Abstract
Study Objectives:
Respiratory-related leg movements (RRLMs) may contribute to the cardiovascular risk associated with obstructive sleep apnea (OSA). Selective serotonin reuptake inhibitors (SSRIs), but not bupropion, increase periodic leg movements in sleep. This study examines whether patients with OSA using SSRIs have more RRLMs than those taking bupropion or no antidepressant.
Methods:
Patients with an apnea-hypopnea index (AHI) of at least 10 events/h during a full-night diagnostic study or split-night study, who were taking bupropion (n = 32), an SSRI (n = 31), or no antidepressant (n = 31), were selected from a database of prestudy questionnaires. RRLMs were scored according to World Association of Sleep Medicine 2016 standards.
Results:
Patients using SSRIs had significantly greater overall RRLM% (defined as the percentage of respiratory events associated with a leg movement, including apneas, hypopneas, and respiratory effort-related arousals), RRLM index, and periodic limb movement index relative to patients using bupropion and control patients. The difference between the RRLM% in the SSRI and bupropion groups was limited to patients undergoing split-night studies, and that of the SSRI and control groups was limited to patients undergoing full-night diagnostic studies.
Conclusions:
The greater number of RRLMs and PLMs in the SSRI group may contribute to treatment-emergent insomnia often seen with SSRI use. Fragmented sleep and elevated autonomic nervous system activation associated with increased RRLMs in patients with OSA taking SSRIs might also limit the tolerability of antidepressant treatment, as well as increase the risk for cardiovascular disease.
Citation:
McCall CA, Winkelman JW. Respiratory-related leg movements of sleep are associated with serotonergic antidepressants but not bupropion. J Clin Sleep Med. 2018;14(9):1569–1576.
Keywords: antidepressants, bupropion, periodic leg movements of sleep, respiratory-related leg movements, SSRIs
BRIEF SUMMARY
Current Knowledge/Study Rationale: Selective serotonin reuptake inhibitors (SSRIs), but not bupropion, are associated with increased periodic limb movements. It was not known whether these medications also increase respiratory-related leg movements, which may further increase sleep fragmentation and cardiovascular risk in patients with obstructive sleep apnea (OSA).
Study Impact: This study demonstrates that leg movements associated with respiratory events occur more frequently in patients with OSA taking SSRIs relative to bupropion or no antidepressant. These results may aid in guiding selection of a nonserotonergic antidepressant such as bupropion in this population to minimize treatment-emergent insomnia and elevated sympathetic activity.
INTRODUCTION
Respiratory-related leg movements (RRLMs) are limb movements occurring at the termination of a respiratory event in patients with obstructive sleep apnea (OSA). In the original scoring rules of the American Sleep Disorders Association, RRLMs were scored and counted in relation to obstructive respiratory events.1 Subsequent updates of scoring rules have specifically excluded RRLMs, on the basis that these events may have a different underlying etiology than periodic limb movements of sleep (PLMS) that are not associated with respiratory events.2,3 Currently, the American Academy of Sleep Medicine guidelines state that a leg movement should not be scored if it occurs during a period from 0.5 seconds before a respiratory event to 0.5 seconds after the event.3 However, this practice has been questioned.4 The duration and latency criteria for excluding these leg movements from scoring was largely unsupported by research data and has contributed to the uncertainty regarding the relationship of PLMS to RRLM. More recently, systematic evaluations of leg movement timing demonstrated that they are increased around respiratory events over a period that is significantly longer than specified in previous scoring criteria.5,6 The World Association of Sleep Medicine (WASM) standards have since recommended scoring and counting leg movements as respiratory related when they occur within 2 seconds before to 10.25 seconds after the termination of a respiratory event.7
Some research has suggested that there may be clinical significance to RRLMs. Individuals without OSA but with PLMS may have increased risk for incident cardiovascular events and mortality, with greater risk attributed to PLMS with arousals.8,9 OSA also confers significant known risk for incident hypertension and cardiovascular disease.10,11 RRLMs have been suggested to be a marker for and/or contribute to this risk.12,13 Respiratory events terminating with a leg movement were found to produce larger heart rate increases than those without a leg movement, even after controlling for the length of, and oxygen desaturation associated with, the event.13 An increased RRLM% (the percent of respiratory events associated with a leg movement) has been associated with greater apnea-hypopnea index (AHI), and arousal index (AI), as well as increased prevalence of chronic obstructive pulmonary disease and nocturnal hypoxemia.4,12
Multiple classes of antidepressants, including selective serotonin reuptake inhibitors (SSRIs), are known to increase the number of periodic limb movements (PLMs) and other forms of electromyogram activity during sleep.14–21 In contrast, bupropion does not increase PLMS, and may even reduce them.16,22 However, it is not known whether antidepressants affect RRLMs. The goal of this study was to examine whether patients with OSA taking SSRI antidepressants have more RRLMs than those taking bupropion or no anti-depressant. Fragmented sleep and elevated autonomic nervous system activation associated with increased RRLMs in patients taking SSRIs could limit the tolerability and effectiveness of antidepressants, as well as increase the risk for cardiovascular disease.
