Abstract
AIM
To characterize the clinical course and outcomes of nasal intermittent mandatory ventilation (NIMV) use in acute pediatric respiratory failure.
METHODS
We identified all patients treated with NIMV in the pediatric intensive care unit (PICU) or inpatient general pediatrics between January 2013 and December 2015 at two academic centers. Patients who utilized NIMV with other modes of noninvasive ventilation during the same admission were included. Data included demographics, vital signs on admission and prior to initiation of NIMV, pediatric risk of mortality III (PRISM-III) scores, complications, respiratory support characteristics, PICU and hospital length of stays, duration of respiratory support, and complications. Patients who did not require escalation to mechanical ventilation were defined as NIMV responders; those who required escalation to mechanical ventilation (MV) were defined as NIMV non-responders. NIMV responders were compared to NIMV non-responders.
RESULTS
Forty-two patients met study criteria. Six (14%) failed treatment and required MV. The majority of the patients (74%) had a primary diagnosis of bronchiolitis. The median age of these 42 patients was 4 mo (range 0.5-28.1 mo, IQR 7, P = 0.69). No significant difference was measured in other baseline demographics and vitals on initiation of NIMV; these included age, temperature, respiratory rate, O2 saturation, heart rate, systolic blood pressure, diastolic blood pressure, and PRISM-III scores. The duration of NIMV was shorter in the NIMV non-responder vs NIMV responder group (6.5 h vs 65 h, P < 0.0005). Otherwise, NIMV failure was not associated with significant differences in PICU length of stay (LOS), hospital LOS, or total duration of respiratory support. No patients had aspiration pneumonia, pneumothorax, or skin breakdown.
CONCLUSION
Most of our patients responded to NIMV. NIMV failure is not associated with differences in hospital LOS, PICU LOS, or duration of respiratory support.
Keywords: Continuous positive airway pressure, Pediatric, Noninvasive positive pressure ventilation, Nasal intermittent mandatory ventilation, High flow nasal cannula, Acute respiratory failure, Bilevel positive airway pressure
Core tip: In our cohort of patients between 0.5 and 28.1 mo of age with acute respiratory failure, the majority of patients were successfully supported with nasal intermittent mandatory ventilation (NIMV) alone or NIMV in conjunction with other modes of noninvasive ventilation (NIV). Use of NIMV with or without NIV was not associated with significant differences in hospital length of stay (LOS), pediatric intensive care unit LOS, or duration of respiratory support. Failure of NIMV with or without NIV was recognized in a median of 6.5 h.
INTRODUCTION
Acute respiratory failure accounts for 46% to 59% of unplanned admissions to the pediatric intensive care unit (PICU) with 68% of these patients requiring advanced respiratory support[1-3]. While endotracheal intubation with mechanical ventilation (MV) is the classic management of respiratory failure, noninvasive ventilation (NIV) is rapidly gaining acceptance as a first line intervention. Continuous positive airway pressure (CPAP), bilevel positive airway pressure (BIPAP), high flow nasal cannula (HFNC), and nasal intermittent mandatory ventilation (NIMV) are examples of NIV.
NIMV is a time-cycled, time-triggered pressure control mode of non-invasive ventilation typically administered through nasal prongs or nasal mask via mechanical ventilator. Unlike BIPAP or endotracheal mechanical ventilation, mandatory breaths on NIMV are often not synchronized to patient breaths, though newer ventilators may synchronize to diaphragmatic stimulation (e.g., Noninvasive neurally adjusted ventilatory assist) or when a negative inspiratory pressure threshold is reached.
NIV modalities have been shown to prevent intubation and reintubation in the adult and neonatal population[4,5]. Relatively few studies have assessed its efficacy in the pediatric critical care setting[6-10]. NIMV in particular is infrequently used outside of the neonatal intensive care unit (NICU) and, to our knowledge, has not been studied in the setting of acute respiratory failure in the PICU. In the absence of data from robust studies, we reviewed our experience with NIMV in critically ill pediatric patients and describe the clinical characteristics and outcomes of pediatric patients with acute respiratory failure who were treated with NIMV.
