Abstract
Study Objectives:
Recent results from the PTSD Initiative, a cross-sectional cohort study in Australian Vietnam veterans (VV) with and without posttraumatic stress disorder (PTSD), demonstrated an increased prevalence of self-reported sleep disturbances in those with PTSD. This study aimed to objectively assess the prevalence of sleep disorders in the same cohort using detailed polysomnography (PSG).
Methods:
Participants from the PTSD Initiative were recruited to undergo PSG. PTSD status was determined with the Clinician Administered PTSD Scale for DSM-5 (CAPS-5). Subjective sleep information was attained via structured questionnaires. Data from single night PSG were compared between trauma-exposed VV with and without PTSD.
Results:
A total of 74 trauma-exposed male VV (40 with PTSD) underwent PSG (prospective n = 59, retrospective n = 15). All PSG parameters were similar between groups. No difference was seen in PSG-diagnosed obstructive sleep apnea (OSA) or periodic limb movements of sleep (PLMS). VV with PTSD showed a trend toward increased duration of sleep with oxygen saturations < 90% (10% versus 1.8%; P = .07). VV with PTSD reported increased sleep onset latency (42.4 versus 13.3 minutes; P < .01); were less likely to report sleeping well (32.5% versus 67.5%; P < .01); had higher OSA risk using Berlin Questionnaire (BQ) (70% versus 38.2%; P < .01); and had higher rates of partner-reported limb movements (56.4% versus 17.6%; P < .01). No association between PSG-diagnosed OSA and PTSD severity was evident.
Conclusions:
In Australian VV with and without PTSD, no difference was seen across all PSG parameters including the diagnosis and severity of OSA and PLMS. However, VV with PTSD demonstrated an increased perception of sleep disturbances.
Citation:
Baird T, Theal R, Gleeson S, McLeay S, O'Sullivan R; PTSD Initiative. Detailed polysomnography in Australian Vietnam veterans with and without posttraumatic stress disorder. J Clin Sleep Med. 2018;14(9):1577–1586.
Keywords: Berlin Questionnaire, BQ, obstructive sleep apnea, OSA, periodic limb movements of sleep, PLMS, polysomnography, posttraumatic stress disorder, PSG, PTSD, sleep architecture, sleep disorders, veterans
BRIEF SUMMARY
Current Knowledge/Study Rationale: Although sleep disturbances are characteristic of posttraumatic stress disorder (PTSD), limited studies have objectively assessed sleep and obstructive sleep apnea risk in veterans with PTSD compared to trauma-exposed control patients. This study examined the prevalence of sleep disorders in a cohort of matched trauma-exposed Australian Vietnam veterans (VV) with and without PTSD using detailed polysomnography.
Study Impact: Compared to trauma-exposed control patients, Australian VV with PTSD demonstrated no difference in the prevalence of objective sleep disorders including obstructive sleep apnea and periodic limb movements. This study suggests that despite the high incidence of self-reported sleep disturbances, VV with PTSD may have a similar prevalence of polysomnography-diagnosed sleep disorders to trauma-exposed controls.
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a mental health condition that may develop after one or more traumatic events, particularly combat-related trauma.1,2 Veterans have a particularly high prevalence of PTSD, with up to 20.9% of Australian Vietnam veterans (VV) having lifetime PTSD.3 Importantly, sleep disturbance including insomnia and nightmares are hallmark features of PTSD, with more than 87% of individuals with PTSD reporting some type of sleep disturbance.4
Although previous studies have reported a high prevalence of sleep disturbances in PTSD,4,5 data from objective measurement of sleep architecture and sleep disorders are variable and few studies investigating sleep in veterans have used trauma-exposed controls. Furthermore, limited studies have utilized polysomnography (PSG) to specifically examine sleep architecture, respiration, periodic limb movements of sleep (PLMS), and obstructive sleep apnea (OSA) in veterans with PTSD and none in the Australian veteran population.
Of note, the prevalence of OSA in veterans exposed to trauma remains uncertain, with inconsistent results across studies and discrepancies between subjective and objective findings.4–6 Moreover, a recent PSG-based study in Dutch veterans importantly suggested that OSA diagnosis and severity, based on respiratory disturbance index (RDI), may be associated with worse PTSD symptoms.6 Finally, no prior studies have examined the utility of the Berlin Questionnaire (BQ) to predict OSA risk in VV or in trauma-exposed individuals.
Recently, our Gallipoli Medical Research Institute (GMRI) PTSD Initiative, a cross-sectional cohort study in 214 trauma-exposed Australian veterans VV with and without PTSD, reported an increased prevalence of a wide variety of sleep disturbances using self-reported subjective structured questionnaires, including OSA and PLMS.7 Given that disturbed sleep is a hallmark symptom of PTSD, we wanted to further explore whether these findings described true differences in our population, or whether the general experience and/or expectation of “poor sleep” in those with PTSD resulted in more negative subjective reporting (including OSA risk evaluated by BQ answers), and increased clinical diagnosis of OSA due to having sought clinical evaluation and treatment.