METHOD
Subjects
Patients were selected from a database containing more than 5,000 questionnaires completed by patients prior to overnight polysomnography (PSG) studies at Massachusetts General Hospital (MGH) between 2011 and 2014. From this database, patients were first identified who reported taking bupropion without other antidepressants, and who underwent either a diagnostic PSG study or a split-night study with an AHI of at least 10 events/h during the diagnostic portion of the study (n = 32). Of these patients, 17 underwent a full-night diagnostic study and 15 underwent a split-night study at the MGH Sleep Center.
Patients taking SSRIs (n = 31, 18 diagnostic and 13 split studies) and control patients not taking any antidepressants (n = 31, 17 diagnostic and 14 split studies) were then matched to the bupropion group based on AHI, type of sleep study, concurrent psychotropic medications (specifically benzodiazepines, anti-convulsants, and antipsychotics), age, and sex. SSRIs included citalopram (n = 14), escitalopram (n = 2), fluoxetine (n = 7), and sertraline (n = 8). Dosages and duration of these medications were not reported. Exclusion criteria included the use of more than one antidepressant (including those prescribed for sleep), and use of a dopamine agonist medication. None of the subjects took an antidepressant other than an SSRI (in the SSRI group) or bupropion (in the bupropion group). There were no significant differences between groups for self-reported medical conditions predisposing to PLMs, such as kidney disease and seizures.
Procedure
Overnight sleep studies were performed according to the American Academy of Sleep Medicine practice standards.23 Channels included six electroencephalogram leads, two electrooculograms, submentalis electromyogram, nasal thermistor, nasal pressure transducer, snore vibration sensor, single-lead electrocardiogram, chest and abdomen respiratory effort belts, finger pulse oximeter, and bilateral anterior tibialis electromyography. Scoring of sleep staging, respiratory events, arousals, and periodic limb movements was performed visually according to standard criteria by trained technicians.24 RRLMs were scored by the first author (CM), blind to medication status, according to WASM 2016 standards for recording and scoring leg movements in polysomnograms, which recommends scoring movements occurring within 2 seconds before to 10.25 seconds after the termination of a respiratory event7 (Figure 1). No events were scored as more than one type of movement; that is, RRLMs also were not scored as PLMs. If an event could have been scored as both an RRLM and a PLM, the RRLM would be scored and the PLM ignored. RRLMs were scored during both diagnostic and treatment portions of split-night studies.
Figure 1. Scoring of respiratory-related leg movements using World Association of Sleep Medicine standards.
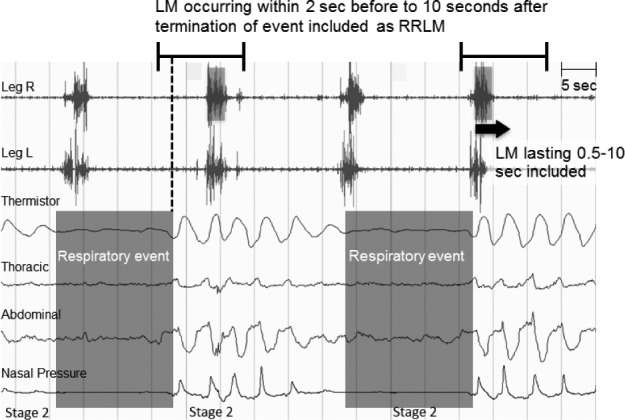
Leg movements were scored as RRLMs if they occurred within 2 seconds before to 10.25 seconds after the termination of a respiratory event. No events were scored as more than one type of leg movement.
Analyses were performed using SAS JMP software (SAS Institute, Inc., Cary, North Carolina, United States). Our primary variable of interest was the RRLM percentage (RRLM%), defined as the total number of RRLMs divided by the total number of respiratory events expressed as a percentage. The total number of respiratory events includes apneas, hypopneas, and respiratory effort-related arousals (RERAS). We also examined the RRLM index (RRLMI), defined as the total number of RRLMs divided by the total hours of sleep time. Variables were compared using analysis of variance, Student t tests, χ2 tests, and Pearson correlations.
