Abstract
Study Objectives:
To assess the role of different levels of adherence and long-term effects of positive airway pressure (PAP) therapy on gas exchange, sleepiness, quality of life, depressive symptoms, and all-cause mortality in patients with obesity hypoventilation syndrome (OHS).
Methods:
A total of 252 patients with newly diagnosed OHS were followed up for a minimum of 2 years after PAP initiation. PAP adherence (h/night) was monitored. Arterial blood gas samples were taken with patients being alert for more than 4 hours after morning awakening. Subjective daytime sleepiness (Epworth Sleepiness Scale [ESS]), quality of life (Short Form 36 [SF-36]) and patient's depressive symptoms (Beck Depression Inventory [BDI]) were assessed before and at the end of the follow-up period, along with all-cause mortality.
Results:
At the end of the follow-up period (median duration [25th–75th percentile], 30 [24–52] months), PaO2 increased from baseline (72.7 ± 10.3 versus 63.2 ± 10.6, P < .001) and both PaCO2 and HCO3− decreased (43.0 [39.2–45.0] versus 50.0 [46.7–55.4] and 27.5 ± 3.2 versus 31.4 ± 4.2, respectively, P < .001). In addition, PAP therapy significantly improved ESS (7 [4–9] versus 14 [11–16], P < .001), BDI (8.8 ± 4.9 versus 15.5 ± 7.3, P < .001) and SF-36 (82 [78–87] versus 74 [67–79], P < .001) scores. Over the follow-up period 11 patients died. Patients who used PAP for > 6 h/night had significant improvements (P < .05) in blood gases and SF-36 scores than less adherent patients.
Conclusions:
Increased hours of use and long-term therapy with PAP are effective in the treatment of patients with OHS. Clinicians should encourage adherence to PAP therapy in order to provide a significant improvement in clinical status and gas exchange in these patients.
Commentary:
A commenary on this article appears in this issue on page 1455.
Clinical Trial Registration:
Title: PAP Therapy in Patients With Obesity Hypoventilation Syndrome, Registry: ClinicalTrials.gov, Identifier: NCT03449641, URL: https://clinicaltrials.gov/ct2/show/NCT03449641
Citation:
Bouloukaki I, Mermigkis C, Michelakis S, Moniaki V, Mauroudi E, Tzanakis N, Schiza SE. The association between adherence to positive airway pressure therapy and long-term outcomes in patients with obesity hypoventilation syndrome: a prospective observational study. J Clin Sleep Med. 2018;14(9):1539–1550.
Keywords: compliance, obesity hypoventilation syndrome, positive airway pressure
BRIEF SUMMARY
Current Knowledge/Study Rationale: There are limited data concerning the effect of long-term effects of positive airway pressure (PAP) therapy on survival and functional status in individuals with obesity hypoventilation syndrome (OHS). This study aimed to assess the role of different levels of adherence and long-term effects of PAP therapy on gas exchange, sleepiness, quality of life, depressive symptoms, and all-cause mortality in patients with OHS after 2 years of PAP therapy.
Study Impact: Our results show that PAP therapy for ≥ 6 h/night, at least for a 2-year period in patients with OHS, resulted in significant improvements of arterial blood gases, quality of life, cardiovascular morbidity, and mortality. Clinicians should encourage PAP adherence to provide a significant improvement in clinical status and gas exchange of these patients.
INTRODUCTION
Obesity is the main risk factor for obstructive sleep apnea (OSA)1 and a determining factor for obesity hypoventilation syndrome (OHS).2 As the prevalence of obesity is increasing rapidly, chronic alveolar hypoventilation resulting in chronic hypercapnic respiratory failure develops in a considerable percentage of severely obese patients.3 Although the prevalence of OHS is currently unknown, 0.3% to 0.48% in the general population are estimated to be affected, whereas in obese patients referred to sleep clinics the prevalence of OHS ranges from 8% to 20%.4,5
Patients with OHS have decreased quality of life, tend to have more comorbidities and increased health care expenses, and are at higher risk of the development of serious cardiovascular disease leading to early mortality, compared to normocapnic morbidly obese patients and normocapnic patients with OSA.6 Despite these worse outcomes, the diagnosis of OHS is typically established late, in the fifth or sixth decade of life; therefore, clinicians need to maintain a high index of suspicion, particularly given that early diagnosis and therapy are considered important to avoid the adverse effects of OHS and reduce the high burden of morbidity and mortality associated with this syndrome.7
Treatment of OHS is based on the underlying pathophysiology of the condition. The upper airway obstruction is an important factor in the pathogenesis of OHS, with 90% of patients exhibiting coexistent OSA. There is evidence that strategies for reversing upper airway obstruction, such as positive airway pressure (PAP), are effective in most patients with stable OHS, particularly in the subgroup with severe OSA. PAP therapy improves blood gases, morning headaches, excessive daytime sleepiness and vigilance, dyspnea, pulmonary hypertension, and secondary erythrocytosis.8–11 Improvements in symptoms and blood gases are directly related to adherence to therapy and maximal improvement in blood gases can be achieved as early as 2 to 4 weeks after initiation of PAP therapy.12
Various forms of PAP therapy are effective in providing short- and long-term benefits in these patients with or without OSA. However, there are limited data concerning the long-term effects of PAP therapy on survival and functional status in individuals with OHS and OSA. Therefore, we aimed to assess the role of different levels of adherence and long-term effects of PAP therapy on gas exchange, sleepiness, quality of life, depressive symptoms, and all-cause mortality in patients with OHS.
METHODS
We conducted a single-center, prospective, long-term follow-up study of patients with a new diagnosis and who fulfilled OHS diagnostic criteria. Between June 2009 and June 2012, consecutive patients aged between 18 and 80 years, who were admitted to the Sleep Disorders Center, Department of Thoracic Medicine, University of Crete Medical School, for evaluation of suspected sleep-disordered breathing, were considered as potential recruits for this study. OHS was determined by an arterial blood gas with partial pressure of carbon dioxide in the arterial blood (PaCO2) at rest > 45 mmHg and a body mass index (BMI) > 30 kg/m2 in the absence of other causes of hypoventilation, such as neuromuscular, chest wall disorders, and chronic obstructive pulmonary disease. To be included in the study, patients had to be clinically stable for at least 4 weeks prior to enrollment. In addition, all included patients had to have achieved higher than elementary-school education. The exclusion criteria were: refusal to participate, refusal of PAP therapy, central sleep apnea syndromes, chronic obstructive pulmonary disease or asthma, restrictive ventilation syndromes, severe congestive heart failure, a history of life-threatening arrhythmias, severe cardiomyopathy, significant chronic kidney disease, untreated hypothyroidism, family or personal history of mental illness, drug or alcohol abuse, sedative use, severe cognitive impairment, concurrent oncological diseases, and history of narcolepsy or restless legs syndrome.