Therefore, the aim of this current study primarily was to objectively compare the prevalence of sleep disorders in the same trauma-exposed Australian VV cohort, using detailed PSG, with a focus on sleep architecture, OSA, and PLMS. Additionally, the study aimed to assess the relationship between OSA and PTSD severity and to directly examine the utility of the BQ in this cohort.
METHODS
This study was performed on a subset of participants from the GMRI PTSD Initiative, a larger cross-sectional cohort study that investigated a range of physical and psychosocial comorbidities, including self-reported sleep disturbances, in 214 trauma-exposed Australian VV.7 In this current study, all 214 PTSD Initiative participants, apart from 4 who had died and 11 who were deemed as living too far away (> 2-hour drive), were invited to undergo assessment with detailed PSG or allow access to previous PSG if already performed. Participants in whom OSA had previously been diagnosed were ineligible for prospective PSG; only their retrospective data were used.
PTSD diagnosis and severity was determined by specialist psychiatric evaluation and psychologist assessment with the Clinician Administered PTSD Scale for DSM-5 (CAPS-5). Trauma exposure was evaluated using Criterion A on the CAPS-5 with those not deemed as trauma exposed excluded.
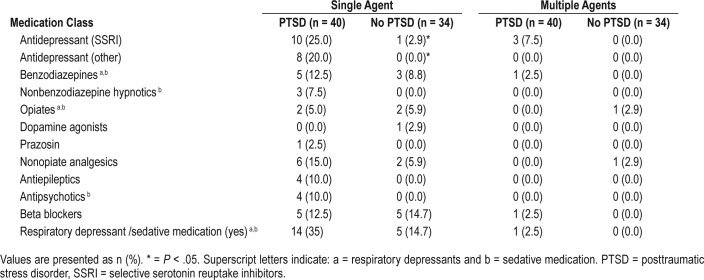
Baseline characteristics including age, body mass index (BMI), and medications were recorded. Participants reporting use of one or more respiratory depressant and/or sedative medication were additionally categorized as “yes” (Table 1). Subjective daytime sleepiness was evaluated using the Ep-worth Sleepiness Scale (ESS); excessive alcohol consumption and alcohol use disorders were screened for using the Alcohol Use Disorders Identification Test (AUDIT); and co-morbid major depressive disorder (MDD) determined using the Mini-International Neuropsychiatric Interview (MINI).8,9 Risk of OSA was assessed using BQ.10 Self-reported restless legs and partner-reported limb movements were assessed using supervised structured questionnaires including the validated Mayo Questionnaire.11
Table 1.
Medication use in Australian Vietnam veterans with and without PTSD.
Participants prospectively underwent 1 night of PSG or had previous PSG data attained. Prospective PSG tests were performed from February 2014 to April 2017. Home PSG studies were conducted where appropriate as per patient preference. PSG data collated included sleep architecture, body position, respiratory and arousal indices, PLMS; oximetry statistics, and the cardiovascular parameters mean heart rate and evening and waking blood pressures. OSA was diagnosed in participants with a RDI > 5 events/h. OSA severity was subcategorized as mild (5–15 events/h), moderate (15–30 events/h), severe (30–50 events/h), or very severe (> 50 events/h) based on RDI.
Statistical analysis was conducted using SPSS (version 24.0, IBM Corp, Armonk, New York, United States). Variables were compared between VV groups with and without PTSD by un-paired Student t tests and Pearson chi-square or Fisher exact tests. Data were assessed for normality with non-normal data assessed by Mann-Whitney U tests.
To examine the relationship between PTSD symptom severity and RDI, both Pearson correlation and linear regression were performed. Logistic regressions were also performed to examine the relationship between PTSD symptom severity and OSA. Separate models were run for risk of OSA determined by BQ and for PSG-diagnosed OSA. Potential risk factors of OSA including age, use of respiratory depressant and/or sedative medications (yes/no), smoking status (yes/no within the past 12 months), MDD (yes/no), and BMI were controlled for within a single step for each analysis. High BMI, a criterion for high risk of OSA on the BQ, was excluded from the OSA risk model.
The accuracy of the BQ to predict OSA was assessed by Fisher exact test. Sensitivity, specificity, positive predictive values, negative predictive values, and diagnostic odds ratios (OR) of the BQ were calculated for total participants as well as veterans with and without PTSD. Values of P < .05 were considered statistically significant.
Ethics approval was obtained from Greenslopes Research and Ethics Committee (reference 16/09) and the Department of Veterans Affairs (reference E016/010).