RESULTS
Baseline Demographic and PSG Characteristics
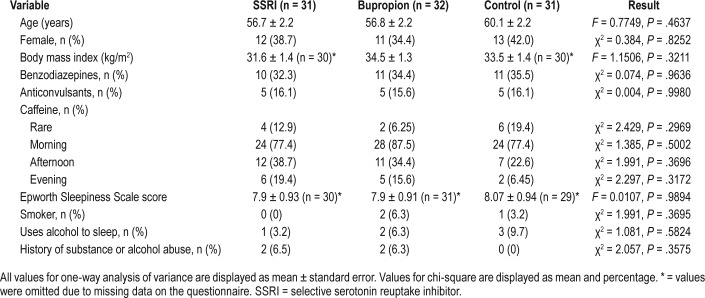
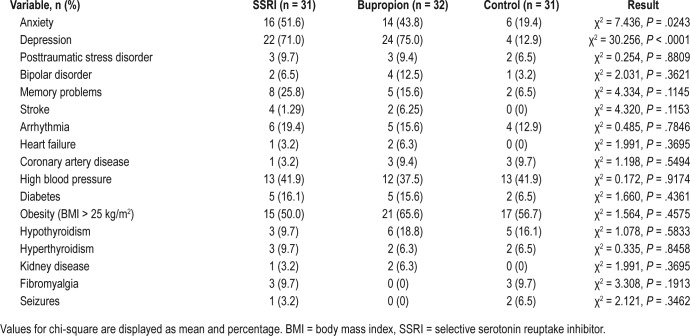
There were no significant differences between groups in characteristics such as age, sex, body mass index, use of pertinent medications or substances, or Epworth Sleepiness Scale scores. (Table 1) Patients taking SSRIs or bupropion had significantly greater prevalence of self-reported depression (χ2 = 30.256, P < .0001) and anxiety (χ2 = 7.436, P = .02) compared to controls. There were no significant differences between groups for other medical and neurological conditions (Table 2).
Table 1.
Demographic characteristics.
Table 2.
Medical comorbidities.
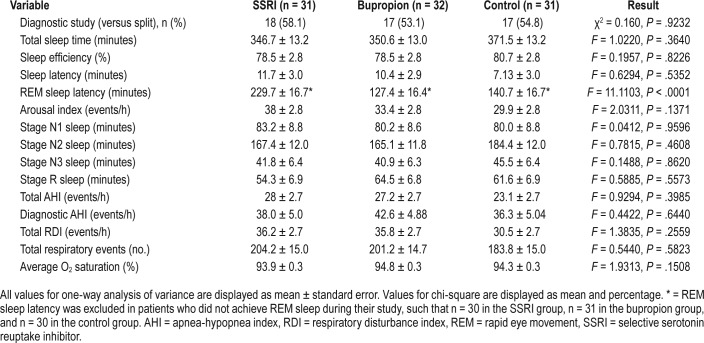
PSG analysis demonstrated greater rapid eye movement (REM) sleep latency in the SSRI group (229.7 minutes, versus 127.4 minutes in the bupropion group and 140.7 minutes in the control group, F = 11.1103, P < .0001), which may be related to the known REM-suppressive effects of SSRIs (Table 3).
Table 3.
Polysomnography and study characteristics.
