Abstract
AIM
To study exercise capacity and determinants of early peak oxygen consumption (VO2peak) in a cohort of de novo heart transplant (HTx) recipients.
METHODS
To determine possible central (chronotropic responses, cardiopulmonary and hemodynamic function) and peripheral factors (muscular exercise capacity and body composition) predictive of VO2peak, a number of different measurements and tests were performed, as follows: Cardiopulmonary exercise testing (CPET) was performed mean 11 wk after surgery in 81 HTx recipients > 18 years and was measured with breath by breath gas exchange on a treadmill or bicycle ergometer. Metabolic/respiratory measures include VO2peak and VE/VCO2 slope. Additional measures included muscle strength testing, bioelectrical impedance analysis, echocardiography, blood sampling and health-related quality of life. Based on the VO2peak (mL/kg per minute) median value, the study population was divided into two groups defined as a low-capacity group and a high-capacity group. Potential predictors were analyzed using multiple regression analysis with VO2peak (L/min) as the dependent variable.
RESULTS
The mean ± standard deviation (SD) age of the total study population was 49 ± 13 years, and 73% were men. This de novo HTx cohort demonstrated a median VO2peak level of 19.4 mL/kg per min at 11 ± 1.8 wk post-HTx. As compared with the high-capacity group, the low-capacity group exercised for a shorter time, had lower maximal ventilation, O2 pulse, peak heart rate and heart rate reserve, while the VE/VCO2 slope was higher. The low-capacity group had less muscle strength and muscular exercise capacity in comparison with the high-capacity group. In order of importance, O2 pulse, heart rate reserve, muscular exercise capacity, body mass index, gender and age accounted for 84% of the variance in VO2peak (L/min). There were no minor or major serious adverse events during the CPET.
CONCLUSION
Although there is great individual variance among de novo HTx recipients, early VO2peak measures appear to be influenced by both central and peripheral factors.
Keywords: Cardiopulmonary exercise testing, Early VO2peak, De novo heart transplant, Health related quality of life, Muscle strength
Core tip: This de novo heart transplant (HTx) cohort demonstrated a median peak oxygen consumption (VO2peak) level of 19.4 mL/kg per min at 11 ± 1.8 wk post-HTx, which is comparable to what is shown in maintenance HTx recipients. VO2peak in this study was determined by both central and peripheral factors. The strongest predictors were O2 pulse, heart rate reserve and muscular exercise capacity. Maximal exercise testing provides valuable information for clinical use and future prognosis and can be safely performed as early as 11 wk post-HTx.
INTRODUCTION
Cardiac rehabilitation, including exercise training to improve exercise capacity and health-related quality of life (HRQoL) is recommended after heart transplant (HTx)[1], but there are no clear and specific guidelines for how, how often or at what intensity exercise training should be performed.
Exercise capacity is often severely reduced shortly after HTx with peak oxygen consumption (VO2peak) levels reported to be between 9.2 and 19.7 mL/kg per min[2-12]. However, early measurement of VO2peak is not routine in most centers. VO2peak is the gold standard to objectively assess functional limitation and give an assessment of the integrative physiology involving cardiovascular, pulmonary, muscular, cellular and oxidative systems[13,14]. It has also been reported that VO2peak is a strong predictor for survival in HTx recipients[15,16]. In studies of maintenance HTx patients, VO2peak seems to be determined by both central (chronotropic incompetence, reduced stroke volume and cardiac output, impaired systolic and diastolic function, pulmonary dysfunction) and peripheral factors (diminished skeletal muscular capacity)[1,17-19]. Other factors, like donor characteristics, diagnosis and deconditioning before transplantation may also be associated with reduced exercise capacity after HTx[18]. However, we have recently reported that the most important variables predicting VO2peak in maintenance HTx patients are mostly of peripheral origin[20,21]. In de novo HTx patients, only two studies exist (n = 43[6] and n = 24[12]), which report limiting factors for VO2peak. These studies indicate that both central and peripheral factors could be involved in the early phase, but the knowledge is scarce and thus, a better understanding of factors that are associated with peak exercise shortly after HTx could guide clinicians and physiotherapist for more individualized therapy and specific exercise recommendations.
We hypothesized that both central and peripheral factors are associated with reduced exercise capacity in de novo HTx recipients. In the present study, we performed cardiopulmonary exercise testing (CPET) in a cohort of de novo HTx patients with the aim to determine clinical, hemodynamic and peripheral factors that contribute to the reduced exercise capacity.
MATERIALS AND METHODS
Patients and settings
This study was conducted in three centers in Scandinavia (Oslo, Gothenburg and Copenhagen). Altogether, 155 de novo HTx patients were assessed for eligibility. Of these, 72 were excluded for various reasons: did not meet inclusion criteria (cognitive issues, physical disabilities, medical complications, language barriers, contagion, no physical therapist available) (n = 43); were not motivated (n = 15); logistic reasons (n = 14). In addition, two were excluded after they had given their consent, one due to medical complications and one withdrawal. A total of 81 patients underwent CPET. The study was approved by the South-East Regional Committee for medical and health research ethics in Norway and the Committee for medical and health research ethics in Sweden and Denmark. The study was conducted in accordance with the recommendations in the Helsinki Declaration.
The current study is based on the baseline data from an ongoing randomized controlled trial (RCT): The High-intensity Interval Training in de novo heart Transplant recipients in Scandinavia (HITTS) study. The design and rationale of this study is described elsewhere[22]. In short, the RCT compares the effect of a 9-mo long two-armed intervention: High-intensity interval training versus moderate intensity continuous training.
Inclusion criteria
The inclusion criteria were: Clinically stable HTx recipients approximately 8-12 wk after HTx; Age > 18 years; Both sexes; Receiving immunosuppressive therapy according to local protocols; Patient willing and able to give written informed consent for study participation, and motivated to participate in the study for nine months.
Measurements
The primary endpoint, VO2peak, was measured on a treadmill or a bicycle ergometer applying an individualized protocol with an incremental workload until exhaustion[23]. The Norwegian populations were tested on a treadmill, except for four subjects, who could not comply and were tested on a bicycle ergometer. All patients in Sweden and Denmark were tested on a bicycle, which is the customary form for exercise testing in these countries. The variables from the CPET have been described previously[22]. Common heart rate (HR) variables and abbreviations used in this study were: Peak heart rate (HRpeak); Percentage of age-predicted maximum HR (% HRmax) = [(HRpeak/220 - age) × 100]; Chronotropic response index (CRI) = (HRpeak -HRrest)/(220 - age/HRrest); Heart rate reserve (HRreserve ) = HRpeak - HRrest; HRrecovery (difference between HRpeak and HR after 30 s, 1, 2, 3 and 4 min).
Secondary endpoints
Potential variables influencing VO2peak, such as lung function, maximum muscle strength and muscular exercise capacity, bioelectrical impedance analysis, echocardiography, blood samples and HRQoL were measured.
Lung function
Different lung function variables were measured in relation to the CPET, both at rest and during exercise. Spirometry was performed at rest before CPET: Peak expiratory flow (PEF), forced expiratory volume at 1 min (FEV1), forced vital capacity (FVC) during exercise, maximum ventilation (Vmax) and ventilatory efficiency (VE/VCO2)[14] were calculated.