RESULTS
Descriptive Analysis
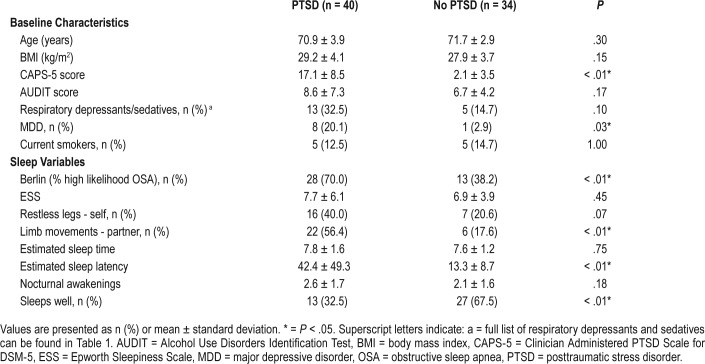
Of the 214 original PTSD Initiative subjects, 74 were included for PSG analysis (4 died; 44 had unavailable previous PSG; and 92 declined or lived too far away) (Figure 1). A total of 59 underwent prospective PSG and 15 had PSG data retrospectively obtained. Most studies (n = 70) were type 1 (in-laboratory) and 4 were type 2 (home studies). Baseline participant characteristics are described in Table 2. Age, BMI, smoking status, and total AUDIT scores were similar between groups. VV with PTSD had significantly higher CAPS-5 scores (17.1 versus 2.09; P < .01) and increased comorbid MDD (20.1% versus 2.9%; P = .03).
Figure 1. Study design.

PTSD = posttraumatic stress disorder.
Table 2.
Baseline characteristics and self-reported sleep variables of Australian Vietnam veterans with and without PTSD.
Use of respiratory depressants and/or sedatives was higher in VV with PTSD, although this did not reach statistical significance (35% versus 14.7%; P = .07). A full description of medication classes is listed in Table 1. Significantly more VV with PTSD reported use of selective serotonin reuptake inhibitor (SSRI) antidepressants (25.0% versus 2.9%; P < .01) and other antidepressants (20.0% versus zero; P < .01). All other medication classes were similar between groups.
Using supervised structured questionnaires, VV with PTSD reported longer sleep latencies (42.4 versus 13.3 minutes; P < .01) and were subjectively less likely to sleep well (32.5% versus 67.5%; P < .01). Estimated total sleep time, nocturnal awakenings, and subjective daytime sleepiness (measured by ESS) were similar between groups. Partner reported limb movements during sleep were significantly higher in VV with PTSD (56.4% versus 17.6%; P < .01) with a trend toward higher self-reported restless legs (40% versus 20.6%; P = .08). The OSA risk determined by the BQ was greater in VV with PTSD (70% versus 38.2%; P < .01) (Table 2).
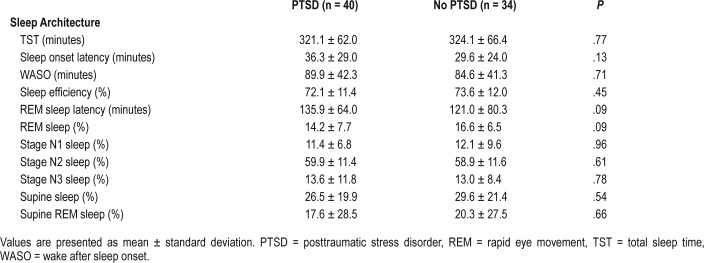
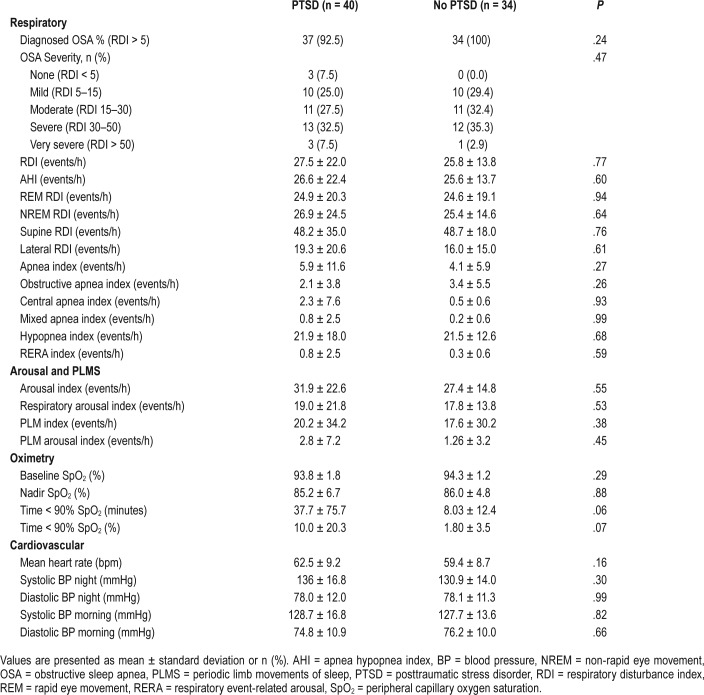
In contrast with self-reported data, all variables measured from single overnight PSG were similar across VV with and without PTSD (Table 3 and Table 4). No difference was seen across sleep architecture, respiratory indices, electroencephalography arousal indices, mean heart rate, and blood pressure. Of the 74 VV without PTSD, 22 (30.2%) had a periodic limb movement index (PLMI) > 15 events/h, and 13 of the 40 VV with PTSD (32.5%) had a PLMI > 15 events/h. No difference in the mean PLMI or severity of PLMS was seen between cohorts (Table 4). OSA was diagnosed in almost all participants on PSG with no difference in prevalence between groups (VV with PTSD n = 37, 92.5%; VV without PTSD n = 34, 100%, P = .25). RDI was not significantly different between groups. There was no difference in the oxygen saturation (SpO2) nadir between groups; however, those with PTSD demonstrated a trend to having an increased duration of sleep time with SpO2 < 90% (10% versus 1.8%; P = .07).