Primary Outcome
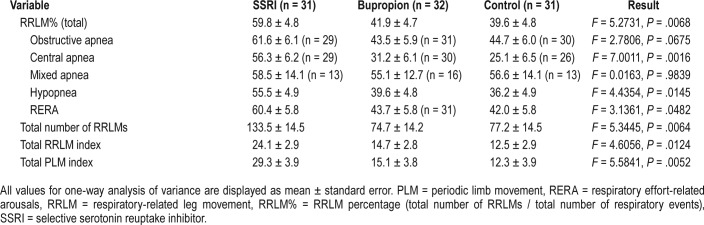
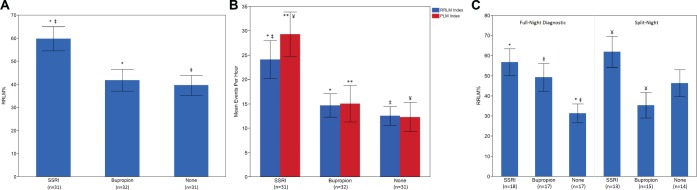
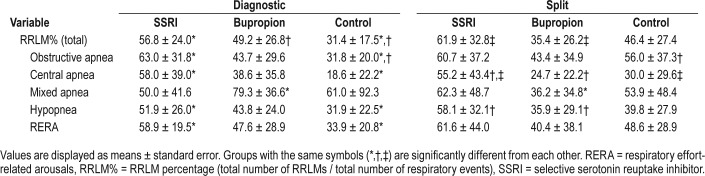
Table 4 shows the mean RRLM%, RRLMI, and periodic limb movement index (PLMI) values for each group. The RRLM% was significantly higher in the SSRI group (59.8%), relative to bupropion (41.9%, P = .01), and to control patients (39.6%), P = .004). (Figure 2A) Patients using SSRIs also demonstrated a significantly greater RRLMI (24.1) compared to control patients (12.5, P = .005) and patients using bupro-pion (14.7, P = .003). Consistent with other studies, patients using SSRIs demonstrated a greater PLMI (29.3) compared to control patients (12.3, P = .02) and patients using bupropion (15.1, P = .01). Among the different types of respiratory events, the SSRI group demonstrated a greater RRLM% specifically for obstructive apneas (61.6%) compared to bupropion (43.5%, P = .04), and a greater RRLM% than both bupropion and control groups for central apneas (56.3% versus 31.2% for bupropion and 25.1% for control patients, P = .005 and .0008 respectively), hypopneas (55.5% for SSRIs versus 39.6% for bupropion and 36.2% for control patients, P = .03 and P = .007, respectively), and RERAS (60.4% for SSRIs versus 43.7% for bupropion and 42% for control patients, P = .043 and P = .03, respectively).The RRLM% for obstructive apneas was also greater in the SSRI group (61.6%) relative to the control group (44.7%); however, this difference did not meet the threshold for significance (P = .07) (Figure 2B).
Table 4.
Comparison of respiratory-related and periodic limb movements.
Figure 2. Respiratory-related leg movement percentage, respiratory-related leg movement index, and periodic limb movement index by group.
All values are mean ± standard error. (A) A RRLM% comparison by group demonstrates a significantly greater overall RRLM% in the SSRI group relative to the bupropion and control groups. * = SSRI RRLM% versus bupropion RRLM%, P = .01. ‡ = SSRI RRLM% versus control RRLM%, P = .004. (B) Similarly, RRLMI and PLMI group comparisons show a greater overall RRLMI and PLMI in the SSRI group compared to the bupropion and control groups. * = SSRI RRLM index versus bupropion RRLM index, P = .02. ‡ = SSRI RRLM index versus control RRLM index, P = .005. ** = SSRI PLM index versus bupropion PLM index, P = .01. ¥ = SSRI PLM index versus control PLM index, P = .003. (C) When comparing groups for full-night diagnostic studies and split-night studies separately, the relative differences between groups show consistently high RRLM% for the SSRI group, however the bupropion group demonstrates elevated RRLM% in diagnostic studies, but low RRLM% in split-night studies. Conversely, the control group demonstrates lower RRLM% in diagnostic studies and higher RRLM% in split-night studies. * = SSRI RRLM% versus control RRLM% during full-night diagnostic PSG, P = .007. ‡ = Bupropion RRLM% versus control RRLM% during full-night diagnostic PSG, P = .045. ¥ = SSRI RRLM% versus bupropion RRLM% during split-night studies, P = .009.
Analysis of these variables by the type of study (full-night diagnostic PSG versus split-night study) demonstrated that the difference in RRLM% between SSRI and bupropion groups was limited to the split-night study subgroups. Table 5 shows summary values for RRLM% by group for each type of study. For full-night diagnostic studies, there was no difference in RRLM% between SSRI (56.8%) and bupropion (49.2%) groups; however, the RRLM% was significantly higher in both the SSRI group and bupropion groups relative to control groups (31.4%, P = .007 and .05, respectively). Conversely, for split-studies, the RRLM% in the SSRI group (61.9%) was greater than the bupropion group (35.4%, P = .009) and the control group (46.4%, P = .1), though the control group did not reach significance with P = .1. (Figure 2C) The differences between split-night and full diagnostic studies between groups appeared to be related to a lower RRLM% in the bupropion group and a higher RRLM% in the control group in the split-night condition, compared to the diagnostic study condition.
Table 5.
Comparison of respiratory-related leg movement percentage by type of study.
Within each group, the overall RRLM% was not significantly different between the full-night diagnostic study condition and the split-night study condition; however, there were differences by the type of event. The bupropion group had a greater RRLM% for mixed apneas in the diagnostic study condition (79.2%) relative to the split-night condition (36.2%, P = .03). The control group had a significantly greater RRLM% for obstructive apneas in the split-night study condition (56.0%) relative to the diagnostic study condition (31.2%, P = .04). For the SSRI group, there were no significant differences between full-night and split-night conditions for any type of respiratory event.