All subjects provided written informed consent and ethical approval was provided by the University Hospital Ethics Committee.
Initial Visit/Evaluation Data Collection
All patients underwent a detailed evaluation that included age, measurement of BMI, medical history focused on sleep-related symptoms, associated conditions and comorbidities, menopausal status, smoking history, and alcohol intake. In addition, we performed spirometry and overnight attended polysomnography (PSG) and collected arterial blood gas (ABG) samples. Furthermore, patients were classified into three groups according to PaCO2 values: (1) mild (PaCO2 46–50 mmHg), moderate (PaCO2 51–55 mmHg), severe (PaCO2 ≥ 56 mmHg), based on the suggestions of Damiani et al.13 Subjective daytime sleepiness was assessed by the Epworth Sleepiness Scale (ESS),14 quality of life by Short Form 36 Health Survey (SF-36), and patient's depressive symptoms by the Beck Depression Inventory (BDI) at baseline and at 2 years after initiation of PAP therapy.
Short Form 36 Health Survey
The SF-36 is a reliable and validated 36-item questionnaire for the assessment of general (physical and mental) health and quality of life, each of which is scored separately from 0 (worst) to 100 (best).15–17
Beck Depression Inventory
The BDI is a 21-item questionnaire that is a widely used and well-validated self-reported inventory of depressive symptoms.18–20 Total scores range from 0 to 63 and represent the sum of the highest levels endorsed on each item. Scores lower than 10 are considered normal.
Spirometry
Spirometry was performed with the patient in the seated position, according to approved standards.21
ABG Samples
ABG samples were taken with patients being alert for more than 4 hours after morning awakening, seated and breathing room air, and having remained at rest for 10 minutes. In patients on long-term oxygen therapy, supplemental oxygen was removed 30 minutes before the measurement of blood gases.
Sleep Study and PAP Treatment
Polysomnography
In-laboratory PSG was performed in clinically stable patients at a median (25th–75th percentile) 58.9 (28.2–47.3) days after the diagnosis. All patients underwent a single-night full diagnostic PSG study (Alice 5, Diagnostics System, Respironics, Murrysville, Pennsylvania, United States) according to standard techniques, with monitoring of the electroencephalogram, electro-oculogram, electromyogram, flow (by oronasal thermistor and nasal air pressure transducer), thoracic and abdominal respiratory effort (by respiratory inductance plethysmography), pulse oximetry (SpO2), and body position. Snoring was recorded by a microphone placed on the anterior neck. In addition, we performed sleep capnography, (CO2SMO, Respironics Novametrix, Wallingford, Connecticut, United States) with transcutaneous carbon dioxide (PtcCO2) monitoring, in order to identify sleep hypoventilation and to guide the titration of PAP therapy, ensuring that ventilation is adequately maintained.22 We identified the average PtcCO2 values by downloading the overnight records. We excluded unreliable records (n = 18) (inexplicable, abrupt, and excessive increases in PtcCO2). PSG recordings were manually interpreted over 30-second periods by experienced staff in accordance with the 2007 American Academy of Sleep Medicine (AASM) guidelines.23 The scorer was blinded to the origin of the data. The definition of apnea and hypopnea followed the AASM standard criteria.23 The apnea-hypopnea index (AHI), calculated as the number of apnea and hypopnea events per hour of sleep, was used to diagnose OSA and assess its severity. OSA was categorized as mild (AHI 5 to < 15 events/h), moderate (AHI 15 to < 30 events/h), and severe (AHI ≥ 30 events/h).
PAP Titration
During in-laboratory PAP titration with full PSG, performed 11.8 ± 8.9 days after the diagnostic PSG, the appropriate PAP settings were established. Continuous positive airway pressure (CPAP) was initially tested in all patients, and manually titrated in order to abolish all nocturnal respiratory events. If oxygen desaturation persisted after obstructive apneas and hypopneas had been eliminated with CPAP, we changed to bilevel PAP ventilation in spontaneous mode (ie, no backup respiratory rate). The level of CPAP that eliminated obstructive apneas and hypopneas was used as the expiratory positive pressure and positive inspiratory pressure was gradually increased until oxygen saturation was steadily over 90% or high inspiratory pressures (equal or above 20 cmH2O) were reached. Oxygen was added when significant oxygen desaturation persisted (SpO2 ≤ 88% for ≥ 5 minutes in the absence of obstructive respiratory events) despite the use of these high inspiratory pressures. In addition, oxygen therapy was added if, prior to the PAP titration, the patient's awake supine SpO2 while breathing room air was ≤ 88%.24 All patients received education prior to the PAP titration night and completed a questionnaire at the end of the first night, reporting their quality of sleep under PAP titration and any side effects.
Follow-Up
All study groups received individual counseling during scheduled clinic appointments, at their initial sleep clinic consultation, and after the completion of overnight in-laboratory polysomnography. After PAP therapy was started, patients were followed up in the outpatient sleep clinic at 1 month, then at 3-month intervals during the first year, and every 6 months thereafter. Patients were advised to bring their PAP device and interface to every sleep clinic visit. The importance of good adherence to PAP therapy was emphasized, encouraging the patients to use PAP therapy throughout the entire sleep period every day. This format adhered to a standardized approach according to our PAP clinic procedures.25 In the first month, separate from treatment adherence encouragement, an ABG analysis was performed, and changes to oxygen therapy or PAP settings were made if necessary. At each visit a clinical nurse, under the supervision of a sleep physician, re-evaluated the exclusion criteria and recorded anthropometric variables, weight, PAP adherence, medications, alcohol and tobacco consumption, and other clinically relevant events. Furthermore, in patients requiring supplemental oxygen, oxygen was withdrawn based on awake ABG levels, effective PAP therapy (PAP adherence), and nocturnal oximetry results.
The study was designed to follow patients for at least 2 years. However, in some patients, follow-up stopped when the patient withdrew informed consent or was unable to complete follow-up.