Muscle strength and muscular exercise capacity
Muscle strength and muscular exercise capacity in the quadriceps and hamstring muscle groups were measured isokinetically. Five repetitions at an angular velocity of 60°/s were performed when measuring muscle maximal strength. For the muscular exercise capacity, 30 isokinetic contractions at 240°/s were performed. In the analyses, we used the bilateral sum of m. quadriceps and m. hamstrings[20,22].
Bioelectrical impedance analysis
Bioelectrical impedance is a simple and fairly valid method to measure body composition[24]. In this study, the Tanita (Tanita, Arlington Heights, IL, United States) system was used to measure body fat, body water, muscle mass, bone mass, visceral fat, metabolic age and basal metabolic rate.
Echocardiography
Standard Doppler-echocardiography was performed by experienced technicians and assessed by cardiologists to determine myocardial size and function.
Biochemistry
All patients underwent blood sampling in the morning in a fasting state. Two EDTA tubes were collected, inverted ten times and immediately placed on ice. Samples were centrifuged within 20 min. Plasma was transferred into four vials and frozen at -80 °C. One serum tube was collected and placed in room temperature for 60 to 120 min for coagulation before centrifugation. The sample was then transferred into two vials and frozen at -80 °C.
Plasma concentrations of N-terminal pro brain natriuretic peptide (NT-proBNP) was determined using an electrochemiluminescence immunoassay on a Modular platform (Roche Diagnostica, Basel, Switzerland), high sensitive C-reactive protein (hs-CRP) levels using a particle-enhanced, high-sensitive immunoturbidimetric assay (hsCRP, Tina-Quant CRP Gen.3), and high-sensitive troponin T (hs-TnT) was measured by electrochemiluminescence immunoassay (hsTnT, Elecsys Troponin T high sensitive, Roche Diagnostics).
HRQoL and symptoms of anxiety and depression
HRQoL was measured with the generic questionnaire short form-36, version 2 (SF-36v2)[25]. The results were transformed into norm-based scores on a standardized scale with a mean of 50 and a standard deviation (SD) of 10[25]. Subscales were aggregated into two sum-scores; physical component summary (PCS) and mental component summary (MCS). Symptoms of anxiety and depression were measured with the Hospital Anxiety and Depression Scale (HADS)[26]. The values were dichotomized using a cut-off score ≥ 8, which was considered to represent symptoms of depression or anxiety.
Statistical analysis
All data were analyzed using IBM SPSS, version 23 and version 25.0 (IBM corporation, United States). Continuous data are expressed as mean ± SD or median first quartile (Q1), third quartile (Q3), and categorical data are presented as percentages. Patients were divided by the median VO2peak (mL/kg per min) value into a low-capacity group (≤ 19.4) and a high-capacity group (> 19.4). Between-group comparisons were performed using two independent samples t or Mann Whitney U test. χ2 or F were used for categorical data, where appropriate. Bivariate relationships were explored and univariate regression analyses were performed with potential predictors (Tables 1 and 2). To identify the degree of association with VO2peak, all relevant variables with P < 0.05 and other potential variables from the univariate analyses of linear regression were selected for further multiple regression analyses. VO2peak (L/min), adjusted for age, sex and BMI, was used as the dependent variable. The final model was built using a series of multiple regression analyses with the enter method (Table 3). Assumptions were checked for normality and linearity.
Table 1.
Clinical characteristics and health-related quality of life of the study population
| 6N = 55-81 | Total | Low-capacity group (n = 41) VO2peak ≤ 19.4 mL/kg per min | High-capacity group (n = 40) VO2peak > 19.4 mL/kg per min | t (P-value) | Univariate regression Standardized coefficient Beta [95%CI], P VO2peak (L/min) | 7R2 |
| Clinical characteristics | ||||||
| Sex (% men) | 73% | 66 | 80 | 0.1521 | -0.45 [-0.61, -0.23], < 0.001 | 0.2 |
| Age (yr) | 49 ± 13 | 51 ± 11 | 46 ± 15 | 0.08 | -0.19 [-0.01, -0.001], 0.093 | 0.04 |
| Body mass index | 25.3 ± 3.7 | 26.3 ± 3.4 | 24.2 ± 3.8 | 0.01 | 0.28 [0.007, 0.056], 0.013 | 0.08 |
| Body fat (%) | 25.1 ± 8.7 | 29.0 ± 8.3 | 21.0 ± 7.1 | <0.001 | -0.34 [-0.03, -0.006], 0.003 | 0.11 |
| Donor age (yr) | 34 (24, 49) | 37 (27, 48) | 33 (23, 52) | 0.8252 | 0.09 [-0.004, 0.009], 0.447 | 0.01 |
| Ischemic time (min) | 210 (95, 237) | 215 (99, 249) | 185 (87, 227) | 0.0722 | -0.01[-0.001, 0.001], 0.938 | 8.2-5 |
| Weeks after HTx | 11 ± 1.8 | 11.3 ± 2 | 10.9 ± 1.5 | 0.307 | -0.001 [-0.05, 0.05], 0.990 | 2.0-5 |
| Duration of HF prior to HTx (yr) | 4 (1.5, 10) | 4 (1.5, 10.5) | 4 (1.0, 9.3) | 0.7182 | -0.05 [-0.02, 0.01], 0.681 | 0.002 |
| Time on HTx waiting list (d) | 75 (24, 193) | 96 (29, 227) | 47 (12, 131) | 0.062 | -0.14 [-0.001, 1.5-4], 0.202 | 0.02 |
| Rejections grade 1-2 (% yes) | 45 | 48 | 43 | 0.6531 | 0.09 [-0.11, 0.27], 0.408 | 0.01 |
| VO2peak preHTx (mL/kg per min) | 11.6 ± 3.3 | 11.1 ± 3 | 12.1 ± 3.5 | 0.248 | 0.03 [-0.032, 0.039], 0.826 | 0.001 |
| LVAD (% yes) | 15 | 22 | 8 | 0.0671 | -0.14 [-0.43, 0.097], 0.211 | 0.02 |