To identify potential differences in demographic, medical conditions, substances, and baseline study characteristics between patients undergoing full-night diagnostic and split-night subgroups, these variables were compared for each group using Student t tests with Bonferroni corrections. There were no significant differences in patient composition between those undergoing full-night diagnostic study and split-night studies, with the exception of the AHI, which was higher in patients undergoing split studies for all three groups, but did not meet significance except in the control group, in which the full-night diagnostic AHI mean was 17.7 ± 8.2 and the split-night study diagnostic AHI was 52.5 ± 25.8 (P < .0001). The full-night diagnostic AHI for the SSRI and bupropion groups was 22.2 ± 12.5 and 29.4 ± 21.0, respectively.
RRLM% correlated with arousal index (r = .435, P < .0001), RDI (r = .237, P = .02), diagnostic AHI (r = .204, P = .05), total AHI (r = .245, P = .02), PLMI (r = .613, P < .001), and REM sleep latency (r = .367, P = .0004), and correlated negatively with stage N3 sleep (r = −.253, P = .01) (Figure 3).
Figure 3. Multivariate analysis of respiratory-related leg movement percentage with periodic limb movement index and arousal index.
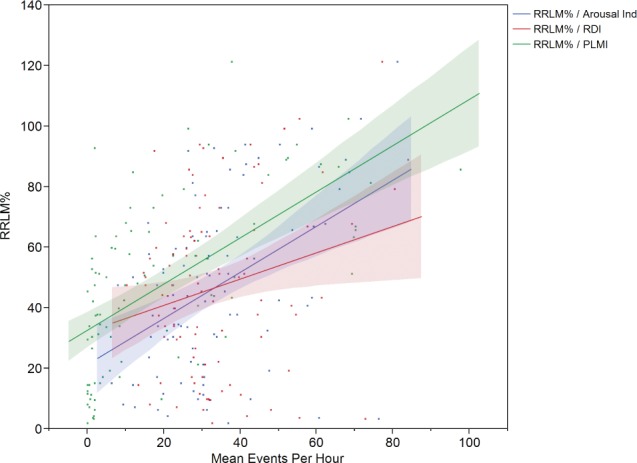
Arousal Ind = arousal index, PLMI = periodic limb movement index, RDI = respiratory disturbance index, RRLM% = RRLM percentage (total number of RRLMs / total number of respiratory events).
DISCUSSION
Our study found that patients with sleep apnea taking SSRIs had a greater RRLM% and RRLM index than patients taking bupropion or no antidepressants. This is consistent with prior studies showing greater PLMS in patients taking serotonergic medications such as SSRIs and serotonin-norepinephrine reuptake inhibitors.14–21
In our study, the difference between RRLM% in the SSRI group and the bupropion group appeared to be limited to patients undergoing split-night studies, during which positive airway pressure (PAP) therapy was initiated for the latter portion of the study. For patients undergoing full-night diagnostic studies, there was no significant difference in RRLM% between patients taking SSRIs or bupropion. Furthermore, although the total RRLM% was greater in patients on SSRIs relative to controls, this difference was nonsignificant in split studies. This effect appears to be related to a lower RRLM% in the bupropion group split-night condition relative to the full-night diagnostic condition, and a lower RRLM% in the control group diagnostic study condition relative to the split-night condition. In contrast, the RRLM% in the SSRI group was similar between conditions. Analysis of differences between full-night studies and split-night studies within groups showed no other differences except a higher AHI in the split-study control group relative to the full-night diagnostic study control group, which may account for the nonsignificant difference between the SSRI RRLM% and control RRLM% for split studies. This AHI difference may also explain the seemingly counterintuitive finding of a higher RRLM% in the split-study control group relative to the diagnostic study control group, which may be related to continued elevated sympathetic tone that is not yet normalized after only a few hours of PAP therapy. The overall RRLM% was significantly correlated with arousal index.