PAP Adherence
PAP usage data included mask type (nasal or full face), number of nights on PAP, average use (h/night), air leakage, and air pressure delivered. Regular PAP adherence was defined as using PAP therapy for an average of 4 h/night for and at least 70% of the nights.26 However, we used the cutoff point of 6 h/ night of PAP use for subgroup analysis, as studies in patients with eucapnic OSA indicate that with use more than 6 h/night a greater percentage of patients will achieve normal levels of objectively measured and self-reported daytime sleepiness, as well as significantly improved memory, daily functioning, and improved survival rates.25,27
Statistical Analysis
All continuous variables were tested for normality using several methods (skewness and kurtosis, the proximity of the mean to the median, visual inspection of their histograms, Q-Q plots, and box plots). Results are presented as mean ± standard deviation (SD) for continuous variables if normally distributed and as median (25th–75th percentile) if not. Qualitative variables are presented as absolute number (percentage). Variables that were normally distributed were compared among the three groups (mild, moderate and severe OHS) using analysis of variance. If analysis of variance was significant, we used Tukey-Kramer post hoc test to compare each pair (moderate versus mild, severe versus mild, and moderate versus mild OHS groups). For variables that are not normally distributed, we used the Kruskal-Wallis test to compare the three groups. If Kruskal-Wallis test results were significant, we used the Mann-Whitney U test to compare each pair. For categorical variables, we used the chi-square test to compare the three groups. To compare changes from baseline to follow-up, the paired samples t test (for normally distributed data) and the Wilcoxon signed-rank test (for non-normally distributed data) were used. Changes of continuous variables from baseline to follow-up were defined as follow-up minus baseline values. Correlation coefficients were calculated using the Pearson or Spearman (for non-normally distributed data) correlation test for all the independent predictors of mean change of PaO2, PaCO2, HCO3−, and questionnaire scores. As independent variables we included clinically relevant variables such as age, sex, BMI, ESS, smoking history, comorbidities, AHI, oxygen desaturation index, mean SpO2 and minimum SpO2 during sleep, awake and asleep transcutaneous CO2 (PtcCO2), sleep time with SpO2 < 90%, ABG, spirometric measurements, h/ night of PAP use, percentage of nights PAP was used, type of PAP (bilevel PAP or fixed CPAP and auto-PAP) mode. Only the variables that were found to be significant were further analyzed. Multivariate linear regression analysis was used to examine any association of PAP treatment with changes in PaO2, PaCO2, HCO3−, and questionnaires scores at follow-up, after controlling for the potential confounders that were found to be significant. Furthermore, we applied a binary logistic regression analysis model to assess the ability of the previous variables to predict the prescription of O2 supplementation to PAP therapy and mortality. As a large proportion of patients were smokers and obesity can falsely increase the forced expiratory volume in one second/forced vital capacity ratio leading to probable false-negative results for the presence of obstructive airways disease, we performed our analysis separately in patients who never smoked. A value of P < .05 was considered statistically significant. Data were analyzed using PAWP 17.0 software (SPSS Inc, Chicago, Illinois, United States).
RESULTS
Baseline Characteristics
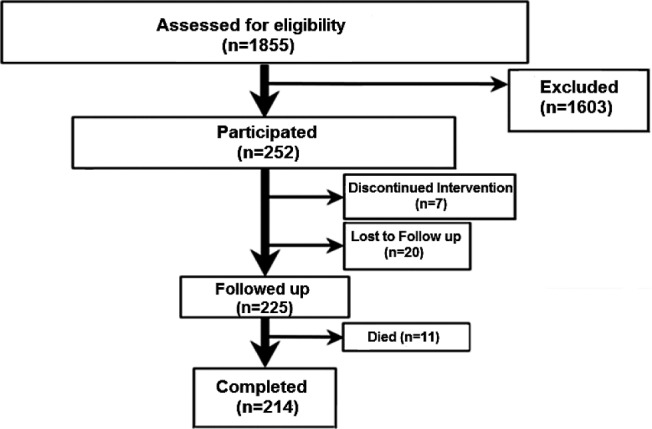
During the study period, 1,855 patients were referred to the sleep disorders clinic for clinical suspicion of OSA. Most were obese (BMI > 30 kg/m2) and had an AHI ≥ 5 events/h (1,623, 87.5%). Among the 1,855 patients, 241 received a diagnosis of OHS and OSA, 11 OHS without OSA (ie, AHI < 5 events/h), 1,227 OSA without hypercapnia, 211 OSA with hypercapnia (mainly due to COPD diagnosis), and 165 no OSA. The overall prevalence of OHS among the 1,855 patients referred was 13.6% and among obese patients with OSA it was 15.5%. All 252 patients with OHS participated, of whom 7 patients abandoned PAP therapy before the 24-month period and 20 did not show up for the final assessment (Figure 1). Thus, the final study sample comprised 225 patients, 108 men (48%) and 117 women (52%). These patients did not differ from those who did not complete the study regarding age, sex, BMI, comorbidities, AHI, mean nighttime SpO2, baseline PaCO2 and PaO2 and type of PAP therapy prescribed (data not shown).
Figure 1. Study flow diagram illustrating how the final group of patients were obtained.
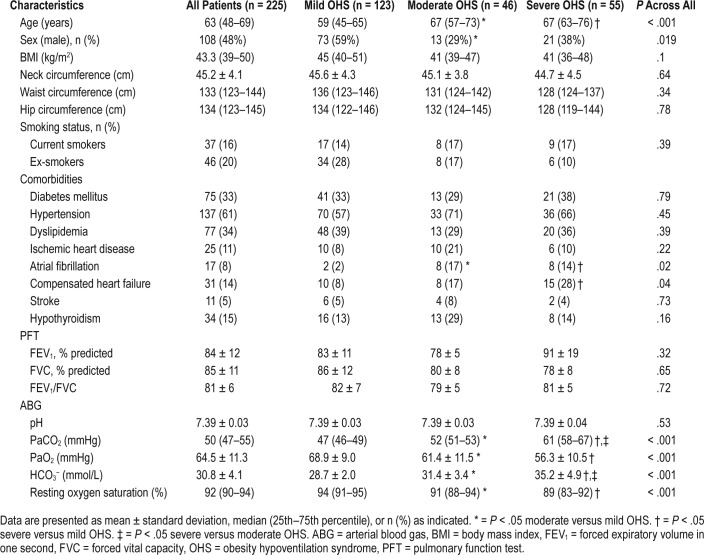
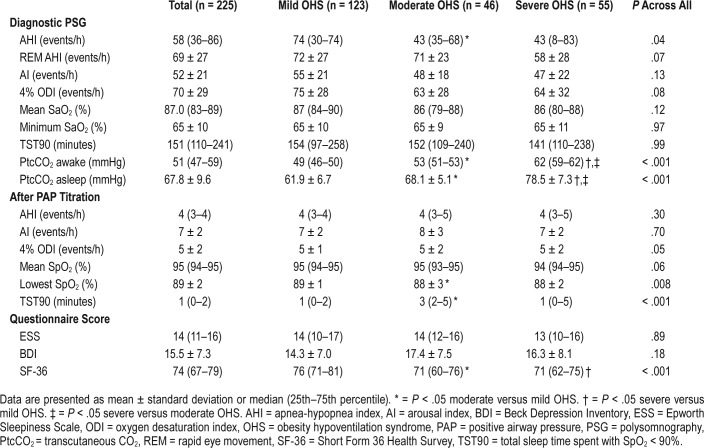
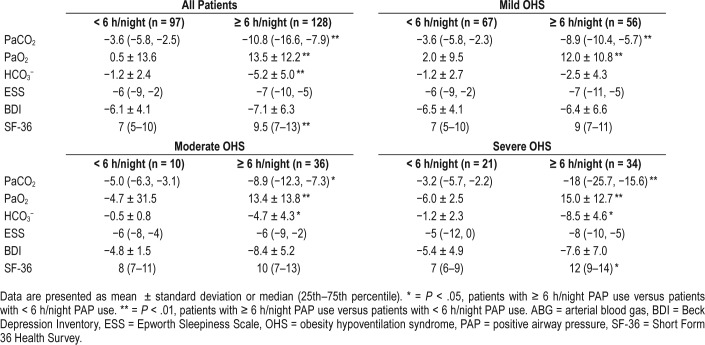
Demographics, spirometric measurements, and ABG analysis of the final sample at baseline are shown in Table 1. PSG data and questionnaires scores are shown in Table 2. Time that PtcCO2 was above 50 mmHg was 72.3 ± 30.1 minutes. None of the patients included in the study had a significant number of central apneas during sleep. Based on OHS grading according to PaCO2 level, 123 patients (55%) were categorized as mild, 46 (20%) as moderate, and 56 (25%) as severe. No difference was found among groups in terms of BMI, current smoking, spirometry results, BDI, and ESS scores (Table 1 and Table 2).