Table 3.
Polysomnographic sleep architecture in Australian Vietnam veterans with and without PTSD.
Table 4.
Polysomnography-measured respiratory, arousal, periodic limb movements, oximetry, and cardiovascular indices in Australian Vietnam veterans with and without PTSD.
Relationship Between RDI and PTSD Severity and BQ OSA Risk and PTSD Severity
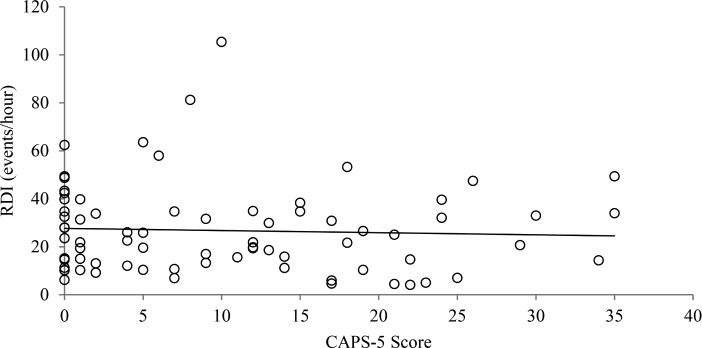
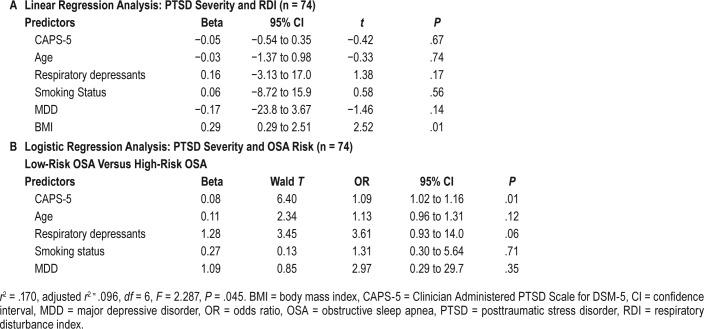
There was no significant correlation between RDI and CAPS scores (r = −.048, P = .684, Figure 2). Multiple regression analysis was performed to assess the relationship between RDI and PTSD severity controlling for potential risk factors (Table 5A). The model explained 10.7% of the variance (adjusted r2 = .107, F6,67 = 2.46, P = .03). However, only BMI significantly contributed to the prediction of RDI (β = .297, P < .01). PTSD severity did not predict a rise in RDI (β = −.052, P = .67).
Figure 2. Correlation between RDI and CAPS-5 in Australian Vietnam veterans with and without PTSD.
n = 74, r = −.05, P = .68. CAPS-5 = Clinician Administered PTSD Scale for DSM-5, PTSD = posttraumatic stress disorder, RDI = respiratory disturbance index.
Table 5.
Regression analysis.
Logistic regression analyses were performed to assess the relationship between OSA risk or diagnosis and PTSD severity while controlling for potential risk factors. Higher CAPS-5 scores were significantly associated with screening positively for high risk of OSA by the BQ (Table 5B). Every 10-point increase in CAPS-5 scores was associated with a 90% increase in the probability for high risk of OSA. Age, medications, smoking status, and MDD were not significantly associated with OSA risk.
Neither PTSD severity nor other OSA risk factors were associated with PSG-diagnosed OSA (χ26, [n = 74] = 7.0, P = .67).
We also explored whether study type (prospective in-laboratory, prospective at-home study, or retrospective) was a potential confounder; however, addition of study type to the models did not significantly affect outcomes (data not shown).
Utility of BQ
The sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic OR were calculated to assess the predictive ability of the BQ in predicting PSG diagnosis of OSA (Table 6). Of those with a high-risk BQ score, 39 of 41 participants (95.1%) had PSG-diagnosed OSA, whereas only 1 of 33 patients (3.03%) with a low-risk BQ score did not have PSG-diagnosed OSA. The association between OSA risk by BQ and OSA diagnosis by PSG was not significant (P = 1.00).
Table 6.
