change in PWVcf sensitivity was observed in either sex.
Conclusions:
CPAP therapy was associated with delayed cardiovagal reactivation after a stressor and down-regulation of the arterial RAS. These findings may have important implications in mitigating cardiovascular risk in both men and women with OSA.
Citation:
Nicholl DD, Hanly PJ, Zalucky AA, Mann MC, MacRae JM, Poulin MJ, Handley GB, Sola DY, Ahmed SB. CPAP therapy delays cardiovagal reactivation and decreases arterial renin-angiotensin system activity in humans with obstructive sleep apnea. J Clin Sleep Med. 2018;14(9):1509–1520.
Keywords: arterial stiffness, continuous positive airway pressure, heart rate variability, obstructive sleep apnea, renin-angiotensin system
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) is associated with increased cardiovascular risk and bradyarrhythmias, including alterations in heart rate variability and arterial stiffness, both validated markers of cardiovascular risk, though the mechanisms remain elusive. Whether adherent continuous positive airway pressure (CPAP) therapy in men and women with OSA is associated with improved measures of cardiovascular risk in response to a stressor remains unknown.
Study Impact: In men and women with OSA, adherent CPAP therapy was associated with (1) delayed cardiovagal reactivation after exposure to an angiotensin II stressor and (2) reduced vascular renin-angiotensin system activity. These findings support a role for the renin-angiotensin system in mediating OSA-induced hypertension and cardiovascular disease and highlight an important mechanism by which CPAP may eradicate bradyarrhythmias during sleep and convey cardiovascular protection.
INTRODUCTION
Obstructive sleep apnea (OSA) is common1 and increases the risk of hypertension,2 bradyarrhythmias,3 and cardiovascular disease (CVD).4,5 Continuous positive airway pressure (CPAP) is an effective treatment for OSA6 and CPAP use is associated with lower rates of CVD.4,5 However, CPAP therapy can be limited by poor adherence7 which mitigates its cardioprotective efficacy.8,9 In fact, several studies have demonstrated cardiovascular benefit in only those subjects who are adherent to CPAP > 4 h/night.8–10 A more complete understanding of the pathophysiology linking OSA to increased CVD may provide alternative therapeutic strategies for those who are unable to tolerate CPAP.
Impaired cardiovascular recovery after a physiologic challenge is associated with increased cardiovascular risk and mortality.11 OSA is associated with impaired heart rate variability (HRV),12–16 reflected by increased cardiac sympathetic and parasympathetic tone, as well as increased arterial stiffness.13,17–19 Upregulation of the renin-angiotensin system (RAS) is detrimental to cardiovascular outcomes,20 but the contribution of the RAS to these validated markers of cardiovascular risk is unclear. Further, established sex differences in RAS activity,21 HRV,22 and arterial stiffness23 may also have important roles in the pathophysiology of OSA in CVD.1,24–26 Although studies have reported improvements in HRV12–16 and arterial stiffness17–19 with CPAP, we sought to determine how these markers of cardiovascular risk responded to the physiological stressor Angiotensin II (AngII) as a marker of vascular RAS activity.22,27,28
In a study designed with the primary objective of investigating the effect of CPAP therapy on the renal RAS and renal hemodynamics,29 we also examined the effects of CPAP on markers of cardiovascular risk in men and women with OSA. We hypothesized that CPAP therapy would increase the cardioprotective parasympathetic HRV response to and recovery from a stressor (AngII), and that the arterial vasculature would be more sensitive to the vasoconstrictor effects of AngII, consistent with a downregulated vascular RAS.22,27,28
METHODS
Subjects
Subjects with OSA were recruited from community patients referred for suspected OSA to the Foothills Medical Centre Sleep Centre and respiratory homecare companies in Calgary, Alberta, Canada, between June 2011 and June 2014. Men and women, age 18–70 years with moderate to severe OSA and significant nocturnal hypoxemia, were eligible to participate in the study. All subjects underwent a medical history, physical examination, and laboratory screening. Exclusion criteria included cardiovascular, cerebrovascular, and kidney disease, uncontrolled hypertension (blood pressure > 140/90 despite maximal use of antihypertensive medications), diabetes mellitus, severe lung disease, current treatment for OSA, current smoking, pregnancy, and use of nonsteroidal anti-inflamma-tory medications or exogenous sex hormones. The primary endpoint of change in renal hemodynamics in response to AngII post-CPAP therapy has been reported elsewhere.29 In this manuscript we report the prespecified exploratory cardiovascular endpoint results. The study protocol was approved by the Conjoint Health Research Ethics Board at the University of Calgary. Written informed consent was obtained from all study subjects in accordance with the Declaration of Helsinki.
Determination of OSA Status
Subjects underwent an unattended, overnight cardiopulmo-nary monitoring study (level 3 sleep study) at home (Remmers Sleep Recorder Model 4.2, Saga Tech Electronic, Calgary, Alberta, Canada).30 The monitor consists of an oximeter to record oxyhemoglobin saturation (SaO2) and HRV, a pressure transducer to record nasal airflow, a microphone to record snoring, and a body position sensor. The oximeter provides the data for an automated scoring algorithm that calculates the respiratory disturbance index (RDI) based on the number of episodes of oxyhemoglobin desaturation ≥ 4% per hour of monitoring. Nocturnal oxygen saturation was sampled at 1 Hz. The Remmers Sleep Recorder has been validated by comparison to attended polysomnography.30
Sleep apnea was defined as a RDI ≥ 15 events/h as this reflects moderate to severe sleep apnea, which is likely to be clinically significant.30 The Remmers Sleep Recorder has a sensitivity of 98% and specificity of 88% for a designation criterion of RDI ≥ 15 events/h.30 Portable monitoring was performed following current guidelines and recommendations of the American Academy of Sleep Medicine.31 The data were reviewed by a sleep medicine physician (PJH) who confirmed that the estimated RDI was accurate and diagnostic of OSA. Nocturnal hypoxemia was defined as in other studies as SaO2 < 90% for ≥ 12% of the duration of nocturnal monitoring.24
Study Protocol
The study protocol for assessment of RAS activity is well established.22,27,28 Subjects were instructed to consume > 200 mmol/d of sodium for 3 days before each study day to ensure maximum RAS suppression.32 Subjects were studied in the supine position in a temperature-controlled, quiet room in the morning after an 8-hour fast to account for circadian variations in the RAS.33 All subjects provided a second morning spot urine test for determination of urinary sodium to verify adherence with the high salt diet.34 All premenopausal female subjects were studied 14 days after the first day of the last menstrual period, determined by counting days and measuring 17β-estradiol levels.35 Subjects on medications that interfere with RAS activity were switched to a calcium-channel blocker (amlodipine) to achieve adequate blood pressure control 2 weeks prior to each study day, as these agents are considered to have a neutral effect on the RAS.36 Amlodipine was taken daily each morning including the morning of the assessment.
At 8:00 am, an 18-gauge peripheral venous cannula was inserted into each antecubital vein (1 for infusion, 1 for blood sampling). After a 90-minute equilibration period, HRV, aortic augmentation index (AIx), and carotid-femoral pulse-wave velocity (PWVcf) were measured at baseline and in response to AngII challenge (3 ng/kg/min·30 minutes, 6 ng/kg/min·30 minutes) as an index of RAS activity22,27,28 followed by a 30-minute recovery period. Blood pressure was recorded every 15 minutes by an automatic recording device (Dinamap; Critikon). Subjects were studied in the supine position using a standard cuff placed on the right arm. The mean of two readings taken by the same Registered Nurse (DYS) were recorded. Mean arterial pressure was calculated as one-third systolic blood pressure + two-thirds diastolic blood pressure.
