Abstract
The outcomes of hepatitis B virus (HBV) infection are closely related to the age at which infection was acquired. Infection acquired in adult life tends to be self-limited, in contrast to perinatal acquirement, for which chronic persistence of the HBV is a general outcome. Innate immunity plays an indispensable role in early virus infection, facilitating virus clearance. However, it has been reported that HBV is under-recognized and poorly eliminated by the innate immune system in the early stages of infection, possibly explaining the long-lasting persistence of viremia afterwards. Furthermore, due to the existence of covalently closed circular DNA, chronic HBV clearance is very difficult, even when patients are given interferon-α and nucleotide/nucleoside analogs for antiviral therapy. The mechanism by which HBV evades innate immune recognition and establishes persistent infection remains a subject of debate. Besides, some researchers are becoming more interested in how to eradicate chronic HBV infection by restoring or boosting innate immunity. This review aimed to summarize the current knowledge on how intrahepatocyte signaling pathways and innate immune cells act after the onset of HBV infection and how these actions are related to the persistence of HBV. We anticipate the insights presented herein to be helpful for future development of novel immune therapeutic strategies to fight HBV infection.
Keywords: Hepatitis B virus, Innate immunity, Immune evasion, Pattern recognition receptor, Toll-like receptor, Natural killer cells, Kupffer cells, Dendritic cells
Core tip: This review covers the following core concepts of hepatitis B virus (HBV) persistence, according to the most up-to-date literature: Hepatocytes lack immune responsiveness to HBV; Innate immune cells display weak responses at the early stages of HBV infection; HBV impairs functions of innate immune cells and select signaling pathways to evade immune recognition and response.
INTRODUCTION
Hepatitis B virus (HBV) infection remains a global health concern, as about 257 million people worldwide are chronically infected with HBV currently[1]. Although the HBV infection rate has been partly controlled by various prophylaxis strategies, researchers have yet to discover a cure for chronic HBV infection. Chronic hepatitis B (CHB) is typically asymptomatic, but harbors the potential for development of life-threatening complications[2]. To overcome this threat, the mechanisms of HBV infection that underlie progression to chronicity need to first be fully elucidated.
HBV, a member of hepadnavirus family, acts as a “stealth” virus, not inducing any obvious innate immune responses in the early stage of infection[3]. Furthermore, the target cells (hepatocytes) do not recognize HBV efficiently through known signaling pathways, indicating the possibility of an HBV immune evasion mechanism[4]. HBV also has the ability to suppress functions of innate immune cells[5-7]. HBV interaction with innate immunity would suggest that HBV persistence is related to a multitude of host and viral factors.
Herein, we summarize the recent knowledge regarding HBV persistence and the evidenced and theorized relations with intrahepatocyte signaling pathways and innate immune cells. Collation of such information will provide a useful overview of the field today, possibly providing new insights into novel therapeutic treatments for CHB.
Fundamentally, the innate immunity system responds to viral infection in three phases. In the first phase, various sensors in the cytoplasm recognize pathogen-associated molecular patterns, such as foreign DNA or RNA, and send a warning message to initiate downstream signals. The second phase involves the proteins of the downstream signaling pathways transmitting the danger message to the nucleus, activating effector elements. In the last phase, the consequently up-regulated effectors [i.e., inflammatory factors or interferon (IFN)-stimulating genes] degrade the exogenous viral elements. Defect or suppression of the involved sensors and signaling pathways tends to result in persistent existence of HBV in the host, since, under such circumstances, HBV cannot be recognized and eliminated in a timely manner. We will begin this review by discussing several intra-hepatocelluar pathways that are closely related to HBV recognition and which exert an anti-HBV effect.
HEPATOCYTES LACK IMMUNE RESPONSIVENESS TO HBV
Pattern recognition receptors
Pattern recognition receptors (PRRs) are the major sensors of exogenous pathogens, and they include the Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MAD5)[8]. These molecules are able to recognize diverse pathogen-associated common loci of specific pathogens, subsequently activating downstream signaling pathways that induce IFNs and inflammatory factors to control virus replication[9]. However, a recent study of liver tissues from CHB patients found that hepatocytes do not respond to HBV, even though they were able to produce IFNs and induce expression of IFN-stimulating genes when stimulated by other infection-related factors, such as those related to TLR3 binding and Sendai virus infection. This finding suggested that HBV is invisible to PRRs on hepatocytes[10].