| Preoperative IABP/ECMO (% yes) | 16 | 15 | 18 | 0.7251 | 0.05 [-0.20, 0.32], 0.637 | 0.003 |
| Postoperative IABP/ECMO (% yes) | 10 | 15 | 5 | 0.2643 | -0.26 [-0.68, -0.066], 0.018 | 0.07 |
| Etiology HF (%) | 0.1383 | |||||
| Cardiomyopathy | 65 | 56 | 75 | |||
| Ischemic heart disease | 25 | 34 | 15 | |||
| Other | 10 | 10 | 10 | |||
| Smoking (%) no/yes/ex-smoker | 49/0/51 | 34/0/66 | 65/0/35 | 0.0051 | -0.19 [-0.34, 0.03], 0.100 | 0.03 |
| 24 h ambulatory blood pressure | ||||||
| Overall systolic BP | 133 ± 12 | 133 ± 13 | 132 ± 10 | 0.672 | ||
| Overall diastolic BP | 81 ± 7 | 80 ± 8 | 82 ± 7 | 0.493 | ||
| Medication (%) | ||||||
| Ciclosporin | 70 | 63 | 78 | 0.1651 | ||
| Tacrolimus | 28 | 32 | 23 | 0.3521 | ||
| Everolimus | 34 | 43 | 25 | 0.0981 | ||
| Mycophenolate | 90 | 81 | 100 | 0.0053 | 0.29 [0.10, 0.71], 0.009 | 0.08 |
| Prednisolone | 100 | 100 | 100 | |||
| Beta-blocker | 28 | 40 | 15 | 0.0121 | -0.19 [-0.39, -0.03], 0.086 | 0.04 |
| Calcium blocker | 25 | 25 | 25 | 1.0001 | ||
| ACE inhibitors | 3 | 3 | 3 | 1.0003 | ||
| AII-blocker | 9 | 13 | 5 | 0.2633 | ||
| Diuretics | 79 | 80 | 78 | 0.7851 | ||
| Statins | 99 | 98 | 100 | 1.0003 | ||
| Blood samples | ||||||
| TG (mmol/L) | 1.7 (1.3, 2.5) | 2.1 (1.5, 2.8) | 1.5 (1.1, 2.2) | 0.0132 | -0.24 [-0,19, -0.002], 0.045 | 0.06 |
| LDL (mmol/L) | 2.9 ± 1.0 | 3.0 ± 1.2 | 2.9 ± 0.7 | 0.416 | 0.12 [-0.05, 0.15], 0.308 | 0.01 |
| HDL (mmol/L) | 1.5 ± 0.5 | 1.5 ± 0.5 | 1.6 ± 0.5 | 0.432 | 0.04 [-0.16, 0.22], 0.755 | 0.001 |
| Cholesterol (mmol/L) | 5.1 ± 1.3 | 5.3 ± 1.5 | 5.0 ± 1.0 | 0.329 | 0.03 [-0.07, 0.09], 0.830 | 0.001 |
| Hemoglobin (g/dL) | 11.8 ± 1.7 | 11.3 ± 1.9 | 12.2 ± 1.4 | 0.017 | 0.38 [0.042, 0.15], 0.001 | 0.14 |
| hs-CRP (mg/L) | 2.3 (1.0, 6.1) | 2.7 (1.3, 6.7) | 1.6 (0.6, 3.9) | 0.0522 | -0.17 [-0.015, 0.002], 0.125 | 0.03 |
| NT-proBNP (ng/L) | 968.3 (625.8, 1680.8) | 1348.9 (765.4,2006.4) | 790.7 (522.2, 1351.0) | 0.0052 | -0.36[-2.7E-4, -6.5-5], 0.002 | 0.13 |
| hs-TnT (ng/L) | 32.5 (20.0, 61.8) | 42.0 (27.8, 66.7) | 24.0 (18.0, 50.8) | 0.0092 | -0.18 [-0.005, 0.001], 0.128 | 0.03 |
| HbA1c (%) | 5.6 ± 0.8 | 5.8 ± 0.9 | 5.4 ± 0.7 | 0.038 | -0.15 [-0.19, 0.04], 0.213 | 0.02 |
| Glucose (mmol/L) | 5.9 ± 1.8 | 6.3 ± 2.1 | 5.5 ± 1.4 | 0.046 | -0.19 [-0.1, 0.01], 0.109 | 0.04 |
| Leukocytes (× 10-9/L) | 5.4 ± 2.3 | 6.0 ± 2.7 | 4.7 ± 1.6 | 0.017 | -0.06 [-0.05, 0.03], 0.580 | 0.004 |
| Creatinine (μmol/L) | 117.4 ± 31.4 | 118.0 ± 31.9 | 116.9 ± 31.3 | 0.868 | -0.05 [-0.004, 0.002], 0.669 | 0.002 |
| Carbamide (mmol/L) | 9.8 ± 3.4 | 9.9 ± 4.0 | 9.7 ± 2.7 | 0.865 | -0.003 [-0.03, 0.03], 0.977 | 1.00E-05 |
| eGFR (mL/min per 1.73 m2) | 55 ± 16 | 54.1 ± 17.0 | 56.1 ± 15.0 | 0.586 | 0.23 [3.9E-5,0.01], 0.049 | 0.05 |
| Muscle strength and muscular exercise capacity | ||||||
| Muscle strength (Nm) | 279 ± 129 | 231 ± 128 | 326 ± 113 | 0.001 | 0.66 [0.002, 0.003], < 0.001 | 0.43 |
| Muscular Exercise capacity (J) | 3229 ± 1660 | 2423 ± 1351 | 4015 ± 1567 | < 0.001 | 0.64 [0.0001, 0.0002], < 0.001 | 0.41 |
| Spirometry | ||||||
| FEV1 (%) | 81 ± 16 | 74 ± 14 | 88 ± 16 | < 0.001 | 0.39 [0.004, 0.02], 0.001 | 0.16 |
| PEF (%) | 85 ± 22 | 79 ± 23 | 91 ± 20 | 0.018 | 0.37 [0.003, 0.01], 0.001 | 0.14 |
| FVC (%) | 86 ± 17 | 81 ± 16 | 90 ± 16 | 0.026 | 0.17 [-0.002, 0.01], 0.152 | 0.03 |
| Echocardiography | ||||||
| EF (%) | 57.9 ± 5.6 | 56.2 ± 5.4 | 59.4 ± 5.4 | 0.011 | 0.26 [0.003, 0.04], 0.025 | 0.07 |
| LVEDD (cm) | 4.9 ± 0.5 | 4.9 ± 0.5 | 4.9 ± 0.4 | 0.996 | 0.42 [0.19, 0.59], < 0.001 | 0.18 |
| FS (%) | 36.7 ± 5.9 | 35.9 ± 6.8 | 37.5 ± 4.9 | 0.242 | 0.23 [-4.7E-5, 0.03], 0.051 | 0.05 |
| CO (L/min) | 6.1 ± 1.2 | 6.0 ± 1.2 | 6.2 ± 1.2 | 0.467 | 0.39 [0.06, 0.21], 0.001 | 0.15 |
| Health-related quality of life | ||||||
| PCS | 43 ± 8 | 41 ± 7 | 45 ± 8 | 0.029 | 0.35 [0.008, 0.03], 0.001 | 0.13 |
| MCS | 54 ± 11 | 53 ± 10 | 55 ± 11 | 0.416 | 0.17 [-0.002, 0.02], 0.127 | 0.03 |
| Symptoms of anxiety and depression | ||||||
| HADS-A ≥ 8 (%)4 | 15 | 17 | 13 | 0.5621 | -0.26 [-0.56, -0.05], 0.02 | 0.07 |
| HADS-D ≥ 8 (%)5 | 5 | 5 | 5 | 1.0003 | -0.16 [-0.73, 0.13], 0.165 | 0.03 |
Groups are divided according to the median VO2peak (mL/kg per min). Variables are presented as percentages, mean ± SD or as median (Q1, Q3) where appropriate.
χ2;
Mann Whitney U-test;
F;
HADS-A score ≥ 8 indicates symptoms of anxiety;
HADS-D score ≥ 8 indicates symptoms of depression;
The actual N varies from 55 to 81 for different variables;
Unadjusted R2. ACE: Angiotensin-converting enzyme; ATII: Angiotensin II; BP: Blood pressure; CO: Cardiac output; ECMO: Extracorporeal membrane oxygenation; EF: Ejection fraction; FEV1: Forced expiratory volume at 1 min; FVC: Forced vital capacity; FS: Fractional shortening; HADS: Hospital anxiety and depression scale; HbA1c: Hemoglobin A1c; HDL: High density lipoprotein; hs-CR: High-sensitive C-reactive protein; hs-TnT: High-sensitive troponin T; HTx: Heart transplantation; IABP: Intra-aortic balloon pump; LVAD: Left ventricle assist device; LVEDD: Left ventricular end diastolic diameter; MCS: Mental component summary; Nm: Newton meter; NT-pro BNP: N-terminal pro brain natriuretic peptide; PEF: Peak expiratory flow; PCS: Physical component summary; Q1: First quartile; Q3: Third quartile; SD: Standard deviation; TG: Triglyceride.