MATERIALS AND METHODS
Inclusion and exclusion criteria
We performed a retrospective chart review of children between 1 d to 28 mo of age in acute respiratory failure admitted to the PICU or intermediate-level pediatric unit of two academic medical centers between January 2013 and December 2015. Institutional review board approval was obtained at each site.
Patients were identified through ICD codes associated with acute respiratory failure and CPT codes for endotracheal intubation or NIV. NIV modalities reviewed include NIMV, HFNC, CPAP, and BIPAP. All patients treated with NIMV were individually reviewed. Due to the paucity of patients utilizing NIMV alone, patients treated with other modes of NIV in series with NIMV during a single admission were included. Exclusion criteria were patients who did not utilize NIMV during hospitalization, managed in the NICU, chronic CPAP or BIPAP dependence, tracheostomy dependence, and post-extubation NIV.
Materials
NIV and NIMV settings were initiated and titrated at clinician discretion based on the patient’s clinical response. NIMV was administered via RAM cannula (Neotech Products, Valencia, CA) nasal prongs sized according to the child’s age and weight and connected to a humidified Avea ventilator (CareFusion, Franklin Lakes, NJ) in NIMV mode. This mode of ventilation was asynchronous to patient breaths at both our institutions. The decision to escalate to invasive mechanical ventilation was made at the discretion of the physician.
Data
Data included (1) demographics--age in days, gender, admission, discharge weight and discharge diagnosis; (2) vital signs on initiation of and with any changes in NIV; (3) characteristics of respiratory support--modality, length of time on each modality, maximum settings (delta P, FIO2, PEEP, and mandatory rate); (4) complications--aspiration pneumonia, pneumothorax, skin breakdown; and (5) outcome data-MV, mortality, pediatric risk of mortality III(PRISM-III) scores, and hospital and PICU length of stay. Respiratory support characteristics were recorded hourly in PICU site A and every 4 h in the pediatric units of site A and PICU site B.
Statistical analysis
Successful NIMV treatment was defined as use of NIMV without the use of invasive mechanical ventilation. Patients successfully treated with NIMV (NIMV responders) were compared to those unsuccessfully treated with NIMV, which we defined as escalation to MV (NIMV non-responders).
Standard descriptive statistics were reported. Medians (min-max, IQR) were analyzed for numerical variables and frequency count (%) for categorical variables. Fisher’s Exact tests were used for the comparison of categorical characteristics and Wilcoxon rank sum tests were used for the comparison of numerical characteristics. P values less than 0.05 were considered statistically significant. All statistical analysis was performed by a biomedical statistician who utilized SAS v9.4 (SAS Institute Inc., Cary, NC).
RESULTS
During our study period, 1164 charts were initially reviewed and 1122 were removed based on exclusion criteria. Forty-two patients used NIMV during their hospital admission. Figures 1 and 2 illustrate the selection process and the sequence of escalation of respiratory support, respectively.
Figure 1.
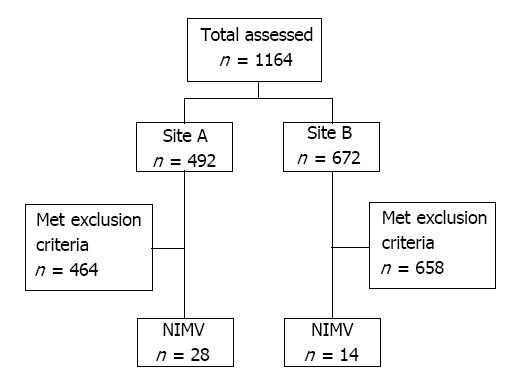
Patient selection by institution.NIMV: Nasal intermittent mandatory ventilation.
Figure 2.