Stimulator of IFN genes
Stimulator of IFN genes (STING) serves as the adaptor protein of multiple cytoplasmic DNA receptors that recognize exogenous pathogens[11]. It has been reported that cytoplasmic DNA-activated cyclic GMP-AMP synthase (commonly known as cGAS) binds to STING, thereby inducing secretion of type I IFN and other cytokines[12]. This pathway is of great importance for eukaryotes (eukaryotic cells) to defend against bacterial, viral and other eukaryotic pathogens[11].
In vivo and in vitro experiments have shown that STING is NOT expressed in human hepatocytes, which might explain the dysfunction of DNA sensing[4]. Hence, HBV DNA may have adapted to this particular “biological niche”, whereby it can escape immune detection. More interestingly, c-GAMP is able to induce a robust cytokine response in a HBV-infected C3AhNTCP hepatoma cell line if added in cell culture, suggesting its capacities of inhibiting HBV replication[13].
INNATE IMMUNE CELLS HAVE WEAK RESPONSIVENESS TO HBV
Natural killer cells
Natural killer (NK) cells constitute 30%-40% of the intrahepatic lymphocytes, and are believed to play an indispensable role in HBV clearance. The NK cells exert their direct antiviral effects via secretion of IFN-γ, tumor necrosis factor (TNF)-α, granulocyte-macrophage stimulating factor, interleukin (IL)-10 and transforming growth factor-β to inhibit HBV replication or stimulate killing of infected cells[14,15]. However, studies of the functions of NK cells in early HBV infection have produced controversial results.
In the woodchuck model of acute hepatitis B, NK cells are activated within 48-72 h after infection, consequently leading to a transient reduction of HBV replication; but, this activation is unable to induce a timely adaptive T cell response[16]. Further, in chimpanzee models with self-limited HBV infection, NK cells do not become activated until the major histocompatibility complex-restricted α/β T cells enter the liver and recognize antigen; the T-specific cells, for the most part, carry out the clearance of HBV[3]. Since these studies were carried out in animal models, it remains unclear to what extent the results mirror the human molecular processes.
In fact, the function of NK cells in early human HBV infection has proven very difficult to determine because the time frame from infection to diagnosis is typically more than 10-12 wk[17]. Regardless of how the NK cells function in this process, T-specific cells are the primary cell-types responsible for HBV clearance. Thus, the inaction of NK cells, along with immature or coincidently impaired functions of T cells, may explain how HBV infection progresses to chronicity.
In addition, in human CHB, immune responses of NK cells are known to be altered. These alterations include impaired cytolytic activity[18] and up-regulation of antiviral T cell death receptors, the latter of which renders the cell susceptible to targeting for destruction by host mechanisms[19]. Given that several studies have demonstrated that activated NK cells and T cells can cause liver damage[20-23], the defective NK cells’ functions and the NK cell-mediated disarming of T-specific cells might be protective for hepatocytes while simultaneously contributing to HBV persistence.
Kupffer cells
Kupffer cells (KCs) are macrophages residing in the liver sinusoids. Programmed to screen and clear pathogens that they engage, the KCs serve as scavengers. They produce pro- and anti-inflammatory cytokines, as well as other molecules known to have antipathogen activities. In the early phase of HBV infection, KCs directly interact with HBV and produce proinflammatory cytokines (i.e., IL-6, TNF-α and IFN-α) to elicit their antiviral effects[24,25]. Subsequently, NK cells and HBV-specific immunity are also activated to aid in eradication of the virus. While the interaction between KCs and hepatitis B surface antigen (HBsAg) is mostly dependent upon the TLRs[25,26], expression of TLR3 is found to be significantly decreased in CHB patients (compared to that in healthy controls); this aberrant expression may contribute to HBV chronicity.