Table 2.
Cardiopulmonary responses to exercise of the study population
| 2N = 63-81 | Total | Low-capacity group VO2peak ≤ 19.4 mL/kg per min (n = 41) | High-capacity group VO2peak > 19.4 mL/kg per min (n = 40) | t (P-value) | Univariate regression Standardized coefficient Beta [95%CI], P VO2peak L/min | 3R2 |
| VO2peak (mL/kg per min) | 20.4 ± 4.9 | 16.4 ± 2 | 24.3 ± 3.6 | < 0.001 | 0.75 [0.05, 0.08], < 0.001 | 0.56 |
| VO2peak (L/min) | 1.6 ± 0.4 | 1.3 ± 0.3 | 1.8 ± 0.4 | < 0.001 | ||
| %expected VO2peak | 55.8 ± 12.4 | 46.5 ± 7.4 | 65.3 ± 8.6 | < 0.001 | 0.60 [0.01, 0.03], < 0.001 | 0.36 |
| RER | 1.2 ± 0.1 | 1.2 ± 0.14 | 1.2 ± 0.10 | 0.898 | ||
| HRrest (echocardiography) | 87 ± 10 | 87 ± 11 | 86 ± 9 | 0.85 | -0.07 [-0.013, 0.007], 0.551 | 0.01 |
| Peak systolic BP (mmHg) | 188 ± 30 | 188 ± 31 | 189 ± 30 | 0.865 | 0.19 [-0.001, 0.006], 0.108 | 0.04 |
| Peak diastolic BP (mmHg) | 82 ± 17 | 82 ± 18 | 82 ± 16 | 0.917 | 0.09 [-0.004, 0.008], 0.467 | 0.01 |
| VE/VCO2slope | 34.8 ± 7.7 | 37.3 ± 7.2 | 32.6 ± 7.6 | 0.008 | -0.42 [-0.035, -0.01], < 0.001 | 0.18 |
| Vmax (L) | 71.4 ± 22.8 | 60.5 ± 17.5 | 81.7 ± 22.7 | < 0.001 | 0.76[0.01, 0.02], < 0.001 | 0.58 |
| O2 pulse (mL/beat) | 12.4 ± 3.3 | 11.0 ± 3 | 13.7 ± 3 | < 0.001 | 0.80 [0.08, 0.12], < 0.001 | 0.65 |
| AT (L/min) | 1.08 ± 0.3 | 0.95 ± 0.2 | 1.2 ± 0.3 | 0.001 | 0.73 [0.74, 1.2], < 0.001 | 0.53 |
| METS | 6.5 ± 1.6 | 5.4 ± 0.8 | 7.8 ± 1.3 | < 0.001 | 0.77 [0.16, 0.24], < 0.001 | 0.59 |
| HRpeak (beats/min) | 128 ± 19 | 121 ± 19 | 134 ± 17 | 0.001 | 0.31 [0.002, 0.01], 0.005 | 0.1 |
| %HRmax | 75 ± 12 | 72 ± 12 | 78 ± 11 | 0.021 | 0.20 [-0.001, 0.02], 0.071 | 0.04 |
| HRreserve (beats/min) | 43 ± 16 | 35 ± 13 | 50 ± 15 | < 0.001 | 0.47 [0.01, 0.02], < 0.001 | 0.22 |
| CRI | 0.51 ± 0.2 | 0.45 ± 0.18 | 0.57 ± 0.2 | 0.004 | 0.31 [0.20, 1.12], 0.005 | 0.1 |
| RPE (Borg scale) | 18.6 ± 0.8 | 18.5 ± 1 | 18.6 ± 0.5 | 0.638 | ||
| Test duration (min) | 9.5 ± 2.8 | 7.8 ± 1.5 | 11.1 ± 2.7 | < 0.001 | ||
| HRrecovery | ||||||
| Beats /min at 2 min | -1.0 (-3.0, 1.0) | -1.0 (-3.0, 1.0) | -2.0 (-3.3, 1.3) | 0.6971 |
Groups are divided according to the median VO2peak (mL/kg per min). Variables are presented as mean ± SD or as median (Q1, Q3) where appropriate.
Mann Whitney U-test;
The actual N varies from 63 to 81 for different variables;
Unadjusted R2. BP: Blood pressure; CI, confidence interval; CRI, chronotropic response index; HR, heart rate; METS, metabolic equivalents; Vmax, maximum ventilation; Q1, first quartile; Q3, third quartile; RER, Respiratory Exchange Ratio; RPE, rated perceived exertion; SD, standard deviation.
Table 3.
Multiple regression analysis
| N = 66 | Model 1 Standardized coefficient Beta [95% CI] | P-value | Model 2 Standardized coefficient Beta [95% CI] | P-value |
| O2 pulse (mL/beat) | 0.707 [0.075, 0.104] | < 0.001 | 0.675 [0.069, 0.102] | < 0.001 |
| HRreserve (beats/min) | 0.382 [0.007, 0.013] | < 0.001 | 0.397 [0.008, 0.013] | < 0.001 |
| Muscular exercise capacity (Joule) | 0.162 [1.1E-5, 7.1E-5] | 0.008 | 0.155 [8.0-5, 7.1-5] | 0.015 |
| BMI (kg/m2) | 0.067 [-0.004, 0.020] | 0.211 | ||
| Sex | -0.029 [-0.142, 0.086] | 0.630 | ||
| Age (yr) | 0.019 [-0.003, 0.004] | 0.719 | ||
| Adjusted R2 | 0.85 | 0.84 |
Dependent variable VO2peak L/min. Final model for n = 66. BMI: Body mass index; CI: Confidence interval; HR: Heart rate.
RESULTS
Clinical characteristics
The mean ± SD age of the total study population was 49 ± 13 years, and 73% were men. Patients were on average 11.1 ± 1.8 wk after HTx. The mean VO2peak was 20.4 mL/kg per min, which is 56% of expected compared to the reference values described in the 9th edition of the American College of Sports Medicine’s (ACSM) guidelines for exercise testing and exercise prescription[27]. Further demographic and clinical characteristics are presented group-wise in Tables 1 and 2.
Compared to the high-capacity group, the low-capacity group was characterized by a higher body mass index (BMI) and a higher fat content, they were more often ex-smokers, had lower PCS score, had less muscle strength and muscular exercise capacity, had lower FEV1, FVC and ejection fraction (EF) as measured by echocardiography. The low-capacity group more often used beta blockers and less mycophenolate, had higher NT-proBNP, hs-TnT, triglycerides and lower hemoglobin (Hgb). Duration of heart failure before HTx, primary diagnosis, donor age, ischemic time, rejection scores, MCS score and HADS depression score were similar between the two groups (Table 1).