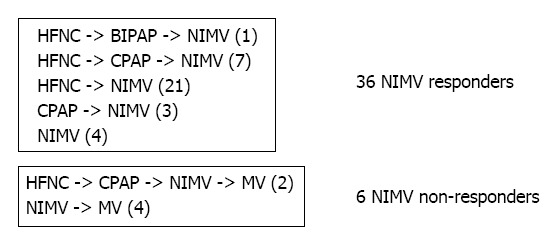
Escalation of respiratory support modalities1. 1Progression of respiratory support indicated by arrows and number of patients (in parentheses) following each pathway. HFNC: High flow nasal cannula; BIPAP: Bilevel positive airway pressure; NIMV: Nasal intermittent mandatory ventilation; CPAP: Continuous positive airway pressure; MV: Mechanical ventilation.
Comparison of NIMV responders and NIMV non-responders
In our 42 patients who received NIMV, 36 (86%) were successfully supported without further escalation, while 6 (14%) subsequently required endotracheal intubation and MV after trial of NIMV with or without other modes of NIV. Eight (19%) of the 42 patients used NIMV exclusively; of these 8 patients, 4 (50%) failed and required mechanical ventilation. Except for the patients requiring MV, NIMV was used as the final mode of noninvasive respiratory support in patients treated with more than one NIV modality (Figure 2).
The median age of these 42 patients was 4 mo (range 0.5-28.1 mo, IQR 7). Half of the patients were males. The leading discharge diagnosis was bronchiolitis (n = 31, 74%). Demographics (weight, age, gender), PRISM-III scores on admission, and vitals prior to initiation of NIMV (heart rate, respiratory rate, blood pressure, oxygen saturation, temperature) between these two subgroups were similar (Table 1). The distribution of diagnoses was similar between NIMV responders and NIMV non-responders (Table 2).
Table 1.
Comparison of nasal intermittent mandatory ventilation responders to nasal intermittent mandatory ventilation non-responders - baseline characteristics1
| Baseline characteristic | NIMV responders (n = 36) | NIMV non-responders (n = 6) | Total (n = 42) | P value |
| Age (mo) | 4 (0.5-20, IQR = 6.4) | 5.2 (0.5-28.1, IQR = 12.6) | 4 (0.5-28.1, IQR = 7) | 0.69 |
| Admission weight (kg) | 4.9 (2.3-12, IQR = 4.3) | 7.1 (2.6-10.9, IQR = 4.8) | 5.1 (2.3-12, IQR = 4.4) | 0.39 |
| T Max in Celsius (range) | 38 (36.6-40, IQR = 1.6) | 38.9 (37.4-39.7, IQR = 1.7) | 38.1 (36.6-40, IQR = 1.6) | 0.2 |
| Respiratory rate (in breaths per minute) | 60 (24-97, IQR = 19) | 56 (42-77, IQR = 32) | 60 (24-97, IQR = 22) | 0.76 |
| O2 saturation (%) | 98 (68-100, IQR = 5.5) | 99 (98-100, IQR = 2) | 98.5 (68-100, IQR = 5) | 0.31 |
| Heart rate (beats per minute) | 152.5 (95-205, IQR = 34.5) | 158.5 (127-210, IQR = 24) | 153.5 (95-210, IQR = 33) | 0.99 |
| Systolic blood pressure (mmHg) | 100 (71-129, IQR = 21) | 112 (81-120, IQR = 25) | 103 (71-129, IQR = 20) | 0.3 |
| Diastolic blood pressure (mmHg) | 51 (37-84, IQR = 20) | 59.5 (43-81, IQR = 25) | 51 (37-84, IQR = 22) | 0.41 |
| PRISM-III score | 0 (0-11, IQR = 0) | 1 (0-7, IQR = 3) | 0 (0-11, IQR = 0) | 0.08 |
1Vital signs were the last recorded values before initiation of NIMV. Values are presented as median (range, IQR). NIMV: Nasal intermittent mandatory ventilation; PRISM-III: Pediatric risk of mortality III.
Table 2.