Table 1.
Baseline demographics, spirometric measurements, and ABG analysis results of the study population.
Table 2.
Baseline PSG data and questionnaires scores of the final sample.
At baseline, 100 patients (45%) required daytime and/ or nocturnal supplemental oxygen (flow 1–3 L/min) therapy (Table S1 in the supplemental material). These patients were older and mostly female, had more comorbidities, higher baseline PaCO2, and lower AHI. In logistic regression models, the prescription of O2 supplementation with PAP therapy was associated with the moderate to severe OHS group (14.1; 95% CI 4.4, 45.4, P < .001) after controlling for the potential confounders.
Adherence With PAP Therapy
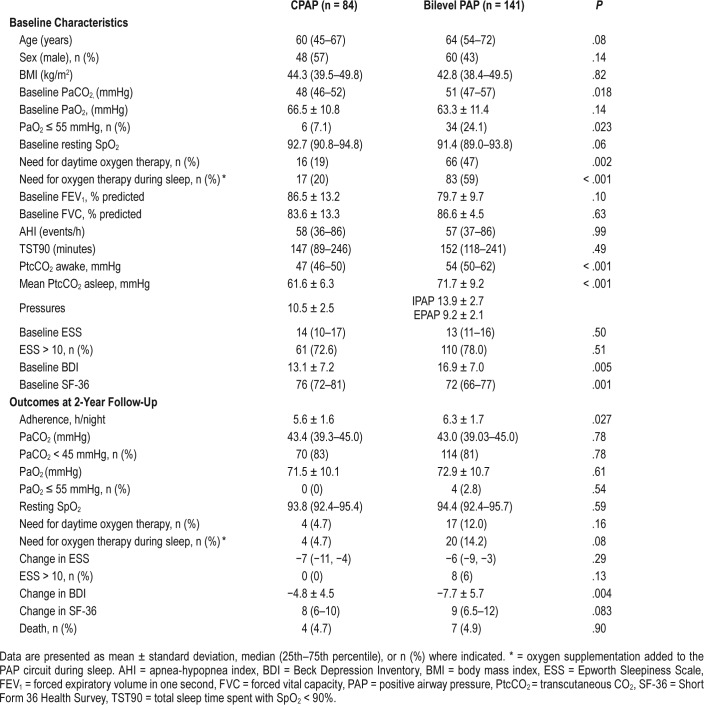
Of 225 patients with OHS, 84 (37.3%) used PAP (either fixed CPAP [17.8%] or auto-PAP [82.2%] with the mean CPAP pressure of 10.5 ± 2.5 cmH2O) and 141 (62.6%) used bilevel PAP (mean IPAP of 13.9 ± 2.7 cmH2O and mean EPAP of 9.2 ± 2.1 cmH2O). In the mild OHS group, 55% of subjects used bilevel PAP; the percentage was 75% in the moderate OHS group and 76% in the severe OHS group, which was slightly but not significantly increased compared to moderate OHS, but was significantly increased compared to mild OHS.
The mean daily use of PAP therapy at the most recent visit was 6.0 ± 1.7 h/night. Most patients (95.9%) had objective usage of ≥ 4 h/night and 56.9% had objective usage of ≥ 6 h/ night. Patients who used bilevel PAP therapy also had greater PAP usage (6.3 ± 1.7 h/night versus 5.6 ± 1.6 h/night, P = .027). PAP usage was also significantly different among OHS severity groups (mild: 5.8 ± 1.4 h/night; moderate: 6.9 ± 1.9 h/night; severe: 5.9 ± 2.0 h/night; P = .03). The moderate group had higher usage compared to the mild group (P = .025).
No significant weight loss or improvement in spirometry tests were observed at the end of the follow-up period (data not shown). Two patients (1 mild, 1 severe) underwent weight reduction surgery, and both lost a significant amount of weight and no longer required PAP therapy. Although initially 45% of the patients required daytime and/or nocturnal supplemental oxygen, oxygen was discontinued in a significant proportion of patients (based on awake ABG levels, effective PAP therapy and nocturnal oximetry results) and only 8.9% were still on oxygen therapy at the end of the follow-up period.
Arterial Blood Gases
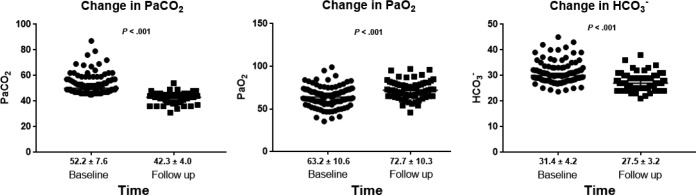
Table 3 describes the observed PaCO2, HCO3−, and PaO2 values as well as the ESS, BDI, and SF-36 scores at baseline, after the end of the follow-up period, and the mean change from baseline. At the end of the follow-up period, PaO2 had increased from baseline (P < .001) and both PaCO2 and HCO3− had decreased (P < .001) (Figure 2). Similar results were found in the subgroup of patients who never smoked (Table S2 in the supplemental material). In all three OHS groups separately, the comparison of pretreatment with the last posttreatment measurements demonstrated significant improvements in median PaCO2 values (P < .001), mean PaO2 values (P < .05), and mean HCO3− values (P < .005) (see supplemental material).