Moreover, if HBV infection progresses to chronicity, the role of KCs will change, just as that of NK cells. The major cytokines secreted by KCs shift towards an anti-inflammatory profile (i.e., increased IL-10, which is known to contribute to the persistent existence of HBV in the host). In virus-persistent mouse models, stimulation of TLR2 by the hepatitis B core antigen leads to up-regulation of IL-10 secretion by KCs, with the ultimate detrimental exhaustion of anti-HBV CD8+ T cells[27]. IL-10 suppresses not only T cell-mediated specific immunity but also humoral immunity[28]. As such, KC depletion or IL-10 deficiency will lead to restoration of CD8+ T cell function, breaking the cycle of humoral immune tolerance and allowing for clearance of the HBV[29]. Thus, the collective results in the literature have revealed that KC secretes IL-10 in chronic HBV infection to disarm humoral and cellular immunity, which leads to HBV persistence.
Dendritic cells
Dendritic cells (DCs) represent the most efficient professional antigen-presenting cell type. As such, although they reside universally throughout the body, their frequency accounts for less than 1% of the total peripheral blood mononuclear cells. Classified by expression of specific surface markers, the DCs are divided into several types, with those most frequently studied in chronic HBV infection being the plasmacytoid (p)DCs and the myeloid (m)DCs.
Besides antigen processing and presenting, the pDCs produce appreciable amounts of IFN-α, which serves to inhibit viral replication via the TLR7/9 signaling pathways, when stimulated. However, some studies have demonstrated that the frequency of pDCs is reduced in CHB patients (as compared with normal controls)[30-32], indicating that HBV persistence is associated with pDC reduction. This hypothesis, however, has been doubted following the finding of pDC frequency in CHB patients being similar to that in healthy individuals[33-36]. Considering the rarity of pDCs in blood, it is likely that different experimental protocols account for the inconsistencies among these findings.
Regardless of the frequencies, the functions of pDCs are indeed hampered in chronic HBV infection. Most studies have reported a significant reduction of IFN-α secretion from pDCs[6,30-34]. Notably, the level of IFN-α has been found to be negatively correlated with alanine aminotransferase levels[33]. This finding could reflect a mechanism of HBV immune evasion and persistence, and an immune regulatory role for pDCs in this infection. Nevertheless, findings from an exceptional study led the authors to argue that pDC function was not impaired in chronic HBV infection[35]. The overall question remains unanswered.
mDCs are primarily responsible for inducing T cell differentiation and producing TNF-α. Unlike the pDCs, most studies on this cell type have found no difference in mDC frequency between CHB patients and healthy people[34-36]. Similar to the pDCs, however, it has been observed that the capacity of TNF-α production is impaired in mDCs when stimulated by HBV[34]. The other primary ability of mDCs, that of inducing T cell differentiation and proliferation, has not yet been defined in CHB patients.
HBV VIRUS-MEDIATED DISRUPTION OF THE HOST IMMUNOLOGICAL RESPONSE
RIG-I
HBV-induced type III IFN expression depends on RIG-I[37]. A recent study has suggested that over-expressed RIG-I could dramatically reduce the levels of HBV mRNA and DNA in vitro[9]. More interestingly, RIG-I can directly exert antivirus function by preventing the HBV P protein from binding with the HBV pregenomic RNA 5’ stem-loop region[37]. MAD5 and RIG-I can recognize many viral RNAs, subsequently initiating downstream signaling pathways by up-regulating the adapter protein mitochondria-antiviral signaling protein [MAVS, also known as virus-induced signaling adaptor (VISA) and interferon promoter stimulator-1 (IPS-1)]. In turn, the IFN regulatory transcription factor 3 (IRF3) and nuclear factor-kappa B (NF-κB), two of the most important proinflammatory transcription factors, become activated[38]. It has been reported, however, that the quantity of MDA5 is obviously decreased in CHB patients (compared to healthy controls)[39]. Moreover, a recent study revealed that HBV-induced miR146a attenuated innate immunity through targeting of RIG-I and RIG-G[40].
TLRs
In rat models, TLR3 activation has been shown to result in the production of type I IFN to control HBV replication[41]. In mammalian cells, TLR2 and TLR4 share the MyD88-dependent signaling pathway, so that they mediate activation of the same downstream signaling pathways, including the NF-κB, MAPK and PI-3k/Akt pathways. Ultimately, the production of proinflammatory cytokines (i.e., TNF-α and IL-8) are up-regulated in hepatocytes, to inhibit the HBV replication[42].
