Abstract
Immune checkpoint inhibition used in combination with standard cisplatin-based chemotherapy regimens is currently under evaluation in clinical trials for head and neck squamous cell carcinoma (HNSCC). The impact of anti–PD-1 therapy on cisplatin-induced ototoxicity and nephrotoxicity has not been established. Here we use a murine model of cisplatin-induced hearing loss to investigate the impact of anti–PD-1 immunotherapy on auditory brainstem responses (ABRs), distortion product otoacoustic emissions (DPOAEs), serum creatinine, and hair cell and renal histology. We demonstrate only mild worsening of DPOAEs at 14.4 and 16 kHz as well as a mild increase in serum creatinine. Renal and hair cell histology as well as ABR measures were unchanged by PD-1 inhibition. Thus, our data suggest that the use of PD-1 inhibition in conjunction with cisplatin results in toxicities that are similar to those of cisplatin alone.
Keywords: cisplatin chemotherapy, PD-1, ototoxicity, nephrotoxicity
Antibodies targeting programmed cell death 1 (PD-1) immune checkpoint pathways have extended therapeutic options to treat recurrent and metastatic head and neck squamous cell carcinoma (HNSCC). Unfortunately, response rates in clinical trials are low, estimated at 13% to 18%.1,2 As most patients will not respond to PD-1 inhibition alone, combination with standard therapies is under exploration in preclinical and clinical studies.3
Cisplatin chemotherapy has historically been a staple in HNSCC management; thus, it is central to many of these combination therapies4,5 (Table 1). Cisplatin causes ototoxicity and nephrotoxicity in a significant number of patients,6,7 and otolaryngologists often see these patients for hearing loss or cancer recurrence due to intolerance of chemoradiotherapy. It is not known how concurrent PD-1 inhibition will affect cisplatin-induced toxicities. However, given that anti–PD-1 therapy may be expected to enhance cisplatin-induced inflammation, we hypothesized that PD-1 blockade might worsen cisplatin-induced toxicities, potentially causing treatment interruptions or affecting posttreatment quality of life. In the current study, we use a murine model of cisplatin-induced ototoxicity to evaluate both ototoxicity and nephrotoxicity in vivo.8,9 We show that PD-1 inhibition only mildly affects these cisplatin-induced toxicities.
Table 1.
Ongoing Clinical Trials Using Checkpoint Inhibitors in Combination with Cisplatin for Head and Neck Cancer Squamous Cell Carcinoma.
| Immunotherapy | Stage of Development | Study Design | Setting |
|---|---|---|---|
| Pembrolizumab | Phase II (NCT02641093) | Pembrolizumab + surgery + RT ± cisplatin | Primary HNSCC |
| Phase II (NCT02777385) | Pembrolizumab after CRT Pembrolizumab before and during CRT |
Primary HNSCC | |
| Phase III (NCT02358031) | Pembrolizumab Pembrolizumab + platinuma+ 5-FU Cetuximab + platinum + 5-FU |
Recurrent/metastatic HNSCC | |
| Phase I/II (NCT02759575) | Pembrolizumab + CRT | Primary laryngeal cancer | |
| Phase I (NCT02586207) | Pembrolizumab + CRT | Primary HNSCC | |
| Phase II (NCT02296684) | Neoadjuvant pembrolizumab + surgery (± adjuvant CRT + pembrolizumab) | Primary HCSCC | |
| Phase III (NCT03040999) | Pembrolizumab + CRT Placebo + CRT |
Primary HCSCC | |
| Phase II (NCT03114280) | Neoadjuvant pembrolizumab, docetaxel, 5-FU + cisplatin followed by RT + carboplatin | Primary unresectable HNSCC | |
| Phase I (NCT02819752) | Pembrolizumab + CRT in HPV-positive tumors Pembrolizumab + CRT in HPV-negative tumors |
Primary HNSCC | |
| Avelumab | Phase III (NCT02952586) | Avelumab + CRT Placebo + CRT |
Primary HNSCC |
| Durvalumab | Phase I (NCT02997332) | Durvalumab, docetaxel, cisplatin + 5-FU induction | Primary HNSCC |
| Nivolumab | Phase I (NCT02764593) | Neoadjuvant nivolumab + RT ± cisplatin or cetuximab | Primary HNSCC |
Abbreviations: CRT, chemoradiotherapy (cisplatin based); 5-FU, fluorouracil; RT, radiation therapy; HPV human papillomavirus; HNSCC, head and neck squamous cell carcinoma.