Berlin Questionnaire predictability for polysomnography-diagnosed OSA in Australian Vietnam veterans with and without PTSD.
Prospective Studies
Although the main aim was to determine rates of PSG-diagnosed OSA in the whole cohort of participants from the PTSD Initiative study, as a secondary investigation, prospective studies (n = 59; Table 1) were analyzed separately. There were no significant changes in PSG parameters in the prospective-only analysis with the exception of an increased apnea index in VV without PTSD (data not shown).
DISCUSSION
This cross-sectional cohort study is the first to objectively investigate the prevalence of sleep disorders in the Australian VV population using PSG. It demonstrates that in trauma-exposed Australian VV with and without PTSD, PSG revealed no difference in OSA, PLMS, and sleep architecture. Although PTSD severity was associated with risk of OSA from BQ, CAPS-5 scores were not associated with OSA diagnosis or OSA severity. Additionally, this study questions the utility of the BQ for screening OSA risk in Australian VV.
Baseline Characteristics
Age, BMI, alcohol use, and smoking history were similar between cohorts. However, VV with PTSD were more likely to have comorbid MDD with more antidepressant use, as well as demonstrating a trend toward increased respiratory depressant and/or sedative medications use. MDD is a well-described comorbidity in individuals with PTSD and has also been suggested to be associated with an increased risk of OSA.1,12 Furthermore, it has been shown that antidepressants, particularly SSRIs, are associated with an increased risk of PLMS.13 The trend toward increased use of respiratory depressants/sedative medications is possibly the result of the high rates of insomnia reported in individuals with PTSD and is an important finding, as it is known that benzodiazepines and opioids are associated with an increased risk of nocturnal hypoxemia as well as both OSA and central sleep apnea.3,14–16
Subjective Sleep Variables
VV with PTSD had poorer sleep, increased self-reported sleep onset latency, and higher rates of partner-reported limb movements during sleep. Additionally, VV with PTSD had a significantly higher risk of OSA on BQ (Table 2). Although these findings are consistent with a number of previous studies, they are in contrast with our objective PSG data discussed in the following paragraphs.3,17–19 Of note, estimated total sleep time and ESS were similar between groups, with ESS being surprisingly low across the entire cohort. Although it is difficult to speculate on the exact cause of this, it may reflect our main finding that individuals with PTSD have a greater prevalence of perceived rather than objective sleep disorders. It would be interesting to examine this more directly with actigraphy as well as the validated Insomnia Severity Index questionnaire, both limitations of this particular study.
PSG Variables
In our study, no difference in sleep architecture was seen between groups including total sleep time, sleep onset latency, wake after sleep onset, rapid eye movement (REM) sleep latency, and sleep stages (N1, N2, N3, and R) (Table 3). This is in contrast to the self-reported measures in our cohort as well as previous studies that suggest PTSD veterans have higher rates of insomnia, greater sleep onset latencies, and more nocturnal awakenings.3,14,20 Moreover, a meta-analysis of PSG studies in PTSD by Kobayashi et al. reported subtle changes in sleep architecture including more stage N1 sleep, less stage N3 sleep, greater REM sleep density, and a reduction in REM sleep in subjects with PTSD.21 Although our study did not specifically assess REM density, it is the largest controlled study comparing PSG data in veterans with and without PTSD, and challenges previous notions of altered sleep architecture in veterans with PTSD.
One of the primary outcomes of our study was OSA diagnosis. Previous uncontrolled studies have reported an increased prevalence of OSA in individuals with PTSD with 69% to 91% of subjects with PTSD demonstrating an apnea-hypopnea index (AHI) > 10 events/h.5,22 In contrast, although our study found a high prevalence of OSA in all VV, no difference was seen in OSA prevalence or severity between trauma-matched veterans with and without PTSD (Table 4). Similar findings have been reported in three prior controlled PSG studies assessing United States active-duty service members, Dutch veterans, and traffic accident victims.6,23 This supports the notion that PTSD may not be associated with an increased prevalence of OSA when objectively assessed using PSG.5,19
To date, there have been limited studies assessing the prevalence of PLMS in individuals with PTSD. In one uncontrolled PSG study in 25 VV with severe PTSD, Brown et al. reported clinically significant PLMS (defined as > 15 events/h) in 76% of subjecs.24 In another study, PLMS were reportedly experienced in 33% of veterans with PTSD and not at all in controls.25 Our study demonstrated 30.2% of participants (32.5% in VV with PTSD) to have significant PLMS. However, no difference in the mean PLM index or PLM severity was seen between VV with and without PTSD (Table 4). Furthermore, those with PTSD reported significantly higher rates of partner reported limb movements and a trend toward increased self-reported restless legs (Table 2). Although it is difficult to speculate on the reasons behind these subjective and objective discrepancies, the results challenge the previously reported higher prevalence of PLMS in PTSD individuals. Interestingly, these findings are in spite of the increased use of antidepressant medications by participants with PTSD, a known risk factor for increased PLMS as previously discussed.13
Of interest in our study, despite no difference being found in PSG-diagnosed OSA, VV with PTSD demonstrated a trend toward an increased duration of sleep with oxygen saturations (SpO2) < 90% (Table 4). Although total AUDIT scores and use of respiratory depressants and/or sedatives were not found to be associated with duration of sleep with SpO2 < 90%, it is important to note that accurate data on specific alcohol and medication use on the night of PSG was not captured. This highlights a possible limitation of the study and although it is plausible that the use of benzodiazepines and/or opioids on the night of PSG may have contributed to the duration of sleep with SpO2 < 90%, this cannot be concluded. Nonetheless, it is an important reminder of appropriate rationalization of medications in individuals with PTSD to ensure nocturnal hypoxemia and sleep disorders are minimized.