Intriguingly, lower expression of TLR2 has been observed in hepatocytes, KCs and peripheral monocytes of hepatitis B e antigen (HBeAg)-positive CHB patients (compared with that in HBeAg-negative CHB and controls)[43]. Further research found that this down-regulated expression correlated with the levels of plasma HBsAg[44] and that HBsAg was able to selectively inhibit TLR2–induced IL-12 production from human monocytes/macrophages in a dose-dependent manner[45]. HBeAg was also shown to specifically inhibit the TLR-mediated activation of NF-κB and IFN-β[46].
MAVS
MAVS is a downstream signaling pathway protein of RIG-I and MAD5. Localized in mitochondria[47], mitochondria-associated endoplasmic reticular membranes[48] and peroxisomes[49], the protein contains an N-terminal CARD-like domain and a C-terminal transmembrane domain, anchoring to the mitochondrial membrane[47]. RIG-I and MAD5 detect exogenous RNA in lymphocytes, subsequently communicating with the mitochondrial membrane and interacting with MAVS. Consequently, NF-κB and IRF3 become activated and induce IFN. Thus, MAVS is regarded as the central hub of the RIG-IFN axis.
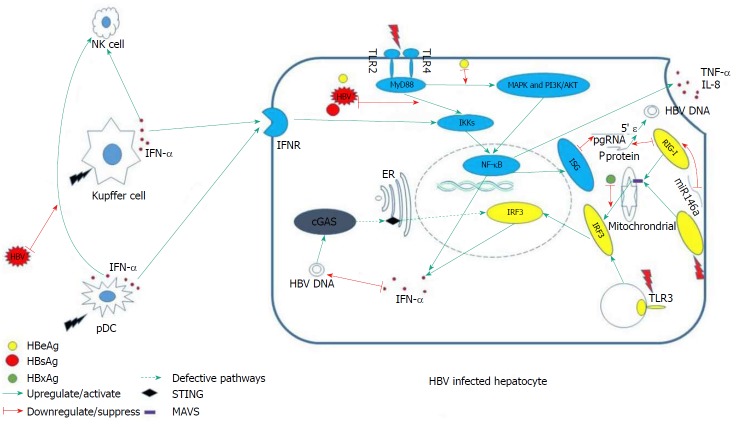
In the absence of MAVS, cells do not produce type I or III IFN or proinflammatory cytokines upon infection with RNA virus[47]. This result was recapitulated in MAVS knockout mice[50]. In addition, the HBV X protein mediates the degradation of MAVS by utilizing Lys(136) ubiquitin (directly on the MAVS protein), thereby suppressing the induction of IFN-γ[51] (Figure 1).
Figure 1.
Relationships between hepatitis B virus and innate immunity system. NK cell: Nature killer cell; IFN-α: Interferon-α; IFNR: Interferon receptor; HBV: Hepatitis B virus; cGAS: Cyclic GMP-AMP synthase; TLRs: Toll-like receptors; pDC: Plasmacytoid dendritic cells; MyD88: Myeloid differentiation primary response 88; MAPK: Mitogen-activated protein kinase; PI3K: Phosphatidylinositol-3 kinase; NF-κB: Nuclear factor-kappa B; IRF3: IFN regulatory transcription factor 3; ER: Endoplasmic reticulum; TNF-α: Tumor necrosis factor alpha; IL8: Interleukin 8; RIG1: Retinoic acid-inducible gene I.
HBV-MEDIATED DYSFUNCTION OF INNATE IMMUNE CELLS
HBV alters NK cell function
A study group found that the pDC-NK cell crosstalk was suppressed by HBV[5], which might explain why the reaction of NK cells in early HBV infection is dull. Activation of NK cells is strongly dependent on IFN-α produced by pDCs[52]. HBsAg and HBeAg were also found to impair NK cell function, through the down-regulation of IFN-γ expression[7,53]. In CHB patients, IFN-γ production is declined, and restoration of the IFN-γ-producing capacity is achievable after viral load reduction[54]. Consequently, the antiviral capacity of NK cells is compromised in chronic HBV infection[55-58]. In addition, HBV can stimulate generation of suppressive monocytes to initiate regulatory NK cell differentiation, resulting in T cell inhibition[59].