Platinum refers to regimens involving either carboplatin or cisplatin.
Materials and Methods
In Vivo Studies
All animal procedures were approved by the National Institute on Deafness and Other Communication Disorders (NIDCD) Animal Care and Use Committee. Forty 10- to 12-week-old CBA/CaJ mice (Jackson Laboratories, Bar Harbor, Maine) underwent hearing testing before and after treatment by recording of auditory brainstem responses (ABRs) and distortion product otoacoustic emissions (DPOAEs) as previously described.9,10 Animals were then randomized to treatment with cisplatin (n = 12), treatment with anti–PD-1 (n = 8), treatment with cisplatin and anti–PD-1 (n = 12), or no treatment (n = 8). Cisplatin treatment consisted of 2 treatment cycles of 3.5 mg/kg/d cisplatin intraperitoneal (IP) for 4 days followed by a 10-day recovery and 1 treatment cycle of 3.5 mg/kg/d cisplatin IP for 3 days followed by an 11-day recovery period for a total of 38.5 mg/kg cisplatin over 6 weeks. Rat anti-mouse PD-1 antibody (BioXCell, West Lebanon, New Hampshire) was administered at 200 mcg twice-weekly IP. Cisplatin-treated animals received fluid and nutritional supplementation as previously described.10
Tissue Preparation
Following euthanasia, cochleas were harvested, fixed, decalcified, microdissected, and tonotopically mapped as previously described.9,10 Immunohistochemistry was performed using mouse anti–myosin VIIa (1:100; Developmental Studies Hybridoma Bank, Iowa City, Iowa) followed by Alexa Fluor 546 donkey anti-mouse IgG (1:500; Invitrogen, Carlsbad, California). Blood was collected (postmortem only) and analyzed with a creatinine assay kit (ab65340; Abcam, Cambridge, Massachusetts) per manufacturer instructions. Kidneys were processed and stained with hematoxylin and eosin by Histoserv (Germantown, Maryland).
Statistical Analysis
Data were analyzed by 1- or 2-way analysis of variance (ANOVA) with Sidak’s multiple comparisons test where appropriate. GraphPad Prism software (GraphPad Software, La Jolla, California) was used for statistical testing. P < .05 was used to determine statistical significance.
Results
Of 40 mice that underwent baseline ABR and DPOAE testing, 5 animals were euthanized due to weight loss or deconditioning, and 4 succumbed to anesthesia during auditory testing. All control and anti–PD-1–treated animals survived, as did 7 of the 12 cisplatin-only and 8 of the 12 cisplatin + anti–PD-1–treated animals. Weight loss did not differ between cisplatin-treated and cisplatin + anti–PD-1–treated animals.
Ototoxicity was assessed functionally via ABR and DPOAE measures and histologically via hair cell counts (Figure 1). Anti–PD-1 treatment alone did not result in ototoxicity relative to room controls. The 38.5-mg/kg cisplatin regimen alone produced a robust hearing loss, most severe in high frequencies. Administration of anti–PD-1 antibody in combination with cisplatin resulted in statistically significant reduction of DPOAE amplitudes at 14.4 and 16 kHz vs cisplatin alone (Figure 1A,B). Differences in ABR threshold shifts (Figure 1C) and hair cell counts (Figure 1D,E) did not reach statistical significance. These data indicate that cisplatin results in severe hearing loss and that anti–PD-1 resulted in very minor worsening of cisplatin-induced hearing loss in this model.
Figure 1.