Heart Rate Variability
HRV represents a balance between cardiac sympathetic and parasympathetic tone with greater variability in HRV considered a marker of a healthy autonomic nervous system; impaired cardiac autonomic tone is associated with increased mortality.37,38 Ambulatory electrocardiogram data were collected with a commercially available Holter monitor using a standard bipolar three-lead configuration (SEER MC recorder, GE Healthcare; Milwaukee, Wisconsin, United States). Holter data were collected continuously for 180 minutes (90 minutes of baseline, 60 minutes of AngII challenge, and 30 minutes of recovery). Ninety minutes of baseline, 30 minutes prior to the 60-minute timepoint for the AngII challenge, and 30 minutes of recovery were used to evaluate HRV at each timepoint, respectively. Power spectral density analysis of HRV, which transforms the electrocardiographic signals into measures of the frequency domain (largely reflecting sympathetic and vagal activity) was carried out by computer-generated algorithms (MARS version 7, GE Healthcare).38 Autonomic activity was categorized into spectral bands: total power (TP), very low frequency (VLF, 0.003–0.04 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (0.15–0.4 Hz) domains.38 VLF represented the parasympathetic effect on heart rate, LF represented a combination of sympathetic and parasympathetic nervous system power, and HF represented the parasympathetic (vagal) power.37,38 LF and HF parameters were evaluated in terms of frequency and amplitude, with amplitude assessed by the area (power) of each component; therefore squared units are used for the absolute value (ms2).39 Absolute LF and HF parameters were not normally distributed; therefore, the values were transformed into the natural logarithm (ln ms2). Absolute LF and HF parameters were also converted to normalized units (nu) to account for changes in the TP and any background contribution from activity within the VLF domain.38–40 Normalized units were calculated as follows: LF or HF divided by the total power (from which VLF has been subtracted) and multiplying by 100 ([HF or LF] / [TP − VLF]) × 100; all units in ms2.39,40 The LF:HF ratio component was calculated by comparing crude LF and HF parameters, representing total cardiosympathovagal balance.38 Time-domain measurements were generated using the beat-to-beat variation in normal R-R intervals, including the standard deviation (SD) of all normal R-R-wave intervals (SDNN; ms), SD of the average normal R-R-wave intervals (SDANN; ms), root mean square of the successive differences (rMSSD; ms), and percentage of normal waves differing more than 50 ms compared to the immediate preceding normal wave (pNN50; %).38 SDNN reflects total HRV, SDANN reflects primarily circadian HRV, while rMSSD and pNN50 reflect para-sympathetic activity.37
Arterial Stiffness Measurement
AIx and PWVcf are validated measures of central and peripheral vascular stiffness, respectively, and are associated with adverse cardiovascular outcomes.41,42 AIx and PWVcf were measured noninvasively with applanation tonometry (Millar Instruments, Houston, Texas, United States) and commercially available acquisition and analysis software (Version 8 SphygmoCor; AtCor Medical, Sydney, Australia) as previously described.43 Subjects were studied in the supine position. Two readings were taken and recorded at each time point by the same registered nurse (DYS) and the mean was reported.
Briefly, the AIx was determined by applanation tonometry of the right femoral artery using a Millar piezoresistive pressure transducer (Miller SPT 301, Millar Instruments) coupled to a SphygmoCor device (PWV Medical). AIx was calculated from the aortic pressure waveform obtained by applying a transfer function to the femoral pressure wave form. The AIx was calculated as the difference between the second systolic peak and inflection point of the aortic pressure waveform, expressed as a percentage of the aortic pulse pressure. Given that aortic pressure waveform is a combination of a forward travelling wave and a reflected wave, AIx is a parameter that reflects the degree to which aortic arterial pressure is enhanced by wave reflection. PWVcf was determined by sequential acquisition of pressure waveforms from the carotid and the femoral arteries by use of the same tonometer. The timing of these waveforms was compared with that of the R wave on a simultaneously recorded electrocardiography (ECG). PWVcf was determined by calculation of the difference in carotid to femoral divided by the difference in R wave to waveform foot times. The distance from the sternal notch to the femoral artery was used to calculate PWVcf.
Measures of Adiposity and Glycemic Control
Weight was measured using a digital scale and height was assessed using a tape measure while the subject was standing. Body mass index was calculated as weight / height2 (kg/m2). Fat mass (FM; kg) was evaluated by bioelectrical impedance measurement (RJL Sciences Quantum II system bioelectrical impedence analyzer). Lean mass (LM; kg/m2) was a calculated by weight-FM and then adjusted for height (LM/[height]2). Homeostatic Model Assessment Insulin Resistance (HOMA-IR) was calculated as ([fasting glucose, mmol/L] × [fasting insulin, μmol/L] / 22.5).
CPAP Treatment
After completing the first study day, subjects were treated with CPAP therapy as per the guidelines for treatment of OSA.6 All subjects underwent an auto-CPAP trial to determine individual CPAP requirement. Initial auto-CPAP settings were 16/6 cmH2O and were automatically titrated according to the CPAP unit titration algorithm to optimize therapy. If airflow limitation or nocturnal hypoxemia were not fully corrected, subjects were switched to fixed CPAP, which was estimated from the CPAP level at the 95th percentile. Adherence to CPAP therapy was monitored by electronic download from the unit each month. After satisfactory CPAP adherence was achieved (defined as CPAP use > 4 h/night on > 70% nights for 4 weeks)6 and correction of OSA and nocturnal hypoxemia was confirmed by a repeat level 3 sleep study while using CPAP, subjects underwent reassessment of HRV, arterial stiffness, and indices of adiposity and glycemic control during a second study day, identical to the pre-CPAP assessment.
Laboratory Measurements
Catecholamines were quantified by liquid chromatography with enzymatic colorimetric assay techniques. Urinary sodium was determined by an indirect potentiometry assay using an ion-selective electrode (Roche Cobas Integra Sodium, Roche). Serum creatinine was quantified by enzymatic colorimetric assay techniques (Roche/Hitachi Creatinine Plus). Fasting serum insulin levels were determined by chemiluminescent immunoassay (Abbott Diagnostics, Abbott Park, Illinois, United States), Hemoglobin A1c levels by turbidometric immunoassay, and fasting serum glucose was determined by a hexokinase-UV colorimetric assay (both Roche Diagnostics, Germany). N-terminal pro B-type natriuretic peptide (pro-BNP) was determined by an electrochemiluminescence immunoassay, or ECLIA (Cobas, Roche Diagnostics).