HBV suppresses the function of TLRs in KCs
As mentioned above, KCs’ ability to combat HBV infection mainly depends on expression of TLRs, but HBV itself can develop the ability to suppress TLR-mediated innate immune responses in the liver[26,60]. Accordingly, HBV replication will be controlled when TLRs are stimulated by agonists; the effective TLRs that potently inhibit HBV replication have been identified as TLR3 and TLR4[41]. In addition, the expression of TLR3 displays restoration after antiviral therapy[61] and hepatic HBV infection triggers a TLR3-dependent immune responses in the absence of HBsAg[62]. Thus, HBsAg might be at the center of the yet-to-be-defined HBV evasion mechanism.
HBV hampers secretion of antiviral cytokines from DCs
HBV lacks the capacity to activate pDCs, and many studies have also revealed that HBV could be able to inhibit IFN-α secretion from these cells[6,30-34]. Similarly, HBV can also reduce TNF-α production by mDCs[34]. Thus, HBV-mediated impairment of functions of both pDCs and mDCs suggests a potential mechanism of HBV persistence; but, the mechanism by which HBV induces such dysfunction remains elusive.
NOVEL THERAPEUTIC STRATEGIES THAT BOOST INNATE IMMUNITY
Currently, the most frequently applied strategy of CHB treatment is PEG-IFN-α/nucleoside analogs (NA) combination which works quite well. However, if altered NK cell functions are corrected, the outcome of the drug combination could possibly be improved. As described above, the functions and number of innate immune cells such as NK cells, KCs and DCs can be impaired in chronic HBV infection. Thus, replacing, restoring or boosting the functions of innate immune cells can be potential therapeutic targets for viral load reduction and further, HBV clearance. And in return, once viral load reduction is achieved, the antiviral capacities of innate immune cells often restore.
IFN-γ is able to inhibit the HBV replication within hepatocytes in the absence of immune cells[63]. Given that IFN-γ-producing capacity of NK cells restores after viral load reduction, it seems to be a positive-feedback when IFN-γ is used in HBV infection. What’s more, compared with IFN-α, IFN-γ serves as efficient as IFN-α but causes less side effects[64]. However, how IFN-γ combat HBV independent from immune cells remains unclear.
Agents enhancing the recognition of HBV could be used to wipe out HBV in the early stages of infection. Some known PRR agonists and TLR agonists could trigger a series of reaction in infected hepatocytes via IRF or NFκB pathways; since PRRs and TLRs are abundantly expressed in innate immune cells, such agonists are capable of rendering immune cells more powerful in recognizing HBV, thus contributing to early HBV clearance[65,66]. Vesatolimod (GS-9620), an oral TLR7 agonist, which was already involved in the 2nd phase clinical trial, was surprisingly found to be able to cause dose-dependent pharmacodynamic induction of ISG15 and a significant increase of serum cytokines[67].
Stimulation of certain innate immune cells to produce direct antiviral cytokines is another theoretical way to fight against human CHB. But in CHB patients, there is a balance between antiviral activity and anti-inflammatory activity. It is unknown whether the agents would break the balance and what consequences they would bring (Table 1).
Table 1.