Diagnoses causing acute respiratory failure in nasal intermittent mandatory ventilation responders vs nasal intermittent mandatory ventilation non-responders1
| Diagnosis | NIMV responders n (%) | NIMV non-responders n (%) | Total (%) | P value |
| Asthma exacerbation | 1 (3) | 2 (33) | 3 (7) | 0.11 |
| Bronchiolitis | 28 (78) | 3 (50) | 31 (74) | |
| Heart failure | 1 (3) | 0 (0) | 1 (2) | |
| Pneumonia | 3 (8) | 1 (17) | 4 (10) | |
| Viral syndrome | 3 (8) | 0 (0) | 3 (7) |
1Values expressed as number (percent). NIMV: Nasal intermittent mandatory ventilation.
We observed no significant difference in maximum NIMV settings (delta P, FIO2, PEEP, and rate), time to escalation to maximum settings, hospital and PICU length of stay, or in total duration of all respiratory support between NIMV responders and non-responders, or those that required MV (Table 3). However, NIMV responders remained on this mode of support for a greater length of time than those who failed (65 h vs 6.5 h, P < 0.0005).
Table 3.
Comparison of nasal intermittent mandatory ventilation responders to nasal intermittent mandatory ventilation non-responders - maximum support and clinical outcomes1
| Support or outcome variable | NIMV responders (n = 36) | NIMV non-responders (n = 6) | Total (n = 42) | P value |
| Max delta P of NIMV (cmH2O) | 16 (6-26, IQR = 6.5) | 17.5 (10-30, IQR = 6) | 16.5 (6-30, IQR = 6) | 0.68 |
| Max FIO2 of NIMV (%) | 40 (25-100, IQR = 22.5) | 42.5 (30-70, IQR = 10) | 40 (25-100, IQR = 15) | 0.84 |
| Max PEEP of NIMV (cmH2O) | 6 (5-8, IQR = 2) | 5 (5-8, IQR = 2) | 6 (5-8, IQR = 2) | 0.37 |
| Max rate of NIMV (breaths per minute) | 30 (20-70, IQR = 3.5) | 30 (20-60, IQR = 5) | 30 (20-70, IQR = 2) | 0.32 |
| Time to max FIO2 (h) | 0 (0-119, IQR = 1.5) | 0 (0-29.5, IQR = 6) | 0 (0-119, IQR = 2) | 0.75 |
| Time to max setting (h) | 1 (0-48, IQR = 9) | 3 (0-29.5, IQR = 20) | 1 (0-48, IQR = 10) | 0.8 |
| Hospital length of stay (d) | 7 (3-30, IQR = 3.5) | 9 (4-14, IQR = 7) | 7.5 (3-30, IQR = 4) | 0.73 |
| PICU length of stay (d) | 6 (3-30, IQR = 3) | 6.5 (3-14, IQR = 5) | 6 (3-30, IQR = 3) | 0.64 |
| Total duration of NIMV (h) | 65 (5-240, IQR = 47.5) | 6.5 (0.5-30, IQR = 24) | 59.5 (0.5-240, IQR = 53) | 0.001 |
| Total duration of all respiratory support (h) | 94.5 (28-254, IQR = 60) | 115 (65-230, IQR = 31.5) | 95.8 (28-254, IQR = 57) | 0.3 |
1Values are presented as median (range, IQR). NIMV: Nasal intermittent mandatory ventilation; PICU: Pediatric intensive care unit.
DISCUSSION
In our cohort of pediatric patients with acute respiratory failure treated with NIMV with or without other NIV modalities, 86% did not require MV. This rate is similar to data on heterogenous modes of NIV modalities in the PICU described in separate studies conducted by Yaman et al[7], Milési et al[8], and Wolfler et al[11] in the PICU. To our knowledge this is the first study that characterizes the patients, pathologies, and clinical outcomes of NIMV for acute respiratory failure in pediatric patients outside of the NICU. The strongest evidence for NIMV in the pediatric population to date are limited to pathologies encountered in the NICU; for example, it shows significant clinical benefit over other modes of NIV in neonatal respiratory distress syndrome, apnea of prematurity, and the prevention of post-extubation failure[4,12,13]. In discordance with the NICU literature, a recent prospective study of NIV for post-extubation support in the PICU showed no difference in respiratory effort when compared between NIMV, HFNC, and CPAP, though NIMV in this study was synchronized to approximately 50%[14].