The pathophysiology of RRLMs is unclear. Some researchers have suggested that RRLMs arise from a different etiology from PLMs. Evidence for this perspective has included studies showing variable response of PLMs to continuous PAP, and lack of response of RRLMs to dopamine agonists.25–27 However, patients with OSA and RRLMs exhibit more PLMs than those without RRLMs, and PLMS and RRLMs are significantly associated even when AHI and restless legs syndrome (RLS) have been controlled, suggesting some commonality in these phenomena.4,5 Individuals with RRLMs also demonstrate nonrespiratory limb movements that are characteristic of PLMS, including a peak in the intermovement interval distribution histogram at approximately 20–24 seconds, and gradually decreasing frequency during the night.4 The results of this study show that both RRLMs and PLMs are increased in the presence of serotonergic medications, further supporting the possibility of a shared or overlapping pathophysiology. Serotonergic medications are hypothesized to increase PLMs and other forms of increased muscle activity during sleep via an interaction between serotonergic and dopaminergic neurotransmitter systems that ultimately decrease dopaminergic transmission.14,18
In contrast, bupropion is a weak dopamine reuptake inhibitor and a strong norepinephrine reuptake inhibitor.28–30 Abnormal dopamine receptor binding and transport have been demonstrated in patients with periodic limb movement disorder, and reductions in dopamine availability coincide with the timing of both RLS and periodic limb movement disorder.31,32 Studies have shown efficacy for dopamine agonist medications in patients with RLS and PLMs, including arousal-associated PLMs.33 If RRLMs share pathophysiology with PLMs, it may be that bupropion's lack of adverse effects on RRLMs, at least in the context of PAP therapy, may be related to its mechanism as a weak dopamine reuptake inhibitor. However, in the setting of untreated OSA, in which obstructive respiratory events increase overall sympathetic tone, one could speculate that increased norepinephrine may contribute to a greater overall propensity for RRLMs. RRLMs have been found to occur more frequently at the termination of obstructive apneas compared to hypopneas, and in the presence of arousals.6 PAP therapy is likely to cause changes in the relative frequency of obstructive apneas, hypopneas, and arousals that may affect the effects of these medications on RRLMs. The dual mechanism of action of bupropion may thus have a different effect on RRLMs in the presence of PAP therapy than without it.
There was greater consistent elevation of RRLMs in the SSRI group, where RRLM% was elevated in both the diagnostic and split-night study conditions. The greater overall number of RRLMs in the SSRI group may contribute to both sleep disruption and cardiovascular risk associated with increased sympathetic activation. Subjective complaints of insomnia or daytime sleepiness are frequent in patients treated with SSRIs, particularly early in treatment.30,34–36 Based on data from the United States Food and Drug Administration study register, complaints of treatment-emergent insomnia ranged from 2% with citalopram to 31.3% with fluvoxamine.36 Frequent arousals from sleep can lead to a subjective sense of reduced sleep quality and excessive daytime fatigue. Sleep disturbance is also associated with increased risk for depression, poor response to antidepressant treatment, depression relapse, and suicide.37 Given that both PLMs and RRLMs are associated with increased arousals and thus increased sleep disturbance, the increased numbers of both types of nocturnal movements in patients with OSA using SSRIs may compound these effects. Understanding the adverse effects of serotonergic medications in this population may guide selection of a nonserotonergic antidepressant such as bupropion in this population to minimize treatment-emergent sleep disruption and elevated sympathetic activity.
It is important to note that RRLM scoring in this study was performed using relatively new scoring guidelines published by the WASM in 2016, which recommends scoring movements occurring beginning 2 seconds before to 10.25 seconds after the termination of a respiratory event as RRLMs. This standard undoubtedly led to the inclusion of events as RRLMs that under other guidelines would be considered isolated or periodic limb movements. The clinical implications of this change are still being determined.
These findings are limited by the absence of medication dosages and durations in the questionnaires. Although groups were matched for the use of benzodiazepines, antipsychotics, and anticonvulsants, the timing and dosages of these medications prior to the studies was unknown and may have influenced RRLMs. Future research could include investigation of dosage or duration-dependent effects or the effects of concurrent bupropion and SSRI administration on RRLMs. Patients in the SSRI group experienced longer REM sleep latency, but did not have less REM sleep, compared to bupropion and control groups. This is consistent with the known effects of SSRIs on REM sleep latency.29,30,35 PLMs are known to have a circadian rhythm, with higher frequency during the first half of the sleep period.38 A recent study found that RRLM occurrence decreased through the sleep period for patients with high PLMs, but did not do so in patients with low PLMs.6 Our study was unable to complete an analysis of time-of-night effects because of limitations in the study reports; however, this would be helpful to better characterize whether changes in frequency of RRLMs over the course of the night influenced our findings. Analysis of changes in RRLMs occurring before and after PAP therapy would also be helpful in identifying interactions between PAP therapy and antidepressant effects. Further research in these areas may help to further elucidate the appropriate medical management of co-morbid sleep-disordered breathing and depression.