Finally, it has been described that individuals with PTSD have an increased resting heart rate and elevated systolic and diastolic blood pressures with a resultant increased risk of coronary artery disease and stroke.26,27 This is thought to be related to the permanent state of hyperarousal or “sympathetic overdrive” associated with PTSD.28 Our study did not demonstrate any difference between groups across these parameters, with no difference being seen in the use of beta blockers between groups. However, despite these findings, the results should be read with caution, as other potentially confounding non beta blocker cardiovascular medications were not directly analyzed.
PTSD Severity and OSA
It has been previously suggested that comorbid sleep disorders, including OSA, are associated with an increased risk of PTSD and worse PTSD symptoms.17,29,30 A recent controlled PSG study by van Liempt et al., assessing younger Dutch veterans with and without PTSD, concluded that OSA severity (based on AHI) directly correlated with PTSD severity (ie, the higher the AHI, the higher the CAPS-5 score).6 In contrast, our study found no relationship between CAPS-5 scores and PSG-diagnosed OSA or severity.
Conversely, CAPS-5 scores significantly predicted high risk of OSA determined by the BQ, indicating PTSD severity may be more related to BQ scoring categories including snoring, perception of fatigue, BMI, and high blood pressure, rather than actual OSA diagnosis. Interestingly, this has also been found in younger United States Iraq and Afghanistan veterans where PTSD severity was associated with increased risk of screening positively for snoring and fatigue on the BQ.18
Utility of BQ
Despite VV with PTSD screening as high risk for OSA at higher rates than those without PTSD, no difference was seen in the diagnosis or severity of OSA with PSG (Table 2 and Table 4). Moreover, the sensitivity and specificity for BQ predicting OSA in VV with PTSD were 70.3% and 33%, respectively (Table 6), and 38.2% and zero, respectively for the trauma-exposed group. Although our assessment of the BQ is limited by the high rates of PSG- diagnosed OSA in our cohort, the findings are in contrast to previous studies, and suggest that BQ may not be an efficacious screening tool in trauma-exposed veterans.18 Further research is needed to assess the validity of BQ and other screening questionnaires for OSA in the veteran and PTSD populations.
Limitations
Our study has several limitations, some already discussed. Although we objectively analyzed participants' sleep with overnight PSG, individuals only underwent a single night study. It is possible that the results may be limited by “first-night effects” and may not have been a true representation of their sleep patterns. Additionally, the 15 retrospective PSG tests were performed over extended periods with 9 prior to 2012 and 7 prior to 2007. As there have been some minor changes in the American Academy of Sleep Medicine scoring criteria over the past decade, it is possible that some of the older PSG tests may have underestimated respiratory events in comparison with more recent PSG tests.31,32 Furthermore, 12 of the 15 participants with retrospective PSG tests were already undergoing active treatment for their OSA at the time of this study (11 with continuous positive airway pressure and 1 with a mandibular advancement splint; all with PTSD). Although this may have confounded the subjective data gathered in these individuals, it should have theoretically improved their symptoms.
It must also be noted that the mean age across our entire study was 69 years, and all participants were male. Although this was dictated by the target cohort being Australian VV, this must be considered in comparison with younger veteran and other PTSD cohorts, as the risk for OSA is likely greater in our subjects. This is supported by the extremely high rates of diagnosed OSA seen across our entire cohort.
Finally, this study did not directly assess parasomnias, including nightmares and REM sleep behavioral disorder, both well-described phenomena in veterans with PTSD. A follow-up study aims to specifically address these parasomnias, in addition to further investigating the newly proposed trauma-associated sleep disorder, a disorder entailing these aforementioned phenomena in trauma-exposed individuals.33,34
CONCLUSIONS
In Australian VV with and without PTSD, no difference was seen across all PSG parameters, including sleep architecture and the presence and severity of OSA and PLMS, with high rates of PSG-diagnosed OSA in both groups. However, VV with PTSD demonstrated an increased perception of sleep disorders. Exploration into these objective and subjective discrepancies is warranted so that more appropriate screening methods for OSA and other sleep disorders in trauma-exposed veterans can be developed.