An ongoing concern of NIV is delay in endotracheal intubation and MV that may lead to worsening physiologic status at time of intubation and thus worse clinical outcomes[15]. Our data do not support this hypothesis. We revealed substantially less time on NIMV in the cohort that proceeded to MV compared to those that responded successfully to NIMV. Our observed median time to intubation of 6.5 h in NIMV nonresponders was similar to treatment failure observed by another study utilizing mask BIPAP in adults[11]. Additionally, similarities in clinical outcomes of hospital length of stay, PICU length of stay, and complications suggest that recovery time may be independent of the mode of respiratory support. There was a single mortality due to an uncorrectable congenital lung pathology.
MV is associated with a number of challenges and complications such as sedation, paralysis, polyneuropathies, iatrogenic pneumonia, chemical pneumonitis, soft tissue trauma, pneumothorax, and other lung injuries[16]. Unlike MV, NIV modalities have three to fivefold fewer rates of these complications, particularly ventilator associated pneumonia and barotrauma[17,18]. Non-invasive ventilation also reduces complications such as mortality and nosocomial infections[7,17,19]. None of our patients had aspiration pneumonia, pneumothorax, barotrauma, or soft tissue injury associated with NIMV use. Additionally, only one patient required mild sedation with an oral benzodiazepine during NIMV support.
The clinical application of NIMV or any NIV is in the hope of avoiding MV. The general practice in our two divisions is to apply NIMV as a last-resort modality prior to MV in young infants. We confirmed this practice pattern in our observation that NIMV, when applied, was used as the final means of noninvasive respiratory support (Figure 2).
Our study is limited in that this was a non-randomized, non-protocolized, retrospective review of chart data with a limited sample size, thus rendering the power of our study low. Limitations in NIMV experience in our PICUs precluded protocols for its application. There is no head-to-head randomization and comparison between other NIV modalities. Treatment with several non-invasive modalities limits the ability to extrapolate the contribution of each mode to successful support or failure. This aspect also limits our data on NIMV alone. Lastly, the criteria to initiate NIV, modify mode of NIV, and decision to intubate was based on clinician judgment and not protocolized.
Despite these limitations, our study provides the first retrospective analysis of outcomes associated with NIMV use in pediatric acute respiratory failure at two academic institutions that are widely disparate geographically. Future goals include verification of this data with a larger cohort and protocolized escalation of respiratory support. Larger and multicenter prospective studies may identify useful clinical parameters that may assist in the identification of patients who may benefit from NIMV. Future goals may include randomization of patients to NIMV alone vs other modes of NIV.
NIMV successfully supported 86% of pediatric patients with acute respiratory failure. The remaining patients who failed NIMV did not have a longer PICU, hospital LOS, or total duration of respiratory support when compared to those successfully supported with NIMV. NIMV failure was recognized within a median of 6.5 h, therefore the use of NIMV did not delay escalation to endotracheal intubation
ARTICLE HIGHLIGHTS
Research background
Nasal intermittent mandatory ventilation (NIMV) is a mode of noninvasive ventilation (NIV) seldomly utilized outside of the neonatal intensive care unit (NICU). To our knowledge NIMV has not been studied in the pediatric intensive care unit (PICU) population.
Research motivation
Acute respiratory failure requiring advanced respiratory support accounts for a large proportion of PICU admissions. NIV is rapidly gaining acceptance as the first mode of oxygenation and ventilatory support for many of these patients. The potential use of NIMV adds to the arsenal of respiratory support strategies. Its success could obviate the need for mechanical ventilation in some patients.
Research objective
Our primary objectives were to review our experience with NIMV-both alone and in conjunction with other modes of NIV-and describe our patient outcome data and compare with existing literature. In particular our interests were intubation rate, PICU length of stay, hospital length of stay, duration of respiratory support, and complications.