Sample Size and Power Calculation
The cardiovascular outcomes of interest outlined in this study are part of a study originally designed to examine the effect of CPAP therapy on renal RAS and renal hemodynamics.29 As such, the sample size is based on a renal outcome (filtration fraction ff). We calculated that 12 subjects would be required to detect a 3% difference in the FF response to AngII infusion before and after OSA treatment with CPAP using 80% power and an alpha of .05. This estimate is based on data from Miller et al. who demonstrated a difference of 3 ± 1% in the FF in response to AngII infusion after RAS blockade with the AT1 receptor blocker (ARB) irbesartan, as compared to before RAS blockade, on a high-salt diet in healthy Caucasians.21
Analyses
Data are reported as mean ± standard error, number (percentage), or mean (range), where appropriate. Our primary outcomes were the changes (Δ) in HRV (VLF, LF, HF, LF/HF, SDNN, SDANN, rMSSD, pNN50), AIx, and PWVcf at baseline and in response to a stressor (AngII), as a measure of vascular RAS activity, pre-CPAP versus post-CPAP therapy. Secondary study outcomes were the changes in indices of adiposity (body mass index, FM, LM), indices of glycemic control (fasting glucose, fasting insulin, HgbA1c, HOMA-IR), serum catecholamines, and pro-BNP at baseline pre-CPAP therapy versus post-CPAP therapy. In an exploratory analysis we also stratified subjects by sex, which is in keeping with Sex and Gender Equity in Research (SAGER) guidelines. Data were assessed for normality, and natural logarithms were applied to non-normally distributed values. Pre-CPAP and post-CPAP comparisons were made using either the Student paired t test (parametric testing for all subjects and male subjects) or Wilcoxon signed-rank test (nonparametric testing for female subjects). To examine the response to AngII on each study day we conducted a repeated-measures analysis of variance (parametric testing for all subjects and male subjects) or a Friedman test (nonparametric for female subjects) for outcomes with multiple measurements. Post hoc analyses with the Wilcoxon signed-rank test were conducted with a Bonferroni correction applied resulting in a significance level set at P < .0167 for outcomes with multiple measurements. Comparisons between men and women were conducted using the Mann-Whitney U test (nonparametric testing). Sensitivity analyses were conducted excluding subjects with controlled hypertension and subjects with persistent nocturnal hypoxia while on CPAP. All statistical analyses were performed with statistical software package SPSS version 17.0 (SPSS Inc., Chicago, Illinois, United States). All statistical analyses were two-tailed with a significance level of .05, with the exception of outcomes with multiple measurements where the significance level was set at P < .0167.
RESULTS
Study Enrollment
A total of 31 subjects completed both study days, but 5 subjects did not have measurement of the endpoints of interest on both study days. One subject ingested a single dose of candesartan (angiotensin II-receptor blocker) the morning of the first study day; this subject was studied in an identical fashion post-CPAP, including ingestion of candesartan, to allow for comparison of pre-CPAP and post-CPAP results. This subject was excluded from the final analysis because the subject deviated from the study protocol by ingesting candesartan but was included in an exploratory analysis as the outcomes of interest were the changes (Δ) in HRV, AIx, and PWVcf at baseline and in response to a stressor (AngII) pre-CPAP versus post-CPAP. Consequently, 25 subjects were included in the final analyses.
Subject Characteristics
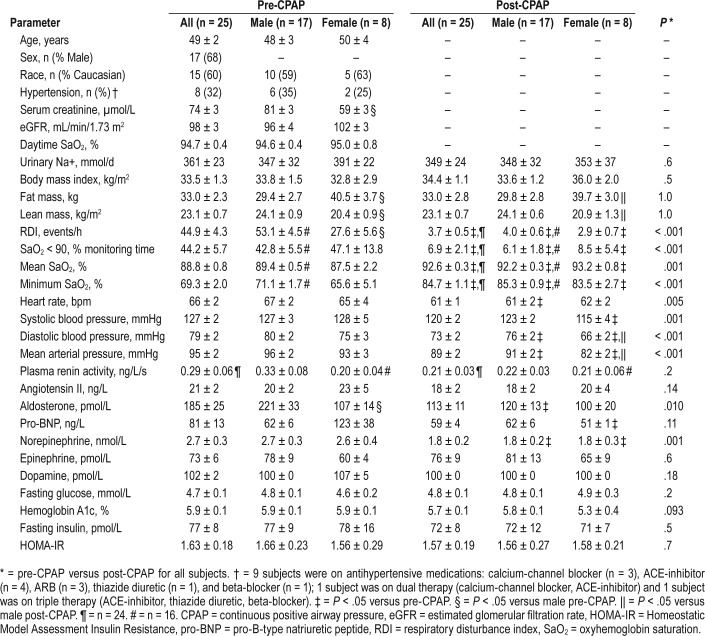
Subject characteristics are presented in Table 1. A total of 25 subjects with newly diagnosed OSA and nocturnal hypoxemia were recruited (17 men, 8 women [2 premenopausal, 6 postmenopausal]; age 49 [26–68] years). All had blood pressure < 140/90 mmHg. RAS-interfering medications were switched to the calcium-channel blocker amlodipine to control blood pressure 2 weeks prior to each assessment. All subjects were nondiabetic and did not smoke, with normal kidney function and in high-salt balance. Subjects had abnormal HRV parameters38 consistent with previous studies of patients with OSA12–16 and increased arterial stiffness18 at baseline. Female subjects had decreased serum creati-nine, aldosterone, and RDI and increased AIx compared to male subjects.
Table 1.
Baseline characteristics pre-CPAP and post-CPAP therapy stratified by sex.
CPAP Therapy
All subjects were adherent with CPAP, which corrected their OSA and nocturnal hypoxemia. Duration to CPAP acclimatization was 149 (64, 327) days. During the 4-week period prior to reassessment of CV risk measures, CPAP was used 92 ± 2% of nights (82 ± 3% with usage > 4 h/night), with an average nightly usage of 6.1 ± 0.2 hours, indicating good CPAP adherence, and an estimated apnea-hypopnea index of 3.3 ± 0.8 events/h, indicating adequate treatment. A total of 17 subjects completed the study on auto-CPAP, whereas 8 subjects were converted to fixed CPAP in order to optimize therapy. All subjects met acceptable criteria for correction of OSA (RDI < 10 events/h; Table 1) and all but four subjects corrected nocturnal hypoxemia (SaO2 < 90% for < 12% monitoring); in those four subjects the post-CPAP mean SaO2 was ≥ 90%. One subject was not available to complete a level 3 sleep study on therapy prior to reassessment, but did have a CPAP adherence download indicating 100% usage > 4 h/night and an apnea-hypopnea index of 0.8 events/h. Consequently, adequate treatment was inferred based on the CPAP download.
Pre-CPAP Versus Post-CPAP Baseline Characteristics
Study subject characteristics pre-CPAP and post-CPAP stratified by sex are reported in Table 1. There were no changes in measures of adiposity or insulin resistance post-CPAP. Blood pressure and heart rate improved post-CPAP. There were significant reductions in serum norepinephrine levels and aldosterone levels. The reduction in aldosterone was observed only in men and a reduction in pro-BNP was only observed in women.
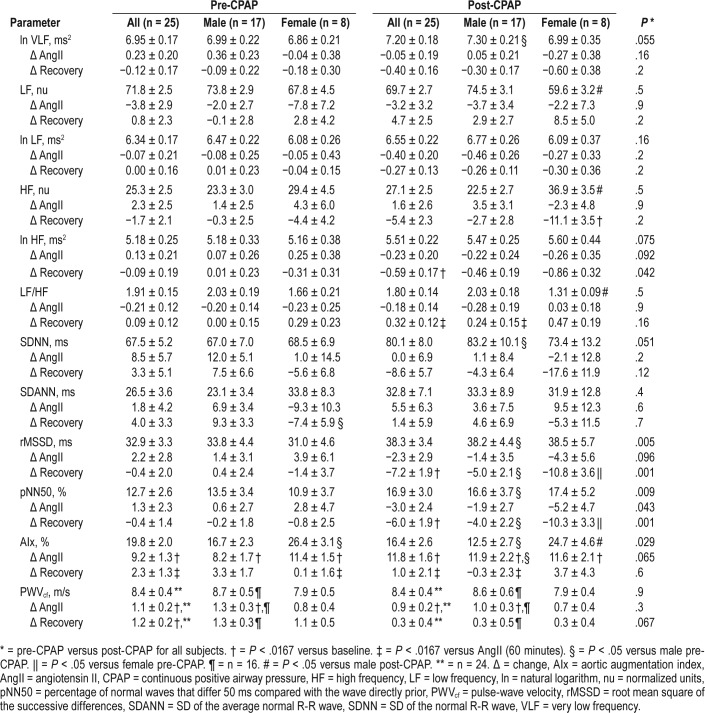
Baseline measures of HRV and arterial stiffness pre-CPAP and post-CPAP stratified by sex are reported in Table 2. Pre-CPAP therapy there were no differences in baseline HRV parameters between men and women. Post-CPAP therapy there were increases in baseline HRV measures (ln VLF, SDNN, rMSSD, and pNN50) in all subjects, though these increases were most prominent among male subjects. Post-CPAP therapy female subjects had decreased LF (nu), increased HF (nu), and increased LF/HF compared to male subjects. Central (AIx) but not peripheral (PWVcf), arterial stiffness was reduced, though the decrease in AIx post-CPAP was only observed in male subjects.