DISCLOSURE STATEMENT
Work for this study was performed at Sleep Care & Gallipoli Medical Research Foundation, Greenslopes Private Hospital, Brisbane, QLD Australia. All authors have seen and approved the manuscript. Funding was provided by Returned & Services League of Australia (Queensland Branch). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
PTSD Initiative Members:
Sarah McLeay, BSc(Hons), PhD1
Wendy Harvey, BSc(Hons), MBBS, MPH1
Madeline Romaniuk, BA, GradDipPsych, BBehSc(Hons), DPsych(Clinical)1,2
Darrell Crawford, MBBS, FRACP, MD1,3,4
David Colquhoun, MBBS, FRACP1,3,4
Ross McD Young, PhD1,5
Miriam Dwyer, BSc, HDipEd1
John Gibson, MBBS, FRANZCP1,4
Robyn O'Sullivan, MBBS, FRACP1,3,4
Graham Cooksley, MBBS, MD, FRACP1,3
Chistopher Strakosch, MD, FRACP1,3,4
Rachel Thomson, MBBS, GradDipClinEpi, PhD, FRACP1,3,4
Joanne Voisey, BSc(Hons), PhD1,2
Bruce Lawford, MBBS, FRANZCP, FAChAM (RACP)1,2,3,4
1Gallipoli Medical Research Foundation, Greenslopes Private Hospital, Newdegate St, Greenslopes
2School of Biomedical Sciences, Faculty of Health and Institute of Health and Biomedical Innovation, Queensland University of Technology, Kelvin Grove, QLD
3School of Medicine, The University of Queensland, Herston, Queensland
4Greenslopes Private Hospital, Newdegate St, Greenslopes, Queensland
5Faculty of Health, Queensland University of Technology, Kelvin Grove, QLD
ABBREVIATIONS
- AUDIT
Alcohol Use Disorders Identification Test
- BMI
body mass index
- CAPS-5
Clinician Administered PTSD Scale for DSM-5
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- MINI
Mini-International Neuropsychiatric Interview
- MDD
major depressive disorder
- OSA
obstructive sleep apnea
- PLMI
periodic limb movement index
- PLMS
periodic limb movements of sleep
- PSG
polysomnography
- PTSD
posttraumatic stress disorder
- BQ
Berlin Questionnaire
- REM
rapid eye movement
- VV
Vietnam veterans
Contributor Information
Collaborators: Sarah McLeay, Wendy Harvey, Madeline Romaniuk, Darrell Crawford, David Colquhoun, Ross McD Young, Miriam Dwyer, John Gibson, Robyn O'Sullivan, Graham Cooksley, Christopher Strakosch, Rachel Thomson, Joanne Voisey, and Bruce Lawford
REFERENCES
- 1.Bisson JI, Cosgrove S, Lewis C, Roberts NP. Post-traumatic stress disorder. BMJ. 2015;351:h6161. doi: 10.1136/bmj.h6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Creamer M, Burgess P, McFarlane AC. Post-traumatic stress disorder: findings from the Australian National Survey of Mental Health and Well-being. Psychol Med. 2001;31(07):1237–1247. doi: 10.1017/s0033291701004287. [DOI] [PubMed] [Google Scholar]
- 3.Khazaie H, Rasoul Ghadami M, Masoudi M. Sleep disturbances in veterans with chronic war-induced PTSD. J Inj Violence Res. 2016;8(2):99–107. doi: 10.5249/jivr.v8i2.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maher MJ, Rego SA, Asnis GM. Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs. 2006;20(7):567–590. doi: 10.2165/00023210-200620070-00003. [DOI] [PubMed] [Google Scholar]
- 5.Krakow BJ, Ulibarri VA, Moore BA, McIver ND. Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Med Rev. 2015;24:37–45. doi: 10.1016/j.smrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 6.van Liempt S, Westenberg HGM, Arends J, Vermetten E. Obstructive sleep apnea in combat-related posttraumatic stress disorder: a controlled polysomnography study. Eur J Psychotraumatol. 2011;2:1–5. doi: 10.3402/ejpt.v2i0.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLeay SC, Harvey WM, Romaniuk MN, et al. Physical comorbidities of post-traumatic stress disorder in Australian Vietnam War veterans. Med J Aust. 2017;206(6):251–257. doi: 10.5694/mja16.00935. [DOI] [PubMed] [Google Scholar]
- 8.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan D, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. [PubMed] [Google Scholar]
- 10.Netzer NC, Sttohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 11.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med. 2013;9(5):475–480. doi: 10.5664/jcsm.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta MA, Simpson FC. Obstructive sleep apnea and psychiatric disorders: a systematic review. J Clin Sleep Med. 2015;11(2):165–175. doi: 10.5664/jcsm.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2017;58(6):510–514. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Neylan TC, Marmar CR, Metzler TJ, et al. Sleep disturbances in the Vietnam generation: findings from a nationally representative sample of male Vietnam veterans. Am J Psychiatry. 1998;155(7):929–933. doi: 10.1176/ajp.155.7.929. [DOI] [PubMed] [Google Scholar]