Cytokines and factors implicated in causing inflammation in atherosclerosis
| Cytokines/factors | Abbreviations |
| Hepatitis B virus | HBV |
| Chronic hepatitis B | CHB |
| Interferon | IFN |
| Pattern recognition receptors | PRRs |
| Toll-like receptors | TLRs |
| Melanoma differentiation-associated gene 5 | MAD5 |
| Retinoic acid-inducible gene I | RIG I |
| Stimulator of IFN genes | STING |
| Cyclic GMP-AMP synthase | cGAS |
| Human sodium taurocholate cotransporting polypeptide | hNTCP |
| Natural killer cell | NK cell |
| Tumor necrosis factor | TNF |
| Interleukin | IL |
| Kupffer cells | KCs |
| Dendritic cells | DCs |
| Plasmacytoid dendritic cells | pDC |
| Myeloid dendritic cells | mDC |
| Mitochondria-antiviral signaling protein | MAVS |
| Virus-induced signaling adaptor | VISA |
| Nucleoside analogs | NA |
CONCLUSION
The crosstalk between innate immunity and HBV persistence has been a controversial topic for a number of years. The collective findings we present in this review demonstrate that HBV is able to hamper innate immunity in many ways, and even to alter the functions of innate immune cells in order to suppress specific immune responses. This partly explains why HBV manages to progress to chronicity and to exist persistently. We hope the field will build upon these insights, leading to a deeper and more comprehensive understanding of HBV persistence and the role of innate immunity. Such knowledge will serve as a foundation for future development of effective immunomodulation treatment for chronic HBV infection. Indeed, therapeutic strategies that aim to restore innate immune responses may represent remarkably potent tools for reducing HBV chronicity and, further, for eradicating HBV infection.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: April 3, 2018
First decision: May 29, 2018
Article in press: August 7, 2018
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chiu KW, Goral V, Hashimoto N S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
Contributor Information
Jian Tang, Department of Infectious Disease, the Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Zhen-Yu Wu, Department of Infectious Disease, the Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Rong-Juan Dai, Department of Infectious Disease, the First Affiliated Hospital of University of South China, Hengyang 421001, Hunan Province, China.
Jing Ma, Department of Infectious Disease, the Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China.
Guo-Zhong Gong, Department of Infectious Disease, the Second Xiangya Hospital, Central South University, Changsha 410011, Hunan Province, China. gongguozhong@csu.edu.cn.
References
- 1.WHO. 2018. Hepatitis B. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/ [Google Scholar]
- 2.Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479-480:672–686. doi: 10.1016/j.virol.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wieland S, Thimme R, Purcell RH, Chisari FV. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci USA. 2004;101:6669–6674. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomsen MK, Nandakumar R, Stadler D, Malo A, Valls RM, Wang F, Reinert LS, Dagnaes-Hansen F, Hollensen AK, Mikkelsen JG, et al. Lack of immunological DNA sensing in hepatocytes facilitates hepatitis B virus infection. Hepatology. 2016;64:746–759. doi: 10.1002/hep.28685. [DOI] [PubMed] [Google Scholar]
- 5.Shi CC, Tjwa ET, Biesta PJ, Boonstra A, Xie Q, Janssen HL, Woltman AM. Hepatitis B virus suppresses the functional interaction between natural killer cells and plasmacytoid dendritic cells. J Viral Hepat. 2012;19:e26–e33. doi: 10.1111/j.1365-2893.2011.01496.x. [DOI] [PubMed] [Google Scholar]