Exercise variables
Exercise variables are shown in Table 2. As compared with the high-capacity group, the low-capacity group exercised for a shorter time, had lower maximal ventilation, O2 pulse, HRpeak and HRreserve, while VE/VCO2 slope was higher (Table 2). The respiratory exchange ratio (RER), rated perceived exertion (RPE) and blood pressure responses were similar between the groups (Table 2).
Predictors of VO2peak
Univariate predictors of VO2peak are shown in Tables 1 and 2. There were strong correlations (P < 0.001) between VO2peak and HRreserve, O2 pulse and muscular exercise capacity (Figures 1-3). In multiple regression analyses, O2 pulse, HRreserve, muscular exercise capacity, BMI, gender and age accounted for 84% of the variance in VO2peak (L/min). Only O2 pulse, HRreserve and muscular exercise capacity were important determinants in the final model (P < 0.001, P < 0.001 and P < 0.015, respectively). Other potential predictors were also analyzed in the multiple regression analyses, but these did not reach statistical significance. VO2peak (L/min) was chosen as the dependent variable in order to be able to adjust for and see the impact of age, gender and BMI directly, as the VO2peak (mL/kg per min) variable is already weight-based.
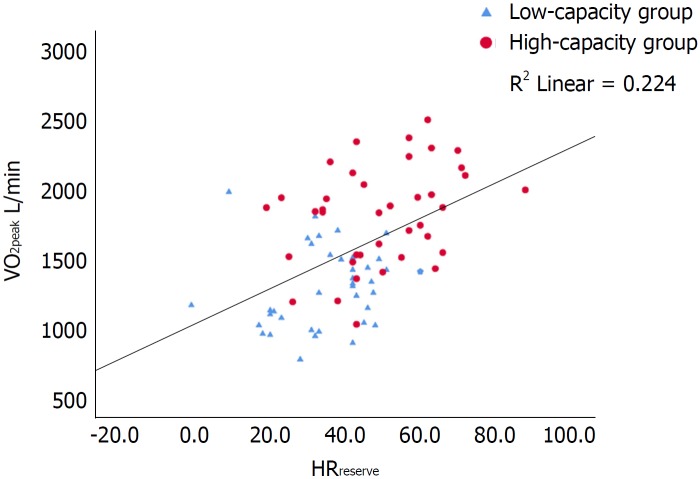
Figure 1.
Scatterplot of the correlation between peak oxygen consumption (L/min) and heart rate reserve with inserted regression line. R2 = 0.224. Pearsons r 0.473, P < 0.001. VO2peak: Peak oxygen consumption; HRreserve: Heart rate reserve.
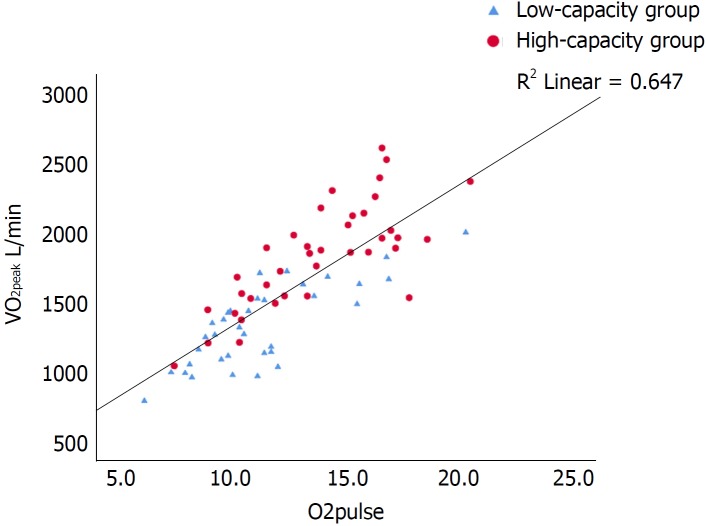
Figure 2.
Scatterplot of the correlation between peak oxygen consumption (L/min) and O2 pulse with inserted regression line. R2 = 0.647. Pearsons r 0.804, P < 0.001. VO2peak: Peak oxygen consumption.
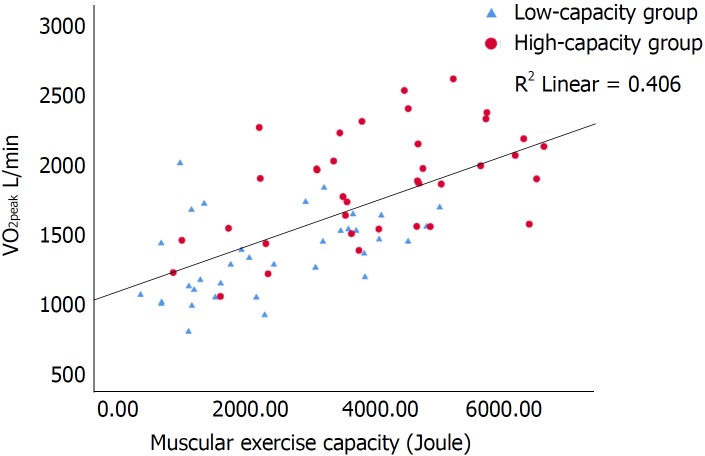
Figure 3.
Scatterplot of the correlation between peak oxygen consumption (L/min) and muscular exercise capacity (Joule) with inserted regression line. R2 = 0.406. Pearsons r 0.637, P < 0.001. VO2peak: Peak oxygen consumption.
Safety
All measurements performed in this study, including the CPET and muscle strength testing, were completed without any minor or serious adverse events.
DISCUSSION
The main findings in this study were that de novo HTx patients display reduced exercise capacity compared with a general population: The reference population in ACSM[27] and Astrand[28], and that maximal exercise capacity was determined by both central (O2 pulse and HRreserve) and peripheral factors (muscular exercise capacity) (Table 3 and Figures 1-3). Furthermore, CPET can be safely performed as early as an average of 11 wk after HTx and is a valuable basis for individual tailoring of the further rehabilitation program.
In addition to the main predictors mentioned above, self-reported physical function was also positively associated with VO2peak in this cohort, which is in accordance with an earlier paper from our research team[15]. Similar findings are reported from the general population in the Norwegian HUNT study, in which physical activity level was associated with VO2peak[29]. Although both groups in our current study had a lower score on the physical function subscale compared to the norm values described in Ware et al[25], the high-capacity group had a clinical meaningful and significantly higher score than the low-capacity group on physical function. The high-capacity group also had higher score on the PCS. On the other hand, there were no differences between the two groups regarding the psychosocial subscales or MCS in SF-36v2.
As previously mentioned, only two previous studies exist that describe determinants for VO2peak in de novo HTx recipients[6,12]. Kitagaki et al[6] found that knee extensor muscle strength and cholinesterase were important predictors for VO2peak 55 d after surgery. Salyer et al[12] found that age was the only predictor of VO2 peak 68 d after HTx, but they did not include muscular exercise capacity or chronotropic variables in their regression analyses. A small study (n = 15) by Oliveira Carvalho et al[30] described that HRreserve, as the only important variable, was associated with VO2peak six months after HTx, while in maintenance HTx recipients, HRreserve was no longer strongly associated with VO2peak. In HRrecovery after exercise, there was an important difference between early and late HTx recipients, suggesting a partial reinnervation in maintenance HTx recipients[30]. However, peripheral factors such as muscular exercise capacity were not measured in Oliveira Carvalho’s study[30]. Borelli et al[31] followed HTx recipients for two years and found that both central and peripheral factors contributed to the reduced VO2peak both early (5.3 mo) and late (2 years) after HTx, but that the improvements in VO2peak seen over two years were mostly related to peripheral factors.