- 15.Zutler M, Holty J-E. Opioids, sleep, and sleep-disordered breathing. Curr Pharm Des. 2011;17(15):1443–1449. doi: 10.2174/138161211796197070. [DOI] [PubMed] [Google Scholar]
- 16.Webster LR, Choi Y, Desai H, Webster L, Grant BJB. Sleep-disordered breathing and chronic opioid therapy. Pain Med. 2008;9(4):425–432. doi: 10.1111/j.1526-4637.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams SG, Collen J, Orr N, Holley AB, Lettieri CJ. Sleep disorders in combat-related PTSD. Sleep Breath. 2015;19(1):175–182. doi: 10.1007/s11325-014-0984-y. [DOI] [PubMed] [Google Scholar]
- 18.Colvonen PJ, Masino T, Drummond SPA, Myers US, Angkaw AC, Norman SB. Obstructive sleep apnea and posttraumatic stress disorder among OEF/OIF/OND veterans. J Clin Sleep Med. 2015;11(5):513–518. doi: 10.5664/jcsm.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ocasio-Tascón ME, Alicea-Colón E, Torres-Palacios A, Rodríguez-Cintrón W. The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath. 2006;10(2):70–75. doi: 10.1007/s11325-005-0043-9. [DOI] [PubMed] [Google Scholar]
- 20.Mysliwiec V, Gill J, Lee H, et al. Sleep disorders in US military personnel: A high rate of comorbid insomnia and obstructive sleep apnea. Chest. 2013;144(2):549–557. doi: 10.1378/chest.13-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi I, Boarts JM, Delahanty DL. Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology. 2007;44(4):660–669. doi: 10.1111/j.1469-8986.2007.537.x. [DOI] [PubMed] [Google Scholar]
- 22.Yesavage JA, Kinoshita LM, Kimball T, et al. Sleep-disordered breathing in Vietnam veterans with posttraumatic stress disorder. Am J Geriatr Psychiatry. 2012;20(3):199–204. doi: 10.1097/JGP.0b013e3181e446ea. [DOI] [PubMed] [Google Scholar]
- 23.Mysliwiec V, McGraw L, Pierce R, Smith P, Trapp B, Roth BJ. Sleep disorders and associated medical comorbidities in active duty military personnel. Sleep. 2013;36(2):167–174. doi: 10.5665/sleep.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown TM, Boudewyns PA. Periodic limb movements of sleep in combat veterans with posttraumatic stress disorder. J Trauma Stress. 1996;9(1):129–136. doi: 10.1007/BF02116838. [DOI] [PubMed] [Google Scholar]
- 25.Mellman TA, Kulick-Bell R, Ashlock LE, Nolam B. Sleep events among veterans with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(1):110–115. doi: 10.1176/ajp.152.1.110. [DOI] [PubMed] [Google Scholar]
- 26.Coughlin SS. Post-traumatic stress disorder and cardiovascular disease. Open Cardiovasc Med J. 2011;5:164–170. doi: 10.2174/1874192401105010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med. 2001;63(4):585–594. doi: 10.1097/00006842-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc. 2007;99(6):642–649. [PMC free article] [PubMed] [Google Scholar]
- 29.Jaoude P, Vermont LN, Porhomayon J, El-Solh AA. Sleep-disordered breathing in patients with post-traumatic stress disorder. Ann Am Thorac Soc. 2015;12(2):259–268. doi: 10.1513/AnnalsATS.201407-299FR. [DOI] [PubMed] [Google Scholar]
- 30.Arnetz B, Templin T, Saudi W, Jamil H. Obstructive sleep apnoea, posttraumatic stress disorder, and health in immigrants. Psychosom Med. 2012;74(8):824–831. doi: 10.1097/PSY.0b013e31826bf1ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT. The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep. 2009;32(2):150–157. doi: 10.1093/sleep/32.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duce B, Milosavljevic J, Hukins C. The 2012 AASM respiratory event criteria increase the incidence of hypopneas in an adult sleep center population. J Clin Sleep Med. 2015;11(12):1425–1431. doi: 10.5664/jcsm.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mysliwiec V, O'Reilly B, Polchinski J, Kwon HP, Germain A, Roth BJ. Trauma associated sleep disorder: a proposed parasomnia encompassing disruptive nocturnal behaviors, nightmares, and REM without atonia in trauma survivors. J Clin Sleep Med. 2014;10(10):1143–1148. doi: 10.5664/jcsm.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mysliwiec V, Brock MS, Creamer JL, O'Reilly BM, Germain A, Roth BJ. Trauma associated sleep disorder: a parasomnia induced by trauma. Sleep Med Rev. 2018;37:94–104. doi: 10.1016/j.smrv.2017.01.004. [DOI] [PubMed] [Google Scholar]