- 6.Martinet J, Dufeu-Duchesne T, Bruder Costa J, Larrat S, Marlu A, Leroy V, Plumas J, Aspord C. Altered functions of plasmacytoid dendritic cells and reduced cytolytic activity of natural killer cells in patients with chronic HBV infection. Gastroenterology. 2012;143:1586–1596.e8. doi: 10.1053/j.gastro.2012.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Han Q, Zhang C, Xiao M, Zhang J. Hepatitis B virus antigens impair NK cell function. Int Immunopharmacol. 2016;38:291–297. doi: 10.1016/j.intimp.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Guo H, Jiang D, Ma D, Chang J, Dougherty AM, Cuconati A, Block TM, Guo JT. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J Virol. 2009;83:847–858. doi: 10.1128/JVI.02008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suslov A, Boldanova T, Wang X, Wieland S, Heim MH. Hepatitis B Virus Does Not Interfere With Innate Immune Responses in the Human Liver. Gastroenterology. 2018;154:1778–1790. doi: 10.1053/j.gastro.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 11.Burdette DL, Vance RE. STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol. 2013;14:19–26. doi: 10.1038/ni.2491. [DOI] [PubMed] [Google Scholar]
- 12.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo F, Tang L, Shu S, Sehgal M, Sheraz M, Liu B, Zhao Q, Cheng J, Zhao X, Zhou T, et al. Activation of Stimulator of Interferon Genes in Hepatocytes Suppresses the Replication of Hepatitis B Virus. Antimicrob Agents Chemother. 2017;61:pii: e00771–17. doi: 10.1128/AAC.00771-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisicaro P, Valdatta C, Boni C, Massari M, Mori C, Zerbini A, Orlandini A, Sacchelli L, Missale G, Ferrari C. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut. 2009;58:974–982. doi: 10.1136/gut.2008.163600. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Han Y, Jin K, Wan Y, Wang S, Liu B, Liu Y, Lu S, Huang Z. Dynamic changes of cytotoxic T lymphocytes (CTLs), natural killer (NK) cells, and natural killer T (NKT) cells in patients with acute hepatitis B infection. Virol J. 2011;8:199. doi: 10.1186/1743-422X-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guy CS, Mulrooney-Cousins PM, Churchill ND, Michalak TI. Intrahepatic expression of genes affiliated with innate and adaptive immune responses immediately after invasion and during acute infection with woodchuck hepadnavirus. J Virol. 2008;82:8579–8591. doi: 10.1128/JVI.01022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webster GJ, Reignat S, Maini MK, Whalley SA, Ogg GS, King A, Brown D, Amlot PL, Williams R, Vergani D, et al. Incubation phase of acute hepatitis B in man: dynamic of cellular immune mechanisms. Hepatology. 2000;32:1117–1124. doi: 10.1053/jhep.2000.19324. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14:465–475. doi: 10.1038/cmi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peppa D, Gill US, Reynolds G, Easom NJ, Pallett LJ, Schurich A, Micco L, Nebbia G, Singh HD, Adams DH, et al. Up-regulation of a death receptor renders antiviral T cells susceptible to NK cell-mediated deletion. J Exp Med. 2013;210:99–114. doi: 10.1084/jem.20121172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriyama T, Guilhot S, Klopchin K, Moss B, Pinkert CA, Palmiter RD, Brinster RL, Kanagawa O, Chisari FV. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science. 1990;248:361–364. doi: 10.1126/science.1691527. [DOI] [PubMed] [Google Scholar]
- 21.Ando K, Moriyama T, Guidotti LG, Wirth S, Schreiber RD, Schlicht HJ, Huang SN, Chisari FV. Mechanisms of class I restricted immunopathology. A transgenic mouse model of fulminant hepatitis. J Exp Med. 1993;178:1541–1554. doi: 10.1084/jem.178.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maini MK, Boni C, Lee CK, Larrubia JR, Reignat S, Ogg GS, King AS, Herberg J, Gilson R, Alisa A, et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J Exp Med. 2000;191:1269–1280. doi: 10.1084/jem.191.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Q, Zhu YY, Chen J, Ye YB, Li JY, Liu YR, Hu ML, Zheng YC, Jiang JJ. Activated natural killer cells accelerate liver damage in patients with chronic hepatitis B virus infection. Clin Exp Immunol. 2015;180:499–508. doi: 10.1111/cei.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hösel M, Quasdorff M, Wiegmann K, Webb D, Zedler U, Broxtermann M, Tedjokusumo R, Esser K, Arzberger S, Kirschning CJ, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773–1782. doi: 10.1002/hep.23226. [DOI] [PubMed] [Google Scholar]