Research methods
During our study period, we identified all patients who utilized NIMV with or without other modes of NIV at two academic institutions. We excluded patients in the NICU, those dependent on chronic continuous positive airway pressure (CPAP) or bilevel positive airway pressure or tracheostomy, and post-extubation NIV. Data included demographics, vitals, characteristics of respiratory support, diagnoses, complications, and outcome data. Patients who did not require escalation to mechanical ventilation (MV) were defined as NIMV responders; those who required escalation to MV were defined as NIMV non-responders. NIMV responders were compared to NIMV non-responders. Standard descriptive statistics are used. All statistical analyses were run by a certified biostatistician using SAS v9.4.
Research results
We identified 42 patients during our three-year study period. Median age of these patients was 4 mo. The majority of patients had a primary diagnosis of bronchiolitis. Six failed NIMV. Baseline demographics, vitals, diagnoses, and pediatric risk of mortality III scores were similar between NIMV responders and NIMV non-responders. However, NIMV non-responders were on this mode of ventilation for a significantly shorter period of time. Outcome data including hospital length of stay, PICU length of stay, and duration of respiratory support were similar between the two groups. No patients had aspiration pneumonia, pneumothorax, or skin breakdown associated with NIMV. There was a single mortality due to an uncorrectable and fatal lung pathology.
Research conclusions
NIMV was utilized in pediatric patients with acute respiratory failure and successfully supported the majority of our patients. Failure of NIMV was quickly identified in a median of 6.5 h. Patients who required intubation did not have a longer PICU length of stay, hospital length of stay, or total duration of respiratory support when compared to those successfully supported with NIMV.
Research perspectives
Based on our data, NIMV appears to be a promising mode of noninvasive respiratory support. Future goals include prospective, and randomized studies to describe and evaluate the efficacy of NIMV.
Footnotes
Institutional review board statement: This study was approved by the UCLA Institutional Review Board.
Conflict-of-interest statement: All authors have no conflicts of interest to report.
STROBE statement: The STROBE Statement have been adopted.
Manuscript source: Invited manuscript
Peer-review started: June 2, 2018
First decision: July 9, 2018
Article in press: August 5, 2018
Specialty type: Critical care medicine
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Adrish M, Inchauspe AA S- Editor: Ma YJ L- Editor: A E- Editor: Wu YXJ
Contributor Information
Billy C Wang, Department of Pediatrics, Division of Critical Care Medicine, Loma Linda University Children’s Hospital, Loma Linda, CA 92354, United States. bcwang@llu.edu.
Theodore Pei, Department of Pediatrics, Division of Pediatric Critical Care, Floating Hospital for Children at Tufts, Boston, MA 02111, United States.
Cheryl B Lin, Department of Pediatrics, Division of Pediatric Critical Care, Floating Hospital for Children at Tufts, Boston, MA 02111, United States.
Rong Guo, Department of Medicine, Biostatistics Core, UCLA David Geffen School of Medicine, Los Angeles, CA 90024, United States.
David Elashoff, Department of Medicine, Biostatistics Core, UCLA David Geffen School of Medicine, Los Angeles, CA 90024, United States.
James A Lin, Department of Pediatrics, Mattel Children’s Hospital at UCLA, Los Angeles, CA 90095, United States.
Carol Pineda, Department of Pediatrics, Division of Pediatric Critical Care, Floating Hospital for Children at Tufts, Boston, MA 02111, United States.
References
- 1.Krmpotic K, Lobos AT. Clinical profile of children requiring early unplanned admission to the PICU. Hosp Pediatr. 2013;3:212–218. doi: 10.1542/hpeds.2012-0081. [DOI] [PubMed] [Google Scholar]
- 2.Mukhija G, Chandra S. Clinical profile of patients admitted to the PICU of a tertiary care teaching hospital. Int J Pediatr Res. 2017;4:127–129. [Google Scholar]
- 3.Society of Critical Care Medicine. Critical Care Statistics. Available from: http://www.sccm.org/Communications/Pages/CriticalCareStats.aspx.