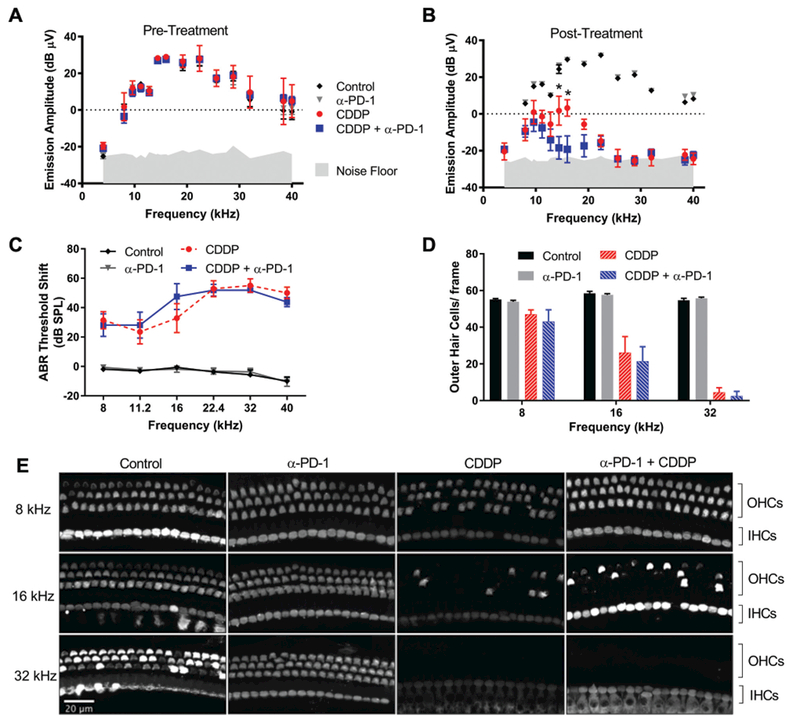
Anti–PD-1 minimally worsens cisplatin ototoxicity. (A-C) Distortion product otoacoustic emission (DPOAE)/auditory brainstem response (ABR) measures (n = 7 ears/7 mice). (D, E) Hair cell counts; images representative of ≥6 animals/condition. *p <0.05, cisplatin alone vs. with anti–PD-1; mean ± SEM. CDDP, cisplatin.
Serum creatinine and kidney histology were used to evaluate nephrotoxicity. Cisplatin or anti–PD-1 treatment alone did not affect serum creatinine. Combination therapy resulted in a slight but statistically significant increase in mean serum creatinine level (Figure 2A). None of the mice demonstrated obvious renal pathology on examination of histologic sections (Figure 2B), suggesting that any nephrotoxicity in this murine model was mild or had largely recovered by this time point.
Figure 2.
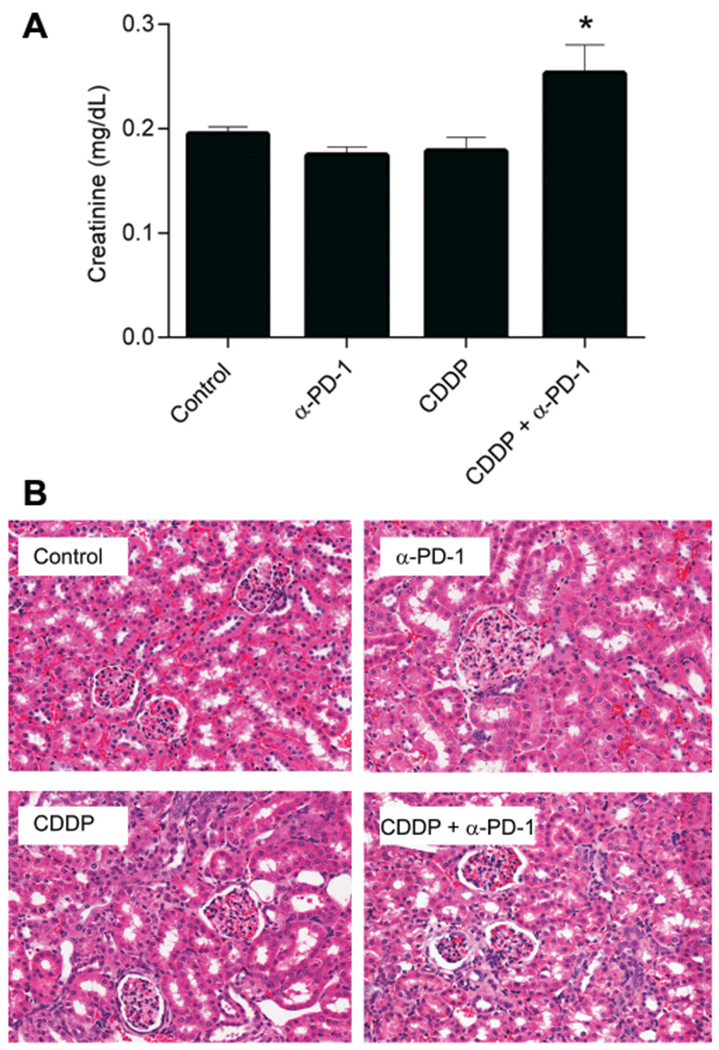
Cisplatin-induced renal toxicity is mildly exacerbated by anti–PD-1. (A) Serum creatinine (mean ± SEM), *P <.05 vs control and cisplatin alone. (B) Kidney histology, representative of ≥3 animals/condition. CDDP, cisplatin.