Table 3.
Comparison of ABG and questionnaire scores at baseline and at the end of the follow-up period for all patients.
Figure 2. Changes in ABG at baseline and at the end of the follow-up period.
Values presented as mean ± standard deviation below graph. ABG = arterial blood gas.
In stepwise multiple linear regression models, the magnitude of change in PaCO2 was associated only with PAP use (β = −1.5 mmHg per average hour of nightly PAP use [95% confidence interval (CI), −2.5, −0.5]; P = .007) and baseline PaCO2 (β = −0.87 [95% CI, −1.2, −0.5]; P < .001) after controlling for the potential confounders (Table S3 in the supplemental material). The change in PaO2 was related only to baseline PaO2 (β = −0.89 [95% CI −1.2, −0.57]; P < .001) and HCO3− change only with baseline HCO3− (β = −0.77 [95% CI −1.1, −0.5]; P < .001).
At the end of the study, 38 patients (18%) had daytime PaCO2 > 45 mmHg (median PaCO2 46.9 mmHg [46.03–48.78]). These patients had higher baseline daytime PaCO2 (54 versus 49 mmHg, P = .03), were categorized mostly as moderate to severe OHS at baseline (82%, P = .02) and were similar in age, sex, BMI, type of PAP therapy, spirometry tests, or presence of comorbidities (P > .05). Mean adherence to PAP therapy was 6.7 ± 1.5 h/night for patients who achieved normocapnia versus 5.7 ± 1.3 h/night for those who remained hypercapnic (P = .038). When we separately analyzed the clinical characteristics of the patients with persistent daytime PaCO2 > 45 mmHg, most of them had a follow-up PaCO2 of 45–49 mmHg, and 5 (2.3%) had a PaCO2 above 50 mmHg. These patients had optimal nighttime SpO2 correction and were clinically asymptomatic and consequently, PAP treatment was maintained.
Questionnaires Scores
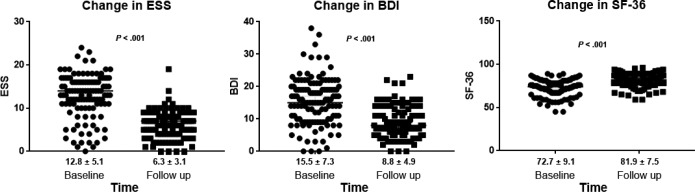
PAP therapy also significantly improved ESS (P < .001), BDI (P < .001), and SF-36 (P < .001) scores in all patients in the three OHS groups and in the subgroup of patients who never smoked (Table 3, Figure 3, and Table S2).
Figure 3. Changes in questionnaires scores at baseline and at the end of the follow-up period.
Values presented as mean ± standard deviation below graph. BDI = Beck Depression Inventory, ESS = Epworth Sleepiness Scale, SF-36 = Short Form 36 Health Survey.
Change in ESS was correlated only with baseline ESS (β = −0.78 [95% CI −0.89, −0.67]; P < .001). Change in BDI was associated with baseline BDI (β = −0.68 [95% CI −0.8, −0.6]; P < .001), baseline SF-36 (β = −0.16 [95% CI −0.25, −0.01]; P = .004), and awake PtcCO2 (β = −0.097 [95% CI −0.26, −0.03]; P = .041). Change in SF-36 was correlated with PAP use (β = 0.8 [95% CI 0.12, 1.45]; P = .007) and baseline SF-36 (β = −0.27 [95% CI −0.45, −0.16]; P < .001) (Table S4 in the supplemental material).
Subgroup Analysis by Hours of PAP Use
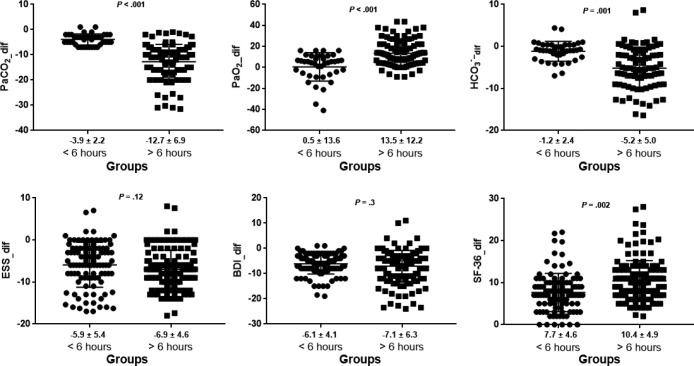
Because PAP use was among the strongest correlated factors in most of the aforementioned parameters, subgroup analysis was performed to compare changes in these parameters between patients with PAP use ≥ 6 and < 6 h/night. Baseline demographics, spirometric measurements, ABG analysis, and PSG parameters (Table S5 and Table S6 in the supplemental material) did not differ between these subgroups (P > .05). Patients who used PAP therapy ≥ 6 h/night had a considerably greater improvement in ABG and SF-36 score than patients who were less adherent (Table 4, Figure 4). Similar results were obtained in the subgroup of patients who never smoked (Table S7 in the supplemental material). Furthermore, the benefit of PAP adherence on PaCO2 and SF-36 seems to continue after 6 hours of PAP use (Table S8 in the supplemental material). The final PaCO2 normalized (< 45 mmHg) in 71% of patients with adherence below 6 h/night compared to 76% of patients with adherence equal or above 6 h/night (P = .18). In more adherent patients, the need for daytime home oxygen therapy decreased from 56% to 5% (P < .001), and in less adherent patients it decreased from 30% to 12% (P = .003). At least one serious cardiovascular event was diagnosed in 15.1% of the patients who used PAP therapy for < 6 h/ night compared to none of the more adherent patients during follow-up (P = .001).
Table 4.
Comparisons of changes in ABG and questionnaire scores over the follow-up according to PAP use per night.
Figure 4. Changes in ABG and in questionnaires scores according to PAP compliance.
Values presented as mean ± standard deviation below graph. ABG = arterial blood gas, BDI = Beck Depression Inventory, ESS = Epworth Sleepiness Scale, PAP = positive airway pressure, SF-36 = Short Form 36 Health Survey.
Mortality
The median follow-up was 30 months (24–52 months). During the entire observation period, a total of 11 deaths (5% of all patients) were recorded. The main cause of death (in 90%) was cardiovascular diseases (heart failure in two patients, sudden death in three patients, ischemic heart disease in three patients, and stroke in two patients). Other causes of death were cancer (10%).