Table 2.
Heart rate variability and arterial stiffness at baseline, in response to and recovery from angiotensin II pre-CPAP and post-CPAP therapy stratified by sex.
HRV Responses to and Recovery From AngII Pre-CPAP Versus Post-CPAP
HRV responses to AngII pre-CPAP and post-CPAP stratified by sex are reported in Table 2. Pre-CPAP, HRV measures remained stable in response to AngII. Post-CPAP, no significant changes in the HRV response to an AngII stressor were observed.
Pre-CPAP, HRV measures remained similar in the recovery period to those during AngII challenge. However, CPAP therapy was associated with delayed cardiovagal reactivation post-AngII as evidenced by decreased ln HF (ms2), rMSSD and pNN50 (Figure 1, Figure 2, Figure 3, and Table 2). There was also delayed cardiovagal reactivation in HF (nu), which was accompanied by an increase in LF/HF for all subjects during the recovery from AngII.
Figure 1. ln high frequency pre-CPAP versus post-CPAP.
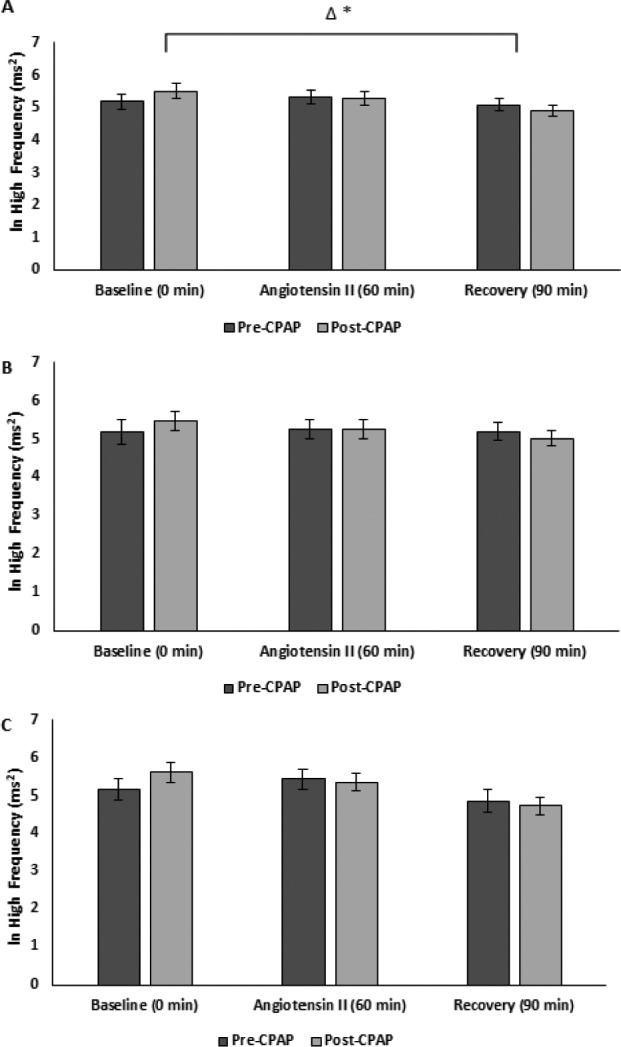
(A) All, (B) male, and (C) female subjects. * = P < .05 versus pre-CPAP. Δ = change, CPAP = continuous positive airway pressure, ln = natural logarithm.
Figure 2. rMSSD pre-CPAP versus post-CPAP.
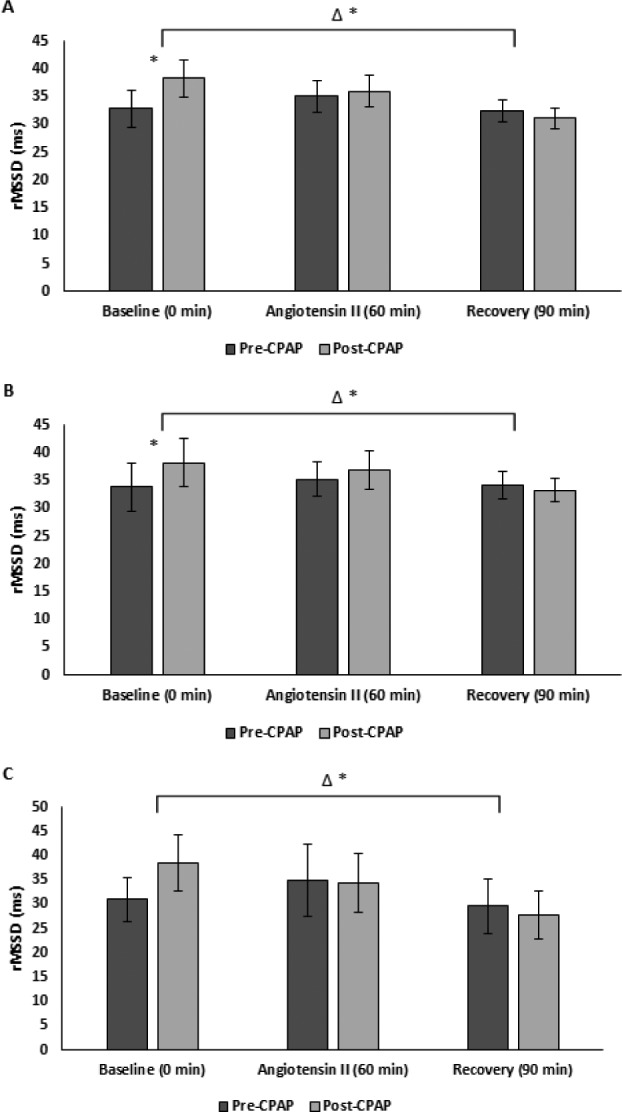
(A) All (B) male, and (C) female subjects. * = P < .05 versus pre-CPAP, Δ = change, CPAP = continuous positive airway pressure, rMSSD = root mean square of the successive differences.
Figure 3. pNN50 pre-CPAP versus post-CPAP.