In the present study, both HRreserve and O2 pulse were independent predictors of VO2peak. The chronotropic responses, CRI, %HRmax and HRpeak were, as expected, lower than normal both in the low-capacity and the high-capacity group. However, the high-capacity group had better chronotropic responses than the low-capacity group (CRI, P = 0.004; %HRmax, P = 0.021, HRpeak, P = 0.001; HRreserve, P < 0.001). HRrecovery was markedly delayed in both groups, with no difference between the groups. Previous studies in maintenance HTx recipients have reported conflicting results whether chronotropic incompetence is associated with a reduced VO2peak or not. Schwaiblmair et al[32] and Kemp et al[33] found a higher VO2peak in patients with a greater HRreserve, compared to patients with a lower HRreserve. In contrast, Squires et al[34] found no difference in VO2peak between patients with high versus low HRreserve (46 ± 15 vs 33 ± 15). In a previous study by our research group, where maintenance HTx recipients demonstrated a close to normal chronotropic response, HRreserve was not a strong determinant of VO2peak[20]. However, in this current study of de novo HTx recipients, it is (Figure 1). The findings described above suggest that as the initially impaired chronotropic responses improve over time, they become less predictive of VO2peak.
O2 pulse derived from CPET is considered a surrogate for stroke volume[14,35,36]. In the current study, there was a strong correlation between VO2 peak and O2 pulse (Figure 2). In line with this, the high-capacity group also had a higher O2 pulse (P < 0.001), increased left ventricular EF, as well as lower NT-proBNP and hs-TnT levels, reflecting a better preserved myocardial function compared with the low-capacity group.
De novo HTx recipients have reduced muscle mass mostly due to inactivity prior to HTx[18]. The high-capacity group had higher muscular exercise capacity (P < 0.001) and muscular strength (P = 0.001) than the low-capacity group (Figure 3), and this finding supports the previously described association between muscle function and VO2peak[20]. Comparing the muscle strength values from our previous study on maintenance recipients[20] with the values in this current study, they are not surprisingly much lower in the de novo recipients. As muscular exercise capacity is the only peripheral predictor for VO2 peak in the current study, peripheral factors might be less dominant than central factors in the early phase after HTx. However, from a clinical point of view, resistance training in the early rehabilitation after HTx is of high importance in order to prevent and restore loss of muscle mass and bone density and is likely to contribute to an improved VO2peak level[37].
In the existing literature, VO2peak in de novo HTx patients is reported to range from 9.2 mL/kg per min up to 19.7 mL/kg per min (1-3 mo after HTx)[2-12]. One small study of nine patients with left ventricle assist device (LVAD) prior to HTx had a mean VO2peak of 24.6 mL/kg per min 12 wk after HTx, which is higher than what has been reported in other studies and may be explained by the LVAD effect and the patients’ relatively high VO2peak before HTx[38]. Except for this study, our cohort’s mean VO2peak level of 20.4 mL/kg per min (measured 11 wk post HTx) is higher than what is previously reported in de novo HTx recipients. Compared to an earlier exercise study in maintenance HTx recipients from our center with a median VO2peak value of 27.3 mL/kg per min[20], this de novo HTx cohort is below this value, but compared to other international studies in maintenance HTx recipients, our current de novo HTx recipients are close to these reported values[18]. This may be partially related to the early and individualized exercise program conducted at our centers, where the patients are attended to daily by a physical therapist from the multidisciplinary HTx team.
Results from a CPET test can be important in many aspects in the early phase after HTx. First of all, a maximal exercise test is of great value to the individual patient in terms of contributing to increased confidence in their new heart and the body’s tolerance to high-intensity exercise. Secondly, an early CPET is useful for deciding and tailoring the individual exercise programs and for the further rehabilitation, both for monitoring patients’ status and prognosis and measuring effect of exercise. In addition to the many gas exchange variables, the CPET also provides other valuable and useful measurements, such as lung function and chronotropic responses. Finally, as we know that measures of physical capacity are strong predictors for long-term survival in HTx recipients[15,16], we suggest that such measures should be routinely included both in the early phase after HTx and at yearly controls thereafter. We underscore that the safety aspect is very important when performing a CPET and it should always be supervised by competent and experienced health personnel.
Limitations
Selection bias is a common risk in all voluntary studies, and although our aim was to include every newly transplanted HTx recipient, the recipients had to be medically stable and able to perform a maximal CPET and other physical tests. Thus, as the median VO2peak value in this de novo cohort is comparable to maintenance HTx recipients’ VO2peak values, this may be due to a possible selection bias.
This is a cross-sectional study, based on the baseline data from an ongoing RCT, and no causal relationships should be drawn from such a study design. We present only associations between VO2peak and different possible determinants. A rather small sample size (n = 81) may also imply type 2 errors, but all the performed statistics were carefully checked for underlying assumptions.
In this de novo HTx cohort, the age-predicted mean VO2 peak value was 56% of age-expected values, which is comparable to previously reported values in maintenance HTx[18]. Predictors for VO2peak in de novo HTx recipients seem to be of both central (O2 pulse and HRreserve) and peripheral (muscular exercise capacity) origin. A CPET and determination of muscular exercise capacity provide important information for patient motivation, rehabilitation and prognosis and thus, measurements for physical function should be considered as routine examinations early after HTx.
ACKNOWLEDGMENTS
We especially thank the transplantation nurses Anne R Authen and Ingelin Grov for help and support throughout the study. From the University of Gothenburg, we thank the PhD student Andreas Lundberg Zachrisson and Professor Stefan Grau for help with the muscle strength testing among the Swedish participants. An abstract with data from this study was presented at the International Society for Heart and Lung Transplantation (ISHLT) 37th Annual Meeting and Scientific Sessions in San Diego 2017 and the ISHLT 38th Annual Meeting and Scientific Sessions in Nice 2018.
ARTICLE HIGHLIGHTS
Research background
Peak oxygen consumption (VO2peak) is reduced after heart transplant (HTx). Both peripheral and central factors are determinants of the reduced VO2peak in maintenance HTx recipients, but there are still few studies among de novo HTx patients. A higher VO2peak is associated with better prognosis after HTx, and knowledge about predictors for VO2peak in de novo HTx is important for the rehabilitation process. A cardiopulmonary exercise test (CPET) is the gold standard for measuring VO2peak and should be performed as a routine test early after HTx.
Research motivation
More knowledge about predictors for VO2peak in de novo HTx patients may contribute to a better understanding of the reduced exercise capacity early after HTx. Individualized exercise prescriptions are very important after HTx, and a CPET early after HTx will guide both clinicians and physiotherapists in this vulnerable phase of the rehabilitation process.
Research objectives
The aim of this study was to investigate determinants of early VO2peak and exercise capacity in a cohort of de novo HTx recipients.
Research methods
This study used baseline data from an ongoing randomized controlled trial investigating high-intensity interval training compared to moderate continuous exercise training among de novo HTx recipients, the HITTS study. A cross sectional analysis was performed on the baseline data from the 81 patients included in the study, and all baseline tests were performed an average of 11 wk after surgery. The primary endpoint was VO2peak measured by CPET. Secondary endpoints were lung function, maximum muscle strength and muscular exercise capacity, bioelectrical impedance analysis, echocardiography, blood samples and health-related quality of life.