With regard to diagnostic OHS subgroups, 2 patients (1.6%) in the mild OHS group died, whereas in the moderate-severe group, there were 9 deaths (9%). Baseline PaCO2 (r = −.2, P = .03), total sleep time spent with SaO2 < 90% (r = .23, P = .02), and hours of PAP use (r = −.26, P = .003) were found to predict mortality. The use of supplemental oxygen therapy was not associated with mortality. In a logistic regression model, after adjusting for important covariates, higher PAP adherence was independently associated with significantly reduced mortality (0.45; 95% CI 0.22, 0.92; P = .029). Patients who used PAP therapy for ≥ 6 h/night had a considerably lower mortality than less adherent patients (8.7% versus 2.5%, P = .038).
Impact of Type of PAP Therapy
Table 5 summarizes the baseline characteristics and outcomes of patients treated with CPAP (n = 84) or bilevel PAP (n = 141). Patients who used bilevel PAP therapy had a higher baseline PaCO2 level (P = .018) as well as awake and asleep PtcCO2 levels (P < .001). Baseline demographics, spirometric measurements, and PSG parameters did not differ between these subgroups (P > .05). Furthermore, patients who used bilevel PAP had greater PAP adherence (P = .027) and experienced a greater improvement in BDI score (P = .004). However, there was no difference in all the other outcomes as well as mortality between the two groups (P = .90).
Table 5.
Baseline characteristic and outcomes at the end of the study based on mode of PAP therapy.
DISCUSSION
This study investigated the effect of PAP treatment on clinical outcomes in a large number of clinically stable patients with OHS. The current results show that in patients with OHS, the use of PAP therapy for ≥ 6 h/night for a period of at least 2 years resulted in significant improvements in gas exchange, quality of life, and all-cause mortality.
A noteworthy aspect of the study was the diagnosis of OSA in most of the patients recruited, confirming the important role of obstructive events in the pathogenesis of hypoventilation in obese patients. Therefore, optimal therapy of chronic hypoventilation should not only focus on improvement of oxygen saturation and normalization of hypercapnia, but also in stabilizing the upper airway. Evidence supporting one mode of PAP therapy over another in patients with OHS and OSA is limited.28 Both continuous and bilevel PAP therapy modes can successfully improve daytime gas exchange.12,29–31 Importantly, adherence to PAP therapy is an important modifiable factor related to the response to treatment. Prior PAP trials have demonstrated that correction of OSA and nocturnal hypoventilation improves diurnal respiratory physiology, quality of life, and morbidity/mortality.28,30,31,32 Several small studies with 1 night or up to 3-month follow-up periods have evaluated such PAP interventions.29,30,33–35 The first prospective study on 23 patients was conducted by Banerjee et al., demonstrating the correction of obstructive events and improvement in sleep architecture during the first CPAP titration night, although a failure to correct nocturnal hypoxemia was noted in 43% of the patients.33 An observational study of 29 patients with OHS treated with CPAP found progressive improvements in mean nocturnal SpO2 and daytime CO2 over a 3-month period.34 Piper and coworkers29 performed a randomized trial that compared CPAP to bilevel PAP in spontaneous mode (no backup respiratory rate) for treatment of selected patients with OHS and OSA. A similar degree of improvement in daytime PaCO2, oxygen saturation, bicarbonate, and daytime sleepiness was noted at 3 months in both treatment groups. In a more recent clinical trial, Howard and colleagues30 compared CPAP to bilevel PAP in ST mode (with a backup respiratory rate) in 60 patients with stable OHS over a 3-month period and found no difference between the two PAP modalities in terms of quality of life, adherence to therapy, or improvement in daytime hypercapnia. In the largest randomized clinical trial (the Pickwick trial), Masa and colleagues randomized 221 patients with stable OHS to bilevel PAP therapy in volume-targeted mode that included a backup respiratory rate, CPAP, or lifestyle modification over a 2-month period. The authors found that both volume-targeted bilevel PAP therapy and CPAP achieved greater improvements in awake PaCO2 and bicarbonate when compared to lifestyle changes. However, there was no significant difference between the two PAP modalities.31 It is worth noting that bicarbonate and PaCO2 improvement with CPAP was dependent on treatment adherence.31 Indeed, similar to prior studies,36,37 we also found that adherence to PAP treatment was essential to obtain clinical benefits such as improvement in ABG and quality of life, irrespective of the modality of PAP therapy (ie, CPAP or bilevel PAP). Although PAP adherence was higher in our clinical cohort than some of the prior studies,30,31 it was similar to that in other studies.33,34 A previous retrospective study by Mokhlesi and associates12 found that the amount of improvement in daytime PaCO2 level is critically dependent on adherence to PAP therapy, regardless of the PAP modality (ie, CPAP or bilevel PAP). There was considerable variability but the PaCO2 level dropped on average by about 1.84 mmHg for every hour of nightly adherence and reached a plateau with longer than 7 hours of nightly use. In our study, the benefit of PAP adherence on PaCO2 level (an average drop of 1.5 mmHg per average hour of nightly PAP use) and SF-36 seems also to continue after 6 hours of nightly PAP use. Furthermore, Carter and colleagues35 in a substudy of a randomized controlled trial in OHS patients found that PAP adherence over a 6-week period had a marked effect on improving surges in nocturnal blood pressure control as well. Therefore, the importance of close clinical follow-up after PAP therapy is initiated should be stressed to ensure adequate adherence and confirm response to therapy, particularly because poor adherence to PAP therapy has also been associated with increased mortality.12,38
OHS has been associated with a fourfold increased mortality risk if untreated.7 Moreover, previous studies showed that patients with OHS may experience higher morbidity and mortality than patients who have OSA without OHS.38–40 Our study showed an even lower mortality rate among patients with OHS (5%) compared to previous studies,10,41–43 probably because of less severe cases of OHS and higher overall PAP adherence. PAP adherence < 6 h/night independently predicted mortality, irrespective of PAP mode, which seems intuitive because good adherence to PAP can improve prognosis, as reported by others.8,41 Surprisingly, mortality was not associated with ABG values, parameters related to obstructive respiratory events (ie, AHI), or gas exchange alterations during sleep, again emphasizing the effectiveness of nocturnal PAP use. Although supplemental oxygen therapy was the only independent predictor of mortality in the study by Priou et al.,41 we did not find an association between supplemental oxygen prescription and increased mortality. This difference may be related to difference in patient population (40.8% with heart disease in the study by Priou et al. versus 25% in our study) and different sample sizes. It is important to note that supplemental oxygen was actively discontinued in a large percentage of our patients. However, the use of supplemental oxygen therapy to prevent nocturnal oxygen desaturation is still an open question, with a more recent study suggesting that low-flow supplemental oxygen is safe at 2 months in patients with OHS as long as they are closely monitored.44 Further research is needed to examine whether long-term supplemental oxygen therapy increases the risk of cardiovascular morbidity and mortality in patients with OHS.