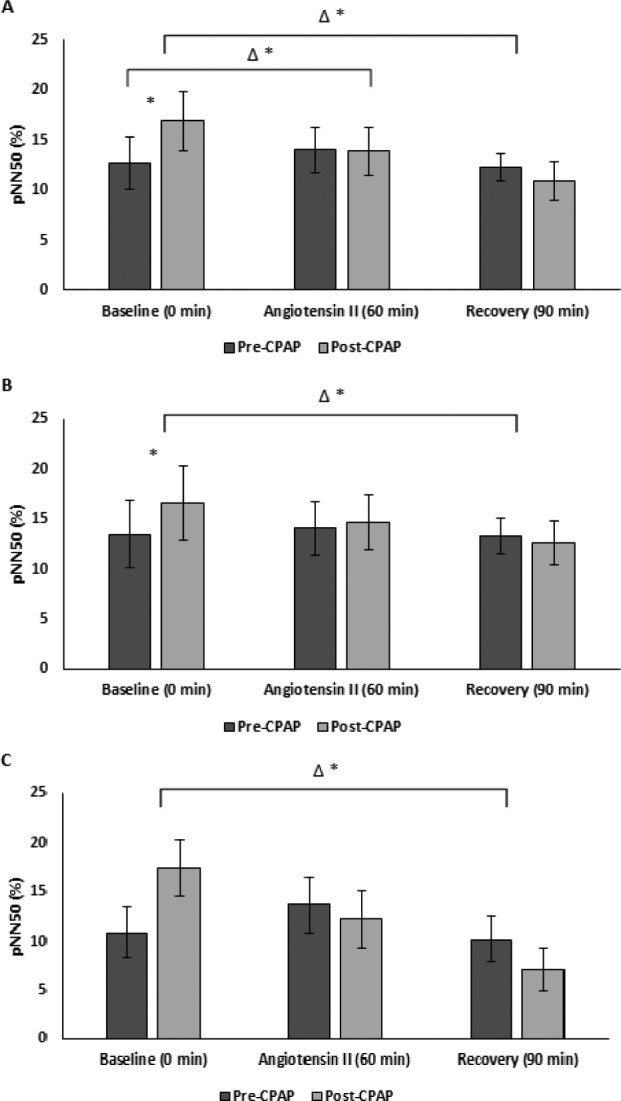
(A) All (B) male, and (C) female subjects. * = P < .05 versus pre-CPAP, Δ = change, CPAP = continuous positive airway pressure, pNN50 = percentage of normal waves that differ 50 ms compared with the wave directly prior.
Sex Differences in HRV in Response to and Recovery From AngII
There were no differences in the HRV responses to AngII between men and women pre-CPAP or post-CPAP therapy.
Similarly, there were no differences in the HRV recovery from AngII challenge between men and women pre-CPAP therapy. However, post-CPAP therapy both men and women demonstrated delayed cardiovagal reactivation in HF (nu) post-AngII challenge, though this finding was only significant among female subjects. Both men and women demonstrated an increase in LF/HF during recovery from the AngII challenge, though the difference was only significant among male subjects. CPAP exaggerated the delayed vagal reactivation in both sexes, but the association was significantly greater in women compared to men as shown by rMSSD and pNN50.
Arterial Stiffness Responses to and Recovery From AngII Pre-CPAP Versus Post-CPAP
Arterial stiffness responses to AngII pre-CPAP and post-CPAP stratified by sex are reported in Table 2. There were increases in AIx and PWVcf in response to AngII pre-CPAP. There was a greater increase in AIx in response to AngII, though this was limited to male subjects (P = .046; Figure 4). There was no change in PWVcf response to AngII post-CPAP (Figure 5).
Figure 4. Aortic augmentation index pre-CPAP versus post-CPAP.
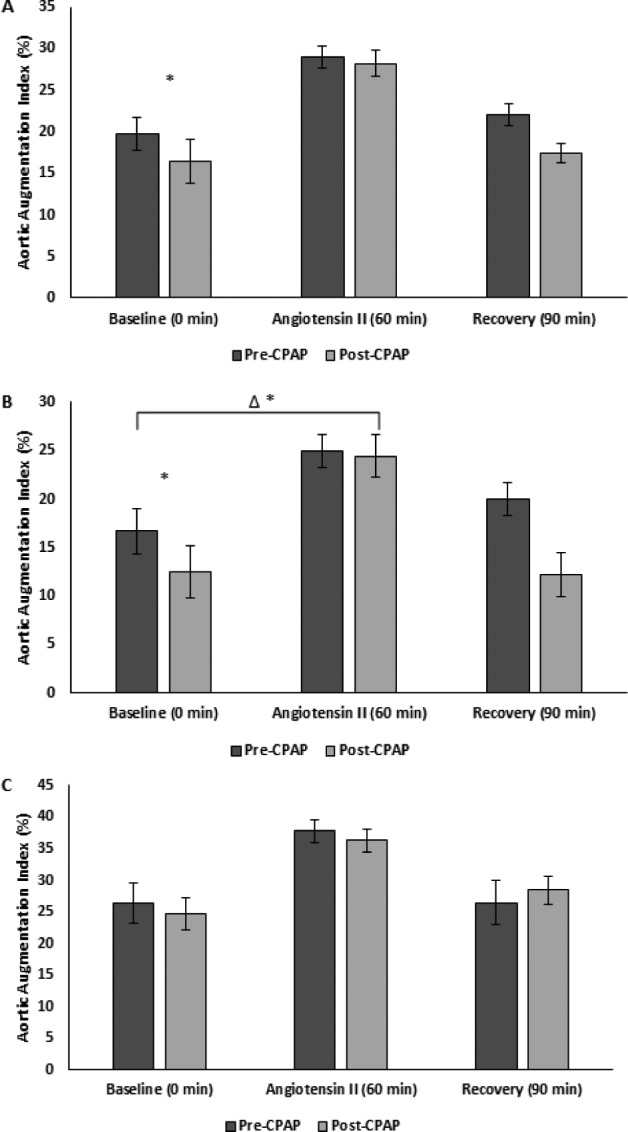
(A) All (B) male, and (C) female subjects. * = P < .05 versus pre-CPAP, Δ = change, CPAP = continuous positive airway pressure.
Figure 5. Carotid-femoral pulse-wave velocity pre-CPAP versus post-CPAP.
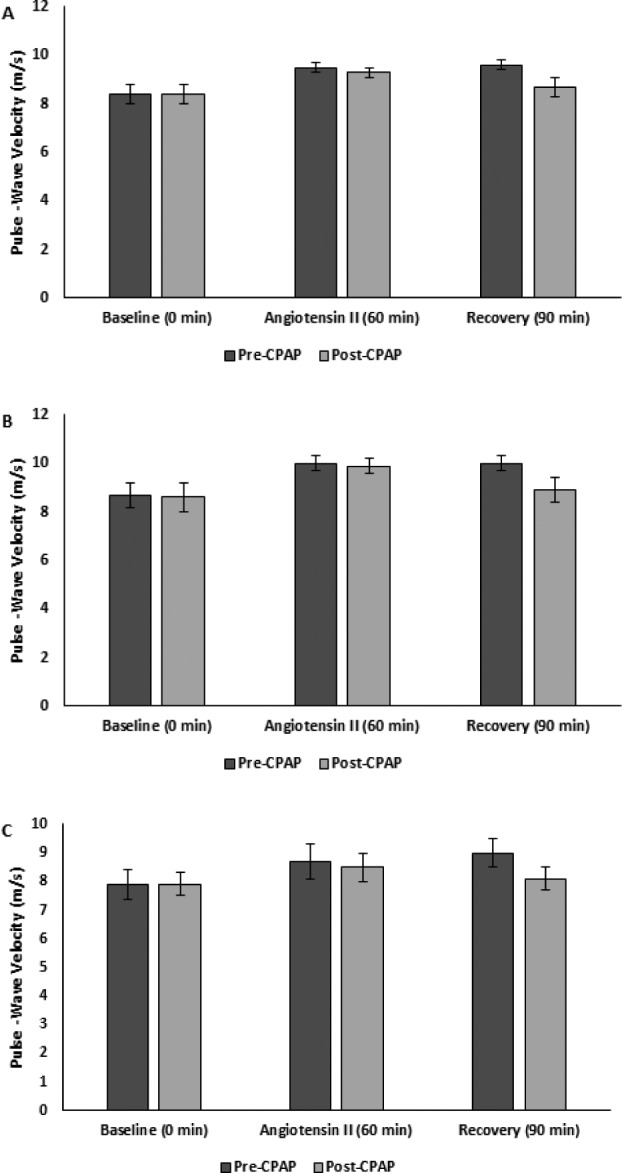
(A) All (B) male, and (C) female subjects. CPAP = continuous positive airway pressure.