- 4.Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst Rev. 2014;9:CD003212. doi: 10.1002/14651858.CD003212.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of Noninvasive Ventilation Delivered by Helmet vs Face Mask on the Rate of Endotracheal Intubation in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA. 2016;315:2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JV, Ramnarayan P, Parslow RC, Fleming SJ. Outcomes for Children Receiving Noninvasive Ventilation as the First-Line Mode of Mechanical Ventilation at Intensive Care Admission: A Propensity Score-Matched Cohort Study. Crit Care Med. 2017;45:1045–1053. doi: 10.1097/CCM.0000000000002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaman A, Kendirli T, Ödek Ç, Ateş C, Taşyapar N, Güneş M, İnce E. Efficacy of noninvasive mechanical ventilation in prevention of intubation and reintubation in the pediatric intensive care unit. J Crit Care. 2016;32:175–181. doi: 10.1016/j.jcrc.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Milési C, Essouri S, Pouyau R, Liet JM, Afanetti M, Portefaix A, Baleine J, Durand S, Combes C, Douillard A, Cambonie G; Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP) High flow nasal cannula (HFNC) versus nasal continuous positive airway pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: a multicenter randomized controlled trial (TRAMONTANE study) Intensive Care Med. 2017;43:209–216. doi: 10.1007/s00134-016-4617-8. [DOI] [PubMed] [Google Scholar]
- 9.Mayordomo-Colunga J, Medina A, Rey C, Díaz JJ, Concha A, Los Arcos M, Menéndez S. Predictive factors of non invasive ventilation failure in critically ill children: a prospective epidemiological study. Intensive Care Med. 2009;35:527–536. doi: 10.1007/s00134-008-1346-7. [DOI] [PubMed] [Google Scholar]
- 10.Bernet V, Hug MI, Frey B. Predictive factors for the success of noninvasive mask ventilation in infants and children with acute respiratory failure. Pediatr Crit Care Med. 2005;6:660–664. doi: 10.1097/01.pcc.0000170612.16938.f6. [DOI] [PubMed] [Google Scholar]
- 11.Wolfler A, Calderini E, Iannella E, Conti G, Biban P, Dolcini A, Pirozzi N, Racca F, Pettenazzo A, Salvo I; Network of Pediatric Intensive Care Unit Study Group. Evolution of Noninvasive Mechanical Ventilation Use: A Cohort Study Among Italian PICUs. Pediatr Crit Care Med. 2015;16:418–427. doi: 10.1097/PCC.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 12.Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics. 2001;108:13–17. doi: 10.1542/peds.108.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Lemyre B, Davis PG, de Paoli AG. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for apnea of prematurity. Cochrane Database Syst Rev. 2002;1:CD002272. doi: 10.1002/14651858.CD002272. [DOI] [PubMed] [Google Scholar]
- 14.Kamerkar A, Hotz J, Morzov R, Newth CJL, Ross PA, Khemani RG. Comparison of Effort of Breathing for Infants on Nasal Modes of Respiratory Support. J Pediatr. 2017;185:26–32.e3. doi: 10.1016/j.jpeds.2017.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teague WG. Noninvasive ventilation in the pediatric intensive care unit for children with acute respiratory failure. Pediatr Pulmonol. 2003;35:418–426. doi: 10.1002/ppul.10281. [DOI] [PubMed] [Google Scholar]
- 16.Principi T, Fraser DD, Morrison GC, Farsi SA, Carrelas JF, Maurice EA, Kornecki A. Complications of mechanical ventilation in the pediatric population. Pediatr Pulmonol. 2011;46:452–457. doi: 10.1002/ppul.21389. [DOI] [PubMed] [Google Scholar]
- 17.Nourdine K, Combes P, Carton MJ, Beuret P, Cannamela A, Ducreux JC. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med. 1999;25:567–573. doi: 10.1007/s001340050904. [DOI] [PubMed] [Google Scholar]
- 18.Carron M, Freo U, BaHammam AS, Dellweg D, Guarracino F, Cosentini R, Feltracco P, Vianello A, Ori C, Esquinas A. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110:896–914. doi: 10.1093/bja/aet070. [DOI] [PubMed] [Google Scholar]
- 19.McCurdy BR. Noninvasive positive pressure ventilation for acute respiratory failure patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser. 2012;12:1–102. [PMC free article] [PubMed] [Google Scholar]