Discussion
An expanding body of evidence suggests that use of cisplatin in combination with immune checkpoint inhibitors may provide antineoplastic benefits beyond the use of these agents individually.11,12 Data from our study suggest that PD-1 inhibitors minimally affect cisplatin-induced toxicities. While the clinical relevance and mechanisms of such minor changes remain unknown, this information is pertinent, as clinical trials investigating these combinations for HNSCC are already under way. Otolaryngologists are likely to see increasing numbers of patients treated with chemoimmunotherapy in the future and should monitor such patients for hearing loss and other toxicities.
This study has several limitations, including mortality secondary to the cisplatin regimen and anesthesia. Toxicity analysis was limited to animals that tolerated the full regimen, and nephrotoxicity may have contributed directly to mortality. There was also a 3-week delay between the final cisplatin dose and postmortem serum collection. As such, the data may only reflect subacute renal toxicity. We speculate that the minor increase in serum creatinine seen in animals receiving combination therapy may reflect a more prolonged recovery from acute nephrotoxicity vs animals treated with cisplatin alone.
Conclusion
Data from a murine model suggest that cisplatin-induced ototoxicity and nephrotoxicity are only minimally affected by PD-1 inhibition. The clinical significance of these findings remains to be determined, and careful monitoring of cisplatin toxicities is warranted.
Acknowledgments
Disclosures
Competing interests: Nicole Schmitt, research funding from Astex Pharmaceuticals (not related to this work).
Sponsorships: None.
Funding source: Supported by the Division of Intramural Research at the National Institute on Deafness and Other Communication Disorders, projects ZIA-DC000079, ZIA-DC000090, and ZIC-DC000080. Intramural funding provided for the work.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- 1.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–965. [DOI] [PubMed] [Google Scholar]
- 3.Economopoulou P, Kotsantis I, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma: combinatorial immunotherapy approaches. ESMO Open. 2017; 1:e000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs G, Lyman E, Velez-Garcia K, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol 1992;10:257–263. [DOI] [PubMed] [Google Scholar]
- 5.Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 2004;350:1945–1952. [DOI] [PubMed] [Google Scholar]
- 6.Osanto S, Bukman A, Van Hoek F, et al. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol 1992;10:574–579. [DOI] [PubMed] [Google Scholar]
- 7.Reddel RR, Kefford RF, Grant JM, et al. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treat Rep 1982;66:19–23. [PubMed] [Google Scholar]
- 8.Roy S, Ryals MM, Van den Bruele AB, et al. Sound preconditioning therapy inhibits ototoxic hearing loss in mice. J Clin Invest 2013;123:4945–4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez K, Jeffers P, Lall K, et al. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci 2015;35:7509–7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breglio A, Rusheen A, Shide E, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun 2017;8:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyranvand N, Van der Sluis TC, Van Duikeren S, et al. Tumor eradication by cisplatin is sustained by CD80/86-mediated costimulation of CD81 T cells. Cancer Res 2016; 76:6017–6029. [DOI] [PubMed] [Google Scholar]
- 12.Tran L, Allen C, Xiao R, et al. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol Res 2017;5:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]