Pre-CPAP AIx returned to baseline after the recovery period, while PWVcf remained elevated. Post-CPAP both AIx and PWVcf returned to baseline after the recovery period (Figure 4 and Figure 5).
Sensitivity Analyses
Inclusion of the subject who took candesartan rendered the increased AIx responsiveness post-CPAP significant for all subjects but otherwise did not affect our findings. Exclusion of the subject who did not have a repeat level-3 assessment did not affect our findings. Similarly, neither exclusion of subjects with controlled hypertension nor subjects with persistent hypoxemia altered our primary findings.
DISCUSSION
We examined two validated physiologic markers of cardiovascular risk, HRV and arterial stiffness, in individuals with OSA before and after CPAP therapy. To our knowledge this is first study to examine the effect of CPAP treatment on the HRV and arterial stiffness responses to an AngII challenge, a well-accepted indirect measure of RAS activity.22,27,28 Our primary findings were that CPAP adherence in OSA was associated with (1) delayed cardiovagal reactivation after exposure to an AngII stressor and (2) reduced vascular RAS activity as reflected by the greater increase in AIx in response to AngII post-CPAP therapy. These findings suggest that OSA treatment with CPAP alters HRV recovery after a stressor. Furthermore, CPAP appears to reduce central arterial stiffness through downregulation of vascular RAS activity. Patients with OSA exhibit bradyarrhythmias during sleep and CPAP therapy has been shown to correct these arrhythmias.3 Chrysostomakis et al.14 postulated that the reduction in parasympathetic activity may be one of the mechanisms responsible for alleviation of bradyarrhythmic episodes following the initiation of CPAP therapy. We extend these findings by demonstrating that adherent CPAP therapy delays parasympathetic reactivation after a stressor, which may be one of the mechanisms by which CPAP therapy eradicates bradyarrhythmias during sleep and confers cardiovascular protection. These findings support a role for the RAS in mediating OSA-induced hypertension and CVD in humans and highlight several mechanisms by which CPAP therapy appears to convey cardiovascular risk reduction.
CPAP therapy is associated with lower rates of cardiovascular complications and mortality, especially among patients who are adherent to treatment.4,5 Nonadherence appears to mitigate the cardioprotective properties of CPAP8 and several studies have demonstrated dose-response relationships between CPAP adherence and cardiovascular endpoints.4,5 The fact that all subjects in our study were adherent to CPAP suggests that the physiological changes observed with CPAP therapy are consistent with an improvement in cardiovascular risk.
Multiple studies have demonstrated abnormalities in sympathetic and parasympathetic activity in OSA and improvement with CPAP, with important differences in HRV during sleep and wakefulness.12–16 It has been suggested that the physiological basis of the OSA-associated HRV abnormalities during sleep is due to upper airway obstruction, with initial stimulation of the parasympathetic nervous system, followed by sympathetic activation due to the resultant intermittent hypoxia,44 intrathoracic pressure changes,45 and recurrent arousals.46 Thus, there is significantly enhanced nocturnal RR variability as a consequence of these alternating strong successive parasympathetic and sympathetic drives in patients with OSA. In contrast, daytime HRV is reduced in OSA and is associated with enhanced sympathetic activity.44
Khoo et al.15 reported no change in HRV measures during sleep in 7 patients with OSA with short-term CPAP, but demonstrated improvement in vagal control with long-term CPAP adherence in 13 men.16 Roche et al.12 reported higher nocturnal measures of parasympathetic activity in patients with OSA compared to controls. In a follow-up study, the same authors reported that CPAP for 3 months decreased nocturnal LF, HF, and LF/HF when patients were asleep.12 Further, the decrease in LF/HF persisted into the daytime and thus the authors concluded that CPAP significantly reduced parameters of cardiac sympathetic tone, which is a favorable effect.12 Chrysostomakis et al.14 found no significant differences in HRV indices during the day in 26 subjects with OSA (18 male) compared to controls, but found that both rMSSD and pNN50, measures of cardiovagal activity, were significantly higher compared to controls at night and that CPAP for 2 months reduced these nighttime vagal indices to resemble that of normal controls. The fact that our study occurred during the daytime may thus explain why our subjects exhibited increased baseline vagal activity with CPAP.
Both AIx and PWVcf are validated measures of central and peripheral vascular stiffness, respectively, which is associated with adverse cardiovascular outcomes.41,42 Kraiczi et al.47 measured vascular sensitivity to an intra-arterial AngII challenge as measured by arterial forearm conductance in 10 subjects with OSA and 10 healthy controls. The authors found the forearm vasoconstrictor response to intra-arterial AngII infusion to be increased in normotensive patients with OSA compared to healthy controls.47 Similar to our findings, there was increased arterial stiffness in subjects with OSA. However, this study differed from ours in that the authors used intra-arterial AngII infusion as opposed to intravenous AngII infusion, evaluated arterial stiffness through a different modality, included only male subjects, did not control for salt intake or kidney function, and importantly the study was not designed to assess the effect of OSA treatment on arterial stiffness. Several studies have reported reductions of arterial stiffness with effective CPAP therapy.13,17–19 Shiina et al.13 studied the effect of CPAP for 3 months on HRV and brachial-ankle PWV in 50 patients with OSA (90% male). They reported a significant independent correlation between the change in LF/HF and brachial-ankle PWV and suggested that improvement of sympathovagal balance with CPAP may be directly related to improvement in arterial stiffness. Upregulation of the vascular AngII system results in the chronic blood pressure changes that occur from episodic hypoxia48 and intermittent hypoxia increases arterial blood pressure in humans through a RAS-dependent mechanism.49 As a blunted vascular response to AngII is a reflection of high local tissue—AngII concentrations and tissue—RAS activity,32 the increased AIx sensitivity to AngII challenge reflects a downregulation in arterial RAS activity with CPAP. Of note, discordance between AIx and PWVcf responses to stress-ors has been reported previously. Kelly et al.28 demonstrated that vasoactive drugs (AngII and nitroglycerine) were capable of producing large effects on AIx but had minimal effect on PWVcf (Δ < 1 m/s) in healthy men. The authors hypothesized that the elastic nature of the aorta may minimize the effects of acute changes on vascular tone and blood pressure on the small arteries and PWVcf.28
We have previously reported no change in circulating levels of PRA and AngII post-CPAP therapy, despite a reduction in serum aldosterone and changes in AngII sensitivity at the level of the kidney.29 In the current study, we report changes in AngII sensitivity at the level of the vasculature despite no changes in circulating PRA or AngII. The most likely explanation for this phenomenon is differences in the local tissue RAS, as local AngII formation in the vasculature may occur independently of the circulating RAS.50 Greater vascular sensitivity during AngII infusion may reflect remodeling in the vessel walls and in particular alterations in sensitivity to vasoconstrictors and vasodilators at the cellular level.47
The sex differences observed in our study deserve comment. Few studies examining the effects of OSA on vascular measures have reported sex-stratified analyses. In the Multi-Ethnic Study of Atherosclerosis, moderate-severe OSA in men was associated with retinal arteriolar narrowing and venular widening but not in women.51 Conversely, severe OSA in women was associated with retinal microaneurysms, but not in men.51 Three months of CPAP significantly improved endothelial function, blood pressure, and glycemic control in male but not female patients with OSA.52 We have previously shown sex differences in HRV responses to AngII challenge in healthy men and women22 and we now extend these findings to men and women with OSA. It is unclear why CPAP was associated with an exaggerated delayed vagal reactivation in women compared to men. The sensitivity of angiotensin type 1 receptors to AngII is much stronger in men than in premenopausal women,21 which may explain the observed sex differences in the HRV and arterial stiffness responses to AngII in patients with OSA. Because six of eight women in our study were postmenopausal, it is possible that the results of our study are not representative of premenopausal women or women using postmenopausal hormone therapy and therefore further study is required.