DISCLOSURE STATEMENT
Work for this study was performed at Massachusetts General Hospital. All authors have seen and approved this manuscript. Dr. Winkelman is a consultant for Flex Pharma. He receives royalties from UpToDate. He has received research grants from NeuroMetrix, NIMH, the RLS Foundation, and Luitpold Pharma. Dr. McCall reports no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- EMG
electromyography
- MGH
Massachusetts General Hospital
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PLM
periodic limb movement
- PLMS
periodic limb movement of sleep
- PLMI
periodic limb movement index (mean number of periodic limb movements per hour of total sleep time)
- PSG
polysomnography
- RDI
respiratory disturbance index (mean number of respiratory events per hour of total sleep time; respiratory events include apneas, hypopneas, and respiratory effort-related arousals)
- REM
rapid eye movement
- RERA
respiratory effort-related arousal
- RLS
restless leg syndrome
- RRLM
respiratory-related leg movement
- RRLM%
percentage of respiratory events associated with a limb movement
- RRLMI
respiratory-related limb movement index (mean number of respiratory-related limb movements per hour of total sleep time)
- SSRI
selective serotonin reuptake inhibitor
- WASM
World Association of Sleep Medicine
REFERENCES
- 1.American Sleep Disorders Association. Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16(8):748–759. [PubMed] [Google Scholar]
- 2.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group. Sleep Med. 2006;7(2):175–183. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 4.Manconi M, Zavalko I, Bassetti CL, Colamartino E, Pons M, Ferri R. Respiratory-related leg movements and their relationship with periodic leg movements during sleep. Sleep. 2014;37(3):497–504. doi: 10.5665/sleep.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manconi M, Zavalko I, Fanfulla F, Winkelman JW, Fulda S. An evidence-based recommendation for a new definition of respiratory-related leg movements. Sleep. 2015;38(2):295–304. doi: 10.5665/sleep.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fulda S, Heinzer R, Haba-Rubio J. Characteristics and determinants of respiratory event associated leg movements. Sleep. 2017;41(2) doi: 10.1093/sleep/zsx206. [DOI] [PubMed] [Google Scholar]
- 7.Ferri R, Fulda S, Allen RP, et al. World Association of Sleep Medicine (WASM) 2016 standards for recording and scoring leg movements in polysomnograms developed by a joint task force from the International and the European Restless Legs Syndrome Study Groups (IRLSSG and EURLSSG) Sleep Med. 2016;26:86–95. doi: 10.1016/j.sleep.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Kendzerska T, Kamra M, Murray BJ, Boulos MI. Incident cardiovascular events and death in individuals with restless legs syndrome or periodic limb movements in sleep: a systematic review. Sleep. 2017;40(3) doi: 10.1093/sleep/zsx013. [DOI] [PubMed] [Google Scholar]
- 9.Winkelman JW, Blackwell T, Stone K, Ancoli-Israel S, Redline S. Associations of incident cardiovascular events with restless legs syndrome and periodic leg movements of sleep in older men, for the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep Study) Sleep. 2017;40(4) doi: 10.1093/sleep/zsx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 11.Yeboah J, Redline S, Johnson C, et al. Association between sleep apnea, snoring, incident cardiovascular events and all-cause mortality in an adult population: MESA. Atherosclerosis. 2011;219(2):963–968. doi: 10.1016/j.atherosclerosis.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aritake S, Blackwell T, Peters KW, et al. Prevalence and associations of respiratory-related leg movements: the MrOS sleep study. Sleep Med. 2015;16(10):1236–1244. doi: 10.1016/j.sleep.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C-K, Jordan AS, White DP, Winkelman JW. Heart rate response to respiratory events with or without leg movements. Sleep. 2006;29(4):553–556. doi: 10.1093/sleep/29.4.553. [DOI] [PubMed] [Google Scholar]
- 14.Armitage R, Trivedi M, Rush AJ. Fluoxetine and oculomotor activity during sleep in depressed patients. Neuropsychopharmacology. 1995;12(2):159–165. doi: 10.1016/0893-133X(94)00075-B. [DOI] [PubMed] [Google Scholar]
- 15.Picchietti D, Winkelman JW. Restless legs syndrome, periodic limb movements in sleep, and depression. Sleep. 2005;28(7):891–898. [PubMed] [Google Scholar]