In line with prior studies, we also found improvements in subjective sleepiness (ESS score),8,13,29–31,34,36,41,45 depressive symptoms (BDI score),46 and quality of life (SF-36 score)29–31,36,37,46 at the end of the follow-up period. We have previously reported that in patients with OSA, > 6 h/night of PAP use is associated with a greater improvement in ESS, BDI, and SF-36 scores.25 In the current study, we only noted a significant association between PAP adherence and improvement in SF-36. The improvement in ESS and BDI scores were mostly related to the baseline values and not to PAP adherence. This may be in part related to the fact that we had high levels of adherence in most patients. This finding is also not surprising if we consider that sleepiness and depressive symptoms may be affected by many factors including socioeconomic status, occupation, and coexisting diseases.
Despite correction of these nocturnal obstructive events with PAP therapy, daytime PaCO2 did not return to normal in 18% of the patients. These patients had higher baseline daytime PaCO2, were categorized mostly as moderate to severe OHS at baseline (as assessed according to PaCO2 values), and had lower hours of PAP use. Nevertheless, these patients had optimal nighttime SpO2 correction and were clinically asymptomatic; consequently, PAP treatment was maintained. Furthermore, a significant proportion of patients will finally require supplemental oxygen in addition to PAP therapy, at least initially, in accordance with previous studies.8,12,30 Our study suggests that patients with moderate to severe OHS may be more likely to require nocturnal oxygen supplementation in addition to PAP therapy. There was no association between comorbidities such as heart failure and need for nocturnal oxygen supplementation, probably because of lower prevalence of heart disease in our cohort. In those requiring long-term oxygen supplementation, close clinical monitoring is necessary, as PAP adherence seems to be a better predictor of mortality than baseline PaO2 and PaCO2. Moreover, as demonstrated in our cohort, a large proportion of patients who are adherent to PAP therapy can successfully discontinue supplemental oxygen therapy. Therefore, early follow-up is imperative and should include repeat measurement of ABG and objective assessment of PAP adherence in order to assess readiness to discontinue supplemental oxygen therapy.
Despite the significant morbidity and mortality associated with this syndrome, diagnosis and initiation of effective treatment usually occurs late in the course of the disease. Unfortunately, OHS is often misdiagnosed as asthma or chronic obstructive pulmonary disease, even in hospitalized patients, delaying or preventing initiation of effective PAP therapy.7,47 Therefore, maintaining a high index of suspicion can lead to early recognition and treatment reducing the high burden of morbidity and mortality and related health care costs associated with undiagnosed and untreated OHS. Finally, separate from PAP treatment, successful management of OHS should consist of a multidisciplinary approach in order to effectively address the various facets of this complex condition including obesity, physical inactivity, and management of cardiometabolic comorbidities.48
Certain limitations of the current study must be addressed. A major limitation of this study is that the results are applicable mainly to the subset of stable patients with OHS with mild to moderate hypercapnia. This limits the applicability of the findings to patients with a higher degree of hypercapnia or those with clinical instability. Our findings may not be applicable to the 10% of patients with OHS who do not have concomitant OSA given that these patients represented a small number of the cohort. Another limitation is that the study was limited by a wide range of follow-up period. Furthermore, although we excluded patients with lung disease, a confounding factor in the selection process of our patients is that age, BMI, and smoking may influence gas exchange.49 However, our results were similar even when we only included never smokers. Last, the observational nature of our study precludes causal inferences. However, the findings from our longitudinal analyses strengthen our hypothesis that a higher level of adherence to PAP therapy is associated with better outcomes in patients with OHS.
In conclusion, higher level of adherence to PAP therapy is associated with better outcomes in patients with OHS. Clinicians should encourage adherence to PAP therapy in order to provide a significant improvement in clinical status and gas exchange in these patients.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- ABG
arterial blood gas
- AHI
apnea-hypopnea index
- BDI
Beck Depression Inventory
- BMI
body mass index
- ESS
Epworth Sleepiness Scale
- OHS
obesity hypoventilation syndrome
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PSG
polysomnography
- PtcCO2
transcutaneous CO2
- SF-36
Short Form 36 Health Survey
REFERENCES
- 1.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 2.Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the art-review. Respir Care. 2010;55(10):1347–1365. [PubMed] [Google Scholar]
- 3.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116(1):1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Piper A. Obesity hypoventilation syndrome: weighing in on therapy options. Chest. 2016;149(3):856–868. doi: 10.1378/chest.15-0681. [DOI] [PubMed] [Google Scholar]
- 5.Balachandran JS, Masa JF, Mokhlesi B. Obesity hypoventilation syndrome epidemiology and diagnosis. Sleep Med Clin. 2014;9(3):341–347. doi: 10.1016/j.jsmc.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc. 2008;5(2):218–225. doi: 10.1513/pats.200708-122MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116(1):1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Perez de Llano LA, Golpe R, Ortiz Piquer M, et al. Short-term and long-term effects of nasal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Chest. 2005;128(2):587–594. doi: 10.1378/chest.128.2.587. [DOI] [PubMed] [Google Scholar]
- 9.Masa JF, Celli BR, Riesco JA, Hernandez M, Sanchez De Cos J, Disdier C. The obesity hypoventilation syndrome can be treated with noninvasive mechanical ventilation. Chest. 2001;119(4):1102–1107. doi: 10.1378/chest.119.4.1102. [DOI] [PubMed] [Google Scholar]
- 10.Heinemann F, Budweiser S, Dobroschke J, Pfeifer M. Non-invasive positive pressure ventilation improves lung volumes in the obesity hypoventilation syndrome. Respir Med. 2007;101(6):1229–1235. doi: 10.1016/j.rmed.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 11.Chouri-Pontarollo N, Borel JC, Tamisier R, Wuyam B, Levy P, Pepin JL. Impaired objective daytime vigilance in obesity-hypoventilation syndrome: impact of noninvasive ventilation. Chest. 2007;131(1):148–155. doi: 10.1378/chest.06-1159. [DOI] [PubMed] [Google Scholar]
- 12.Mokhlesi B, Tulaimat A, Evans AT, et al. Impact of adherence with positive airway pressure therapy on hypercapnia in obstructive sleep apnea. J Clin Sleep Med. 2006;2(1):57–62. [PMC free article] [PubMed] [Google Scholar]