The prevalence, clinical presentation, and severity of OSA are known to differ between men and women. OSA is more common in men with an estimated male to female ratio ranging from 3:1 to 5:1 in the general population.1 This is partly because of underrecognition of OSA in women whose clinical presentation is often atypical and different from that in men. OSA is often less severe in women because of differences in the pathogenesis including upper airway anatomy and collapsibility.25
Our study has strengths and limitations. First, our study sample was large compared to the published literature12,14–17,19 but was limited to subjects with OSA without comorbidities who were CPAP adherent, thereby potentially limiting the generalizability of our results. However, by studying a healthier population of subjects with OSA, we were able to examine the effect of CPAP on HRV, arterial stiffness, and the RAS without confounders. Further, by design, we included only subjects with both moderate-severe OSA and significant nocturnal hypoxemia. Thus, it remains unclear whether it was the correction of apnea or intermittent hypoxemia that was responsible for our findings. Although no control group was included, conditions during the assessment of RAS and vascular function were standardized to minimize any potential effect of confounders. Specifically, subjects were studied supine in the morning to account for circadian variations in HRV38 and the RAS,33 in a high-salt state to ensure maximal RAS suppression,32 during the same stage of the menstrual cycle to eliminate estrogen-mediated RAS differences,35 and all subjects had a blood pressure < 140/90 with no other coexisting diseases and were free of RAS-interfering medications and exogenous sex hormones. Further, through our pre-post study design, whereby each subject acted as their own control, we attempted to minimize the risk introduced by interindividual variability in factors such as differences in age and other unmeasured confounders on our study outcomes. As such, it is unlikely that the observed changes in HRV and vascular function were due to factors other than CPAP. Third, the gold standard of HRV measurement is with a 24-hour Holter ECG monitor. However, many studies routinely use short-term ECG recordings, as the accuracy of these more practical HRV analyses has been substantiated against long-term measurements.53 This study included a 180-minute period of Holter ECG monitoring in a calm, temperature-controlled room, diminishing the effect of heavy respiratory, parasympathetic activity, which has been suggested to alter short-term HRV analysis.54 We used carotid-femoral PWV, which is considered the “gold standard” measurement of arterial stiffness.43 Fourth, the duration of CPAP usage prior to reassessment varied because of differences in the ability of individual patients to acclimatize to it, which is well recognized in patients with OSA.7 However, by ensuring that all subjects were on effective CPAP therapy for the same amount of time (4 weeks) before their RAS and vascular function was reassessed, we were able to standardize this intervention and determine the effect of CPAP on HRV and arterial stiffness. Consequently, by design our subjects had the same standardized amount of adequate and adherent CPAP therapy (defined as CPAP use > 4 h/night on > 70% nights for 4 weeks) prior to reassessment of the study outcomes of interest and therefore we cannot comment on the effect that duration of CPAP therapy has on our study outcomes. Fifth, we used portable monitoring instead of polysomnography both to diagnose OSA and to evaluate patients' response to CPAP. However, the use of portable monitoring was appropriate for the population we studied according to current guidelines.6 Further, the findings were objective and unequivocal. The duration of CPAP therapy may have been insufficient to demonstrate its full benefits on RAS activity. However, the treatment period we chose has been shown to improve other cardiovascular outcomes in previous studies.55 Last, we reported sex-stratified analyses and included subjects with controlled hypertension, thereby increasing the generaliz-ability and clinical implications of our results.
In a community-based OSA population, adherence to CPAP was associated with delayed cardiovagal reactivation after a stressor and decreased arterial RAS activity, suggesting a physiological mechanism by which CPAP therapy may prevent CVD. Sex differences in these findings may have important implications in mitigating cardiovascular risk in patients with OSA.
DISCLOSURE STATEMENT
An abstract reporting on these data was presented at the American Society of Nephrology Kidney Week, Chicago, Illinois, USA, November 15–20, 2016. All authors have seen and approved the manuscript. This study was funded by an Establishment Grant from Alberta Innovates–Health Solutions. SBA is supported by Alberta Innovates–Health Solutions and a joint initiative between Alberta Health and Wellness and the Universities of Alberta and Calgary. MJP holds the Brenda Strafford Foundation Chair for Alzheimer Research. Funding sources had no role in the design, conduct, or reporting of this study. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the FMC Sleep Centre for patient recruitment and diagnostic testing. Author contributions: concept and design of study: DDMN, PJH, SBA; acquisition, analysis and interpretation of the data: DDMN, PJH, AAZ, MCM, JMM, MJP, GBH, DYS, SBA; drafting of the manuscript: DDMN, SBA; review of manuscript for important intellectual content: DDMN, PJH, AAZ, MCM, JMM, MJP, GBH, DYS, SBA; final approval of manuscript: DDMN, PJH, AAZ, MCM, JMM, MJP, GBH, DYS, SBA; study supervision: SBA, PJH.
ABBREVIATIONS
- AIx
aortic augmentation index
- AngII
angiotensin II
- CPAP
continuous positive airway pressure
- CVD
cardiovascular disease
- ECG
electrocardiography
- FF
filtration fraction
- FM
fat mass
- HF
high frequency
- HOMA-IR
Homeostatic Model Assessment Insulin Resistance
- HRV
heart rate variability
- LM
lean mass
- LF
low frequency
- ln
natural logarithm
- nu
normalized units
- OSA
obstructive sleep apnea
- pNN50
percentage of normal waves that differ 50 ms compared with the wave directly prior
- PRA
plasma renin activity
- pro-BNP
pro-B-type natriuretic peptide
- PWVcf
carotid-femoral pulse-wave velocity
- rMSSD
root mean square of the successive differences
- RAS
renin-angiotensin system
- RDI
respiratory disturbance index
- SaO2
oxyhemoglobin saturation
- SD
standard deviation
- SDANN
SD of the average normal R-R wave
- SDNN
SD of the normal R-R wave
- TP
total power
- VLF
very low frequency
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25(12):1070–1076. doi: 10.1016/j.ehj.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Marti S, Sampol G, Munoz X, et al. Mortality in severe sleep apnoea/ hypopnoea syndrome patients: impact of treatment. Eur Respir J. 2002;20(6):1511–1518. doi: 10.1183/09031936.02.00306502. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Rodriguez F, Martinez-Garcia MA, de la Cruz-Moron I, Almeida-Gonzalez C, Catalan-Serra P, Montserrat JM. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med. 2012;156(2):115–122. doi: 10.7326/0003-4819-156-2-201201170-00006. [DOI] [PubMed] [Google Scholar]
- 6.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 8.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Zhou Z, McEvoy RD, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318(2):156–166. doi: 10.1001/jama.2017.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abuzaid AS, Al Ashry HS, Elbadawi A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea. Am J Cardiol. 2017;120(4):693–699. doi: 10.1016/j.amjcard.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Panaite V, Salomon K, Jin A, Rottenberg J. Cardiovascular recovery from psychological and physiological challenge and risk for adverse cardiovascular outcomes and all-cause mortality. Psychosom Med. 2015;77(3):215–226. doi: 10.1097/PSY.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roche F, Court-Fortune I, Pichot V, et al. Reduced cardiac sympathetic autonomic tone after long-term nasal continuous positive airway pressure in obstructive sleep apnoea syndrome. Clin Physiol. 1999;19(2):127–134. doi: 10.1046/j.1365-2281.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 13.Shiina K, Tomiyama H, Takata Y, et al. Effects of CPAP therapy on the sympathovagal balance and arterial stiffness in obstructive sleep apnea. Respir Med. 2010;104(6):911–916. doi: 10.1016/j.rmed.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Chrysostomakis SI, Simantirakis EN, Schiza SE, et al. Continuous positive airway pressure therapy lowers vagal tone in patients with obstructive sleep apnoea-hypopnoea syndrome. Hellenic J Cardiol. 2006;47(1):13–20. [PubMed] [Google Scholar]