Research results
The main findings in this study were that de novo HTx patients display reduced exercise capacity compared to a general population, but comparable with maintenance HTx recipients. This de novo HTx cohort demonstrated a median VO2peak level of 19.4 mL/kg per min at 11 ± 1.8 wk post-HTx. Maximal exercise capacity was determined by both central (O2 pulse and HRreserve) and peripheral factors (muscular exercise capacity). The CPET tests were performed without any serious adverse events mean 11 wk after HTx. This is a cross-sectional study, and no causal relationships should be drawn from such a study design. We present only associations between VO2peak and different possible determinants.
Research conclusions
In this de novo HTx cohort, the age-predicted mean VO2 peak value was 56% of age-expected values, which is comparable to previously reported values in maintenance HTx. Predictors for VO2peak in de novo HTx recipients seem to be of both central and peripheral origin.
Research perspectives
A CPET and determination of muscular exercise capacity provide important information for patient motivation, rehabilitation and prognosis and thus, measurements for physical function should be considered as routine examinations early after HTx.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: Norway
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
Institutional review board statement: The study was approved by the South-East Regional Committee for medical and health research ethics in Norway and the Committee for medical and health research ethics in Sweden and Denmark.
Clinical trial registration statement: This study is registered at ClinicalTrials.gov. The registration identification number is NCT01796379.
Informed consent statement: All study participants gave their written consent prior to study inclusion.
Conflict-of-interest statement: None of the authors have any conflict of interest to declare.
CONSORT 2010 statement: We have prepared the manuscript according to the CONSORT 2010 statement, where appropriate. A pdf version of the document is uploaded.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 24, 2018
First decision: July 19, 2018
Article in press: August 6, 2018
P- Reviewer: Boteon YL, Gonzalez F, Hanna R, Hibberd AD S- Editor: Ji FF L- Editor: Filipodia E- Editor: Yin SY
Contributor Information
Katrine Rolid, Department of Cardiology, Oslo University Hospital, Oslo 0424, Norway; the Norwegian Health Association, Oslo 0307, Norway; Faculty of Medicine, University of Oslo, Oslo 0316, Norway; KG Jebsen Center for Cardiac Research, and Center for Heart Failure Research, University of Oslo, Oslo 0316, Norway. katrine.rolid@medisin.uio.no.
Arne K Andreassen, Department of Cardiology, Oslo University Hospital, Oslo 0424, Norway.
Marianne Yardley, Department of Cardiology, Oslo University Hospital, Oslo 0424, Norway; the Norwegian Health Association, Oslo 0307, Norway; Faculty of Medicine, University of Oslo, Oslo 0316, Norway.
Elisabeth Bjørkelund, Department of Cardiology, Oslo University Hospital, Oslo 0424, Norway.
Kristjan Karason, Department of Cardiology, Sahlgrenska University Hospital, Gothenburg 41345, Sweden.
Julia P Wigh, Department of Physical Therapy, Sahlgrenska University Hospital, Gothenburg 41345, Sweden.
Christian H Dall, Department of Cardiology, Bispebjerg University Hospital, Copenhagen 2400, Denmark.
Finn Gustafsson, Department of Cardiology, Rigshospitalet University Hospital, Copenhagen 2100, Denmark.
Lars Gullestad, Department of Cardiology, Oslo University Hospital, Oslo 0424, Norway; Faculty of Medicine, University of Oslo, Oslo 0316, Norway; KG Jebsen Center for Cardiac Research, and Center for Heart Failure Research, University of Oslo, Oslo 0316, Norway.
Kari Nytrøen, Department of Cardiology, Oslo University Hospital, Oslo 0424, Norway; Faculty of Medicine, University of Oslo, Oslo 0316, Norway; KG Jebsen Center for Cardiac Research, and Center for Heart Failure Research, University of Oslo, Oslo 0316, Norway.
References
- 1.Anderson L, Nguyen TT, Dall CH, Burgess L, Bridges C, Taylor RS. Exercise-based cardiac rehabilitation in heart transplant recipients. Cochrane Database Syst Rev. 2017;4:CD012264. doi: 10.1002/14651858.CD012264.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habedank D, Ewert R, Hummel M, Wensel R, Hetzer R, Anker SD. Changes in exercise capacity, ventilation, and body weight following heart transplantation. Eur J Heart Fail. 2007;9:310–316. doi: 10.1016/j.ejheart.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Braith RW, Schofield RS, Hill JA, Casey DP, Pierce GL. Exercise training attenuates progressive decline in brachial artery reactivity in heart transplant recipients. J Heart Lung Transplant. 2008;27:52–59. doi: 10.1016/j.healun.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Chen SY, Lan C, Ko WJ, Chou NK, Hsu RB, Chen YS, Chu SH, Lai JS. Cardiorespiratory response of heart transplantation recipients to exercise in the early postoperative period. J Formos Med Assoc. 1999;98:165–170. [PubMed] [Google Scholar]
- 5.Hsu CJ, Chen SY, Su S, Yang MC, Lan C, Chou NK, Hsu RB, Lai JS, Wang SS. The effect of early cardiac rehabilitation on health-related quality of life among heart transplant recipients and patients with coronary artery bypass graft surgery. Transplant Proc. 2011;43:2714–2717. doi: 10.1016/j.transproceed.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Kitagaki K, Nakanishi M, Ono R, Yamamoto K, Suzuki Y, Fukui N, Yanagi H, Konishi H, Yanase M, Fukushima N. Cholinesterase levels predict exercise capacity in cardiac recipients early after transplantation. Clin Transplant. 2018:32. doi: 10.1111/ctr.13170. [DOI] [PubMed] [Google Scholar]
- 7.Jaski BE, Lingle RJ, Kim J, Branch KR, Goldsmith R, Johnson MR, Lahpor JR, Icenogle TB, Piña I, Adamson R, et al. Comparison of functional capacity in patients with end-stage heart failure following implantation of a left ventricular assist device versus heart transplantation: results of the experience with left ventricular assist device with exercise trial. J Heart Lung Transplant. 1999;18:1031–1040. doi: 10.1016/s1053-2498(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 8.Kobashigawa JA, Leaf DA, Lee N, Gleeson MP, Liu H, Hamilton MA, Moriguchi JD, Kawata N, Einhorn K, Herlihy E, et al. A controlled trial of exercise rehabilitation after heart transplantation. N Engl J Med. 1999;340:272–277. doi: 10.1056/NEJM199901283400404. [DOI] [PubMed] [Google Scholar]
- 9.Daida H, Squires RW, Allison TG, Johnson BD, Gau GT. Sequential assessment of exercise tolerance in heart transplantation compared with coronary artery bypass surgery after phase II cardiac rehabilitation. Am J Cardiol. 1996;77:696–700. doi: 10.1016/s0002-9149(97)89202-8. [DOI] [PubMed] [Google Scholar]