- 13.Damiani MF, Falcone VA, Carratù P, et al. Using PaCO2 values to grade obesity-hypoventilation syndrome severity: a retrospective study. Multidiscip Respir Med. 2017;12:14. doi: 10.1186/s40248-017-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, MA: New England Medical Center Hospital, Health Institute; 1994. [Google Scholar]
- 16.Ware JE, Jr, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):264–279. [PubMed] [Google Scholar]
- 17.Martinez TY, Pereira CA, dos Santos ML, Ciconelli RM, Guimarães SM, Martinez JA. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with idiopathic pulmonary fibrosis. Chest. 2000;117(6):1627–1632. doi: 10.1378/chest.117.6.1627. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7(0):151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 19.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- 20.Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31(3):160–168. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 22.Murphy PB, Davidson C, Hind MD, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax. 2012;67(8):727–734. doi: 10.1136/thoraxjnl-2011-201081. [DOI] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 25.Bouloukaki I, Giannadaki K, Mermigkis C, et al. Intensive versus standard follow-up to improve continuous positive airway pressure compliance. Eur Respir J. 2014;44(5):1262–1274. doi: 10.1183/09031936.00021314. [DOI] [PubMed] [Google Scholar]
- 26.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1993;147(4):887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 27.Weaver TE, Mailsin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce AM, Brown LK. Obesity hypoventilation syndrome: current theories of pathogenesis. Curr Opin Pulm Med. 2015;21(6):557–562. doi: 10.1097/MCP.0000000000000210. [DOI] [PubMed] [Google Scholar]
- 29.Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomized trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63(5):395–401. doi: 10.1136/thx.2007.081315. [DOI] [PubMed] [Google Scholar]
- 30.Howard M, Piper A, Stevens B, et al. A randomised controlled trial of CPAP versus non-invasive ventilation for initial treatment of obesity hypoventilation syndrome. Thorax. 2017;72(5):437–444. doi: 10.1136/thoraxjnl-2016-208559. [DOI] [PubMed] [Google Scholar]
- 31.Masa JF, Corral J, Alonso ML, et al. Efficacy of different treatment alternatives for obesity hypoventilation syndrome. Pickwick study. Am J Respir Crit Care Med. 2015;192(1):86–95. doi: 10.1164/rccm.201410-1900OC. [DOI] [PubMed] [Google Scholar]
- 32.Pépin JL, Borel JC, Janssens JP. Obesity hypoventilation syndrome: an underdiagnosed and undertreated condition. Am J Respir Crit Care Med. 2012;186(12):1205–1207. doi: 10.1164/rccm.201210-1922ED. [DOI] [PubMed] [Google Scholar]
- 33.Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome. Hypoxemia during continuous positive airway pressure. Chest. 2007;131(6):1678–1684. doi: 10.1378/chest.06-2447. [DOI] [PubMed] [Google Scholar]
- 34.Salord N, Mayos M, Miralda RM, et al. Continuous positive airway pressure in clinically stable patients with mild-to-moderate obesity hypoventilation syndrome and obstructive sleep apnoea. Respirology. 2013;18(7):1135–1142. doi: 10.1111/resp.12131. [DOI] [PubMed] [Google Scholar]
- 35.Carter JR, Fonkoue IT, Grimaldi D, et al. Positive airway pressure improves nocturnal beat-to-beat blood pressure surges in obesity hypoventilation syndrome with obstructive sleep apnea. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R602–R611. doi: 10.1152/ajpregu.00516.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hida W, Okabe S, Tatsumi K, et al. Nasal continuous positive airway pressure improves quality of life in obesity hypoventilation syndrome. Sleep Breath. 2003;7(1):3–12. doi: 10.1007/s11325-003-0003-1. [DOI] [PubMed] [Google Scholar]
- 37.Tsolaki V, Pastaka C, Kostikas K, et al. Noninvasive ventilation in chronic respiratory failure: effects on quality of life. Respiration. 2011;81(5):402–410. doi: 10.1159/000317138. [DOI] [PubMed] [Google Scholar]
- 38.Castro-Añón O, Pérez de Llano LA, De la Fuente Sánchez S, et al. Obesityhypoventilation syndrome: increased risk of death over sleep apnea syndrome. PLoS One. 2015;10(2):e0117808. doi: 10.1371/journal.pone.0117808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trakada GP, Steiropoulos P, Nena E, Constandinidis TC, Bouros D. Prevalence and clinical characteristics of obesity hypoventilation syndrome among individuals reporting sleep-related breathing symptoms in northern Greece. Sleep Breath. 2010;14(4):381–386. doi: 10.1007/s11325-010-0360-5. [DOI] [PubMed] [Google Scholar]
- 40.Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest. 2001;120(2):377–383. doi: 10.1378/chest.120.2.377. [DOI] [PubMed] [Google Scholar]
- 41.Priou P, Hamel JF, Person C, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest. 2010;138(1):84–90. doi: 10.1378/chest.09-2472. [DOI] [PubMed] [Google Scholar]
- 42.Budweiser S, Riedl SG, Jörres RA, Heinemann F, Pfeifer M. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med. 2007;261(4):375–383. doi: 10.1111/j.1365-2796.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 43.Marik PE, Chen C. The clinical characteristics and hospital and post-hospital survival of patients with the obesity hypoventilation syndrome: analysis of a large cohort. Obes Sci Pract. 2016;2(1):40–47. doi: 10.1002/osp4.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masa JF, Corral J, Romero A, et al. The effect of supplemental oxygen in obesity hypoventilation syndrome. J Clin Sleep Med. 2016;12(10):1379–1388. doi: 10.5664/jcsm.6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palm A, Midgren B, Janson C, Lindberg E. Gender differences in patients starting long-term home mechanical ventilation due to obesity hypoventilation syndrome. Respir Med. 2016;110:73–78. doi: 10.1016/j.rmed.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 46.Argun Baris S, Tuncel D, Ozerdem C, et al. The effect of positive airway pressure therapy on neurocognitive functions, depression and anxiety in obesity hypoventilation syndrome. Multidiscip Respir Med. 2016;11:35. doi: 10.1186/s40248-016-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marik PE, Desai H. Characteristics of patients with the “Malignant Obesity Hypoventilation Syndrome” admitted to an ICU. J Intens Care Med. 2013;28(2):124–130. doi: 10.1177/0885066612444261. [DOI] [PubMed] [Google Scholar]
- 48.Borel JC, Borel AL, Monneret D, Tamisier R, Levy P, Pepin JL. Obesity hypoventilation syndrome: from sleep-disordered breathing to systemic comorbidities and the need to offer combined treatment strategies. Respirology. 2012;17(4):601–610. doi: 10.1111/j.1440-1843.2011.02106.x. [DOI] [PubMed] [Google Scholar]
- 49.Glaser S, Ittermann T, Koch B, et al. Influence of smoking and obesity on alveolar-arterial gas pressures differences and dead space ventilation at rest and peak exercise in healthy men and women. Respir Med. 2013;107(6):919–926. doi: 10.1016/j.rmed.2013.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.