- 15.Khoo MC, Kim TS, Berry RB. Spectral indices of cardiac autonomic function in obstructive sleep apnea. Sleep. 1999;22(4):443–451. doi: 10.1093/sleep/22.4.443. [DOI] [PubMed] [Google Scholar]
- 16.Khoo MC, Belozeroff V, Berry RB, Sassoon CS. Cardiac autonomic control in obstructive sleep apnea: effects of long-term CPAP therapy. Am J Respir Crit Care Med. 2001;164(5):807–812. doi: 10.1164/ajrccm.164.5.2010124. [DOI] [PubMed] [Google Scholar]
- 17.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(7):706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 18.Buchner NJ, Quack I, Stegbauer J, Woznowski M, Kaufmann A, Rump LC. Treatment of obstructive sleep apnea reduces arterial stiffness. Sleep Breath. 2012;16(1):123–133. doi: 10.1007/s11325-010-0465-x. [DOI] [PubMed] [Google Scholar]
- 19.Phillips CL, Yee B, Yang Q, et al. Effects of continuous positive airway pressure treatment and withdrawal in patients with obstructive sleep apnea on arterial stiffness and central BP. Chest. 2008;134(1):94–100. doi: 10.1378/chest.07-3121. [DOI] [PubMed] [Google Scholar]
- 20.Brewster UC, Setaro JF, Perazella MA. The renin-angiotensin-aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci. 2003;326(1):15–24. doi: 10.1097/00000441-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Miller JA, Cherney DZ, Duncan JA, et al. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. 2006;17(9):2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 22.Mann MC, Exner DV, Hemmelgarn BR, Turin TC, Sola DY, Ahmed SB. Impact of gender on the cardiac autonomic response to angiotensin II in healthy humans. J Appl Physiol. 2012;112(6):1001–1007. doi: 10.1152/japplphysiol.01207.2011. [DOI] [PubMed] [Google Scholar]
- 23.Woznicka-Leskiewicz L, Posadzy-Malaczynska A, Juszkat R. The impact of ankle brachial index and pulse wave velocity on cardiovascular risk according to SCORE and Framingham scales and sex differences. J Hum Hypertens. 2015;29(8):502–510. doi: 10.1038/jhh.2014.80. [DOI] [PubMed] [Google Scholar]
- 24.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 25.O'Connor C, Thornley K, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 26.Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms? Arch Intern Med. 1996;156(21):2445–2451. [PubMed] [Google Scholar]
- 27.Shoback DM, Williams GH, Swartz SL, Davies RO, Hollenberg NK. Time course and effect of sodium intake on vascular and hormonal responses to enalapril (MK 421) in normal subjects. J Cardiovasc Pharmacol. 1983;5(6):1010–1018. doi: 10.1097/00005344-198311000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse-wave velocity in healthy men. Hypertension. 2001;37(6):1429–1433. doi: 10.1161/01.hyp.37.6.1429. [DOI] [PubMed] [Google Scholar]
- 29.Nicholl DDM, Hanly PJ, Poulin MJ, et al. Evaluation of continuous positive airway pressure therapy on renin-angiotensin system activity in obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(5):572–580. doi: 10.1164/rccm.201403-0526OC. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55(4):302–307. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3(7):737–747. [PMC free article] [PubMed] [Google Scholar]
- 32.Shoback DM, Williams GH, Moore TJ, Dluhy RG, Podolsky S, Hollenberg NK. Defect in the sodium-modulated tissue responsiveness to angiotensin II in essential hypertension. J Clin Invest. 1983;72(6):2115–2124. doi: 10.1172/JCI111176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams GH, Cain JP, Dluhy RG, Underwood RH. Studies of the control of plasma aldosterone concentration in normal man. I. Response to posture, acute and chronic volume depletion, and sodium loading. J Clin Invest. 1972;51(7):1731–1742. doi: 10.1172/JCI106974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol. 1993;20(1):7–14. doi: 10.1111/j.1440-1681.1993.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 35.Chidambaram M, Duncan JA, Lai VS, et al. Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol. 2002;13(2):446–452. doi: 10.1681/ASN.V132446. [DOI] [PubMed] [Google Scholar]
- 36.Seifarth C, Trenkel S, Schobel H, Hahn EG, Hensen J. Influence of antihypertensive medication on aldosterone and renin concentration in the differential diagnosis of essential hypertension and primary aldosteronism. Clin Endocrinol. 2002;57(4):457–465. doi: 10.1046/j.1365-2265.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- 37.Kleiger RE, Stein PK, Bigger JT. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10(1):88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 39.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84(2):482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 40.Burr RL. Interpretation of normalized spectral heart rate variability indices in sleep research: a critical review. Sleep. 2007;30(7):913–919. doi: 10.1093/sleep/30.7.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 42.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 43.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 44.Roche F, Gaspoz JM, Court-Fortune I, et al. Screening of obstructive sleep apnea syndrome by heart rate variability analysis. Circulation. 1999;100(13):1411–1415. doi: 10.1161/01.cir.100.13.1411. [DOI] [PubMed] [Google Scholar]
- 45.Pressman GS, Orban M, Leinveber P, et al. Effects of the Mueller maneuver on functional mitral regurgitation and implications for obstructive sleep apnea. Am J Cardiol. 2015;115(11):1563–1567. doi: 10.1016/j.amjcard.2015.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol. 1989;67(5):2101–2106. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]
- 47.Kraiczi H, Hedner J, Peker Y, Carlson J. Increased vasoconstrictor sensitivity in obstructive sleep apnea. J Appl Physiol. 2000;89(2):493–498. doi: 10.1152/jappl.2000.89.2.493. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol. 2002;92(2):627–633. doi: 10.1152/japplphysiol.00152.2001. [DOI] [PubMed] [Google Scholar]
- 49.Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension. 2010;56(3):369–377. doi: 10.1161/HYPERTENSIONAHA.110.152108. [DOI] [PubMed] [Google Scholar]
- 50.Danser AH. Local renin-angiotensin systems. Mol Cell Biochem. 1996;157(1-2):211–216. doi: 10.1007/BF00227900. [DOI] [PubMed] [Google Scholar]
- 51.Lin GM, Redline S, Klein R, et al. Sex-specific association of obstructive sleep apnea with retinal microvascular signs: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2016;5(7):e003598. doi: 10.1161/JAHA.116.003598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kallianos A, Panoutsopoulos A, Mermigkis C, et al. Sex differences of continuous positive airway pressure treatment on flow-mediated dilation in patients with obstructive sleep apnea syndrome. Clin Interv Aging. 2015;10:1361–1366. doi: 10.2147/CIA.S84199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumert M, Lambert GW, Dawood T, et al. Short-term heart rate variability and cardiac norepinephrine spillover in patients with depression and panic disorder. Am J Physiol Heart Circ Physiol. 2009;297(2):H674–H679. doi: 10.1152/ajpheart.00236.2009. [DOI] [PubMed] [Google Scholar]
- 54.Hayano J, Mukai S, Sakakibara M, Okada A, Takata K, Fujinami T. Effects of respiratory interval on vagal modulation of heart rate. Am J Physiol. 1994;267(1 Pt 2):H33–H40. doi: 10.1152/ajpheart.1994.267.1.H33. [DOI] [PubMed] [Google Scholar]
- 55.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163(2):344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]