- 16.Yang C, White DP, Winkelman JW. Antidepressants and periodic leg movements of sleep. Biol Psychiatry. 2005;58(6):510–514. doi: 10.1016/j.biopsych.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Hao Y, Jia F, et al. Sertraline and periodic limb movements during sleep: an 8-week open-label study in depressed patients with insomnia. Sleep Med. 2013;14(12):1405–1412. doi: 10.1016/j.sleep.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Goerke M, Rodenbeck A, Cohrs S, Kunz D. The influence of the tricyclic antidepressant amitriptyline on periodic limb movements during sleep. Pharmacopsychiatry. 2013;46(3):108–113. doi: 10.1055/s-0032-1331702. [DOI] [PubMed] [Google Scholar]
- 19.Fulda S, Kloiber S, Dose T, et al. Mirtazapine provokes periodic leg movements during sleep in young healthy men. Sleep. 2013;36(5):661–669. doi: 10.5665/sleep.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorsey C, Lukas S, Cunningham S. Fluoxetine-induced sleep disturbance in depressed patients. Neuropsychopharmacology. 1996;14(6):437–442. doi: 10.1016/0893-133X(95)00148-7. [DOI] [PubMed] [Google Scholar]
- 21.Vendrame M, Zarowski M, Loddenkemper T, Steinborn B, Kothare SV. Selective serotonin reuptake inhibitors and periodic limb movements of sleep. Pediatr Neurol. 2011;45(3):175–177. doi: 10.1016/j.pediatrneurol.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Nofzinger EA, Fasiczka A, Berman S, Thase ME. Bupropion SR reduces periodic limb movements associated with arousals from sleep in depressed patients with periodic limb movement disorder. J Clin Psychiatry. 2000;61(11):858–862. doi: 10.4088/jcp.v61n1108. [DOI] [PubMed] [Google Scholar]
- 23.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales AD. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 25.Baran AS, Richert AC, Douglass AB, May W, Ansarin K. Change in periodic limb movement index during treatment of obstructive sleep apnea with continuous positive airway pressure. Sleep. 2003;26(6):717–720. doi: 10.1093/sleep/26.6.717. [DOI] [PubMed] [Google Scholar]
- 26.Manconi M, Vitale G, Ferri R, Zucconi M, Ferini-Strambi L. Periodic leg movements in Cheyne-Stokes respiration. Eur Respir J. 2008;32(6):1656–1662. doi: 10.1183/09031936.00163507. [DOI] [PubMed] [Google Scholar]
- 27.Carelli G, Krieger J, Calvi-Gries F, Macher JP. Periodic limb movements and obstructive sleep apneas before and after continuous positive airway pressure treatment. J Sleep Res. 1999;8(3):211–216. doi: 10.1046/j.1365-2869.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell H, Weinshenker D. Good night and good luck: norepinephrine in sleep pharmacology. Biochem Pharmacol. 2010;79(6):801–809. doi: 10.1016/j.bcp.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stahl SM, Grady MM. Differences in mechanism of action between current and future antidepressants. J Clin Psychiatry. 2003;64(Suppl 13):13–17. [PubMed] [Google Scholar]
- 30.Thase ME. Depression, sleep, and antidepressants. J Clin Psychiatry. 1998;59(Suppl 4):55–65. [PubMed] [Google Scholar]
- 31.Staedt J, Stoppe G, Kögler A, et al. Dopamine D2 receptor alteration in patients with periodic movements in sleep (nocturnal myoclonus) J Neural Transm Gen Sect. 1993;93(1):71–74. doi: 10.1007/BF01244940. [DOI] [PubMed] [Google Scholar]
- 32.Trenkwalder C, Hening WA, Walters AS, Campbell SS, Rahman K, Chokroverty S. Circadian rhythm of periodic limb movements and sensory symptoms of restless legs syndrome. Mov Disord. 1999;14(1):102–110. doi: 10.1002/1531-8257(199901)14:1<102::aid-mds1017>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Erichsen D, Ferri R, Gozal D. Ropinirole in restless legs syndrome and periodic limb movement disorder. Ther Clin Risk Manag. 2010;6:173–182. doi: 10.2147/tcrm.s6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wichniak A, Wierzbicka A, Walęcka M, Jernajczyk W. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63. doi: 10.1007/s11920-017-0816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson S, Argyropoulos S. Antidepressants and sleep a qualitative review of the literature. Drugs. 2005;65(7):927–947. doi: 10.2165/00003495-200565070-00003. [DOI] [PubMed] [Google Scholar]
- 36.Doghramji K, Jangro WC. Adverse effects of psychotropic medications on sleep. Psychiatr Clin North Am. 2016;39(3):487–502. doi: 10.1016/j.psc.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Krystal AD. Psychiatric disorders and sleep. Neurol Clin NA. 2012;30(4):1389–1413. doi: 10.1016/j.ncl.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duffy JF, Lowe ASW, Silva EJ, Winkelman JW. Periodic limb movements in sleep exhibit a circadian rhythm that is maximal in the late evening/early night. Sleep Med. 2011;12(1):83–88. doi: 10.1016/j.sleep.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]