- 10.Mandak JS, Aaronson KD, Mancini DM. Serial assessment of exercise capacity after heart transplantation. J Heart Lung Transplant. 1995;14:468–478. [PubMed] [Google Scholar]
- 11.Keteyian S, Shepard R, Ehrman J, Fedel F, Glick C, Rhoads K, Levine TB. Cardiovascular responses of heart transplant patients to exercise training. J Appl Physiol (1985) 1991;70:2627–2631. doi: 10.1152/jappl.1991.70.6.2627. [DOI] [PubMed] [Google Scholar]
- 12.Salyer J, Jewell DV, Quigg RJ. Predictors of early post-cardiac transplant exercise capacity. J Cardiopulm Rehabil. 1999;19:381–388. doi: 10.1097/00008483-199911000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary Exercise Testing: What Is its Value? J Am Coll Cardiol. 2017;70:1618–1636. doi: 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, Fletcher GF, Forman DE, Kitzman DW, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. doi: 10.1161/CIR.0b013e31826fb946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yardley M, Havik OE, Grov I, Relbo A, Gullestad L, Nytrøen K. Peak oxygen uptake and self-reported physical health are strong predictors of long-term survival after heart transplantation. Clin Transplant. 2016;30:161–169. doi: 10.1111/ctr.12672. [DOI] [PubMed] [Google Scholar]
- 16.Yardley M, Gullestad L, Nytrøen K. Importance of physical capacity and the effects of exercise in heart transplant recipients. World J Transplant. 2018;8:1–12. doi: 10.5500/wjt.v8.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notarius CF, Levy RD, Tully A, Fitchett D, Magder S. Cardiac versus noncardiac limits to exercise after heart transplantation. Am Heart J. 1998;135:339–348. doi: 10.1016/s0002-8703(98)70103-6. [DOI] [PubMed] [Google Scholar]
- 18.Nytrøen K, Gullestad L. Exercise after heart transplantation: An overview. World J Transplant. 2013;3:78–90. doi: 10.5500/wjt.v3.i4.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobashigawa J, Olymbios M. Physiology of the Transplanted Heart. In: Kobashigawa J, editor. Clinical Guide to Heart Transplantation. Cham: Springer International Publishing; 2017. pp. 81–93. [Google Scholar]
- 20.Nytrøen K, Rustad LA, Gude E, Hallén J, Fiane AE, Rolid K, Holm I, Aakhus S, Gullestad L. Muscular exercise capacity and body fat predict VO(2peak) in heart transplant recipients. Eur J Prev Cardiol. 2014;21:21–29. doi: 10.1177/2047487312450540. [DOI] [PubMed] [Google Scholar]
- 21.Yardley M, Ueland T, Aukrust P, Michelsen A, Bjørkelund E, Gullestad L, Nytrøen K. Immediate response in markers of inflammation and angiogenesis during exercise: a randomised cross-over study in heart transplant recipients. Open Heart. 2017;4:e000635. doi: 10.1136/openhrt-2017-000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nytrøen K, Yardley M, Rolid K, Bjørkelund E, Karason K, Wigh JP, Dall CH, Arora S, Aakhus S, Lunde K, et al. Design and rationale of the HITTS randomized controlled trial: Effect of High-intensity Interval Training in de novo Heart Transplant Recipients in Scandinavia. Am Heart J. 2016;172:96–105. doi: 10.1016/j.ahj.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Working Group on Cardiac Rehabilitation & Excercise Physiology and Working Group on Heart Failure of the European Society of Cardiology. Recommendations for exercise testing in chronic heart failure patients. Eur Heart J. 2001;22:37–45. doi: 10.1053/euhj.2000.2388. [DOI] [PubMed] [Google Scholar]
- 24.Jaffrin MY. Body composition determination by bioimpedance: an update. Curr Opin Clin Nutr Metab Care. 2009;12:482–486. doi: 10.1097/MCO.0b013e32832da22c. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Kosinski M, Bjorner BJ, Turner-Bowker D, Gandek B and Maruish ME. 2008. User’s manual for the SF36V2© Health survey second edition. QualityMetric Inc; pp. 1–310. [Google Scholar]
- 26.Snaith RP, Zigmond AS. 1994. The Hospital Anxiety and Depression Scale Manual. GL Assessment Limited; pp. 1–15. [Google Scholar]
- 27.American College of Sports Medicine, Arena R, Riebe D, Thompson PD, editors. ACSM’s guidelines for exercise testing and prescription. 9th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams Wilkins, cop; 2014. [Google Scholar]
- 28.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol Scand Suppl. 1960;49:1–92. [PubMed] [Google Scholar]
- 29.Aspenes ST, Nauman J, Nilsen TI, Vatten LJ, Wisløff U. Physical activity as a long-term predictor of peak oxygen uptake: the HUNT Study. Med Sci Sports Exerc. 2011;43:1675–1679. doi: 10.1249/MSS.0b013e318216ea50. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira Carvalho V, Barni C, Teixeira-Neto IS, Guimaraes GV, Oliveira-Carvalho V, Bocchi EA. Exercise capacity in early and late adult heart transplant recipients. Cardiol J. 2013;20:178–183. doi: 10.5603/CJ.2013.0031. [DOI] [PubMed] [Google Scholar]
- 31.Borrelli E, Pogliaghi S, Molinello A, Diciolla F, Maccherini M, Grassi B. Serial assessment of peak VO2 and VO2 kinetics early after heart transplantation. Med Sci Sports Exerc. 2003;35:1798–1804. doi: 10.1249/01.MSS.0000093610.71730.02. [DOI] [PubMed] [Google Scholar]
- 32.Schwaiblmair M, von Scheidt W, Uberfuhr P, Ziegler S, Schwaiger M, Reichart B, Vogelmeier C. Functional significance of cardiac reinnervation in heart transplant recipients. J Heart Lung Transplant. 1999;18:838–845. doi: 10.1016/s1053-2498(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 33.Kemp DL, Jennison SH, Stelken AM, Younis LT, Miller LW. Association of resting heart rate and chronotropic response. Am J Cardiol. 1995;75:751–752. doi: 10.1016/S0002-9149(99)80674-2. [DOI] [PubMed] [Google Scholar]
- 34.Squires RW, Leung TC, Cyr NS, Allison TG, Johnson BD, Ballman KV, Wagner JA, Olson LJ, Frantz RP, Edwards BS, et al. Partial normalization of the heart rate response to exercise after cardiac transplantation: frequency and relationship to exercise capacity. Mayo Clin Proc. 2002;77:1295–1300. doi: 10.4065/77.12.1295. [DOI] [PubMed] [Google Scholar]
- 35.Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 36.Whipp BJ, Higgenbotham MB, Cobb FC. Estimating exercise stroke volume from asymptotic oxygen pulse in humans. J Appl Physiol (1985) 1996;81:2674–2679. doi: 10.1152/jappl.1996.81.6.2674. [DOI] [PubMed] [Google Scholar]
- 37.Braith RW, Edwards DG. Exercise following heart transplantation. Sports Med. 2000;30:171–192. doi: 10.2165/00007256-200030030-00003. [DOI] [PubMed] [Google Scholar]
- 38.de Jonge N, Kirkels H, Lahpor JR, Klöpping C, Hulzebos EJ, de la Rivière AB, Robles de Medina EO. Exercise performance in patients with end-stage heart failure after implantation of a left ventricular assist device and after heart transplantation: an outlook for permanent assisting? J Am Coll Cardiol. 2001;37:1794–1799. doi: 10.1016/s0735-1097(01)01268-2. [DOI] [PubMed] [Google Scholar]