- 25.Boltjes A, van Montfoort N, Biesta PJ, Op den Brouw ML, Kwekkeboom J, van der Laan LJ, Janssen HL, Boonstra A, Woltman AM. Kupffer cells interact with hepatitis B surface antigen in vivo and in vitro, leading to proinflammatory cytokine production and natural killer cell function. J Infect Dis. 2015;211:1268–1278. doi: 10.1093/infdis/jiu599. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Meng Z, Jiang M, Pei R, Trippler M, Broering R, Bucchi A, Sowa JP, Dittmer U, Yang D, et al. Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology. 2009;49:1132–1140. doi: 10.1002/hep.22751. [DOI] [PubMed] [Google Scholar]
- 27.Li M, Sun R, Xu L, Yin W, Chen Y, Zheng X, Lian Z, Wei H, Tian Z. Kupffer Cells Support Hepatitis B Virus-Mediated CD8+ T Cell Exhaustion via Hepatitis B Core Antigen-TLR2 Interactions in Mice. J Immunol. 2015;195:3100–3109. doi: 10.4049/jimmunol.1500839. [DOI] [PubMed] [Google Scholar]
- 28.Xu L, Yin W, Sun R, Wei H, Tian Z. Kupffer cell-derived IL-10 plays a key role in maintaining humoral immune tolerance in hepatitis B virus-persistent mice. Hepatology. 2014;59:443–452. doi: 10.1002/hep.26668. [DOI] [PubMed] [Google Scholar]
- 29.Tian Y, Kuo CF, Akbari O, Ou JH. Maternal-Derived Hepatitis B Virus e Antigen Alters Macrophage Function in Offspring to Drive Viral Persistence after Vertical Transmission. Immunity. 2016;44:1204–1214. doi: 10.1016/j.immuni.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beckebaum S, Cicinnati VR, Dworacki G, Müller-Berghaus J, Stolz D, Harnaha J, Whiteside TL, Thomson AW, Lu L, Fung JJ, et al. Reduction in the circulating pDC1/pDC2 ratio and impaired function of ex vivo-generated DC1 in chronic hepatitis B infection. Clin Immunol. 2002;104:138–150. doi: 10.1006/clim.2002.5245. [DOI] [PubMed] [Google Scholar]
- 31.Duan XZ, Wang M, Li HW, Zhuang H, Xu D, Wang FS. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. J Clin Immunol. 2004;24:637–646. doi: 10.1007/s10875-004-6249-y. [DOI] [PubMed] [Google Scholar]
- 32.Shi B, Ren G, Hu Y, Wang S, Zhang Z, Yuan Z. HBsAg inhibits IFN-α production in plasmacytoid dendritic cells through TNF-α and IL-10 induction in monocytes. PLoS One. 2012;7:e44900. doi: 10.1371/journal.pone.0044900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woltman AM, Op den Brouw ML, Biesta PJ, Shi CC, Janssen HL. Hepatitis B virus lacks immune activating capacity, but actively inhibits plasmacytoid dendritic cell function. PLoS One. 2011;6:e15324. doi: 10.1371/journal.pone.0015324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Molen RG, Sprengers D, Binda RS, de Jong EC, Niesters HG, Kusters JG, Kwekkeboom J, Janssen HL. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 35.Tavakoli S, Mederacke I, Herzog-Hauff S, Glebe D, Grün S, Strand D, Urban S, Gehring A, Galle PR, Böcher WO. Peripheral blood dendritic cells are phenotypically and functionally intact in chronic hepatitis B virus (HBV) infection. Clin Exp Immunol. 2008;151:61–70. doi: 10.1111/j.1365-2249.2007.03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gehring AJ, Haniffa M, Kennedy PT, Ho ZZ, Boni C, Shin A, Banu N, Chia A, Lim SG, Ferrari C, et al. Mobilizing monocytes to cross-present circulating viral antigen in chronic infection. J Clin Invest. 2013;123:3766–3776. doi: 10.1172/JCI66043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, et al. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123–132. doi: 10.1016/j.immuni.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 38.Cao X, Ding Q, Lu J, Tao W, Huang B, Zhao Y, Niu J, Liu YJ, Zhong J. MDA5 plays a critical role in interferon response during hepatitis C virus infection. J Hepatol. 2015;62:771–778. doi: 10.1016/j.jhep.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Ebrahim M, Mirzaei V, Bidaki R, Shabani Z, Daneshvar H, Karimi-Googheri M, Khaleghinia M, Afrooz MR, Yousefpoor Y, Arababadi MK. Are RIG-1 and MDA5 Expressions Associated with Chronic HBV Infection? Viral Immunol. 2015;28:504–508. doi: 10.1089/vim.2015.0056. [DOI] [PubMed] [Google Scholar]
- 40.Hou Z, Zhang J, Han Q, Su C, Qu J, Xu D, Zhang C, Tian Z. Hepatitis B virus inhibits intrinsic RIG-I and RIG-G immune signaling via inducing miR146a. Sci Rep. 2016;6:26150. doi: 10.1038/srep26150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Lu M, Meng Z, Trippler M, Broering R, Szczeponek A, Krux F, Dittmer U, Roggendorf M, Gerken G, et al. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology. 2007;46:1769–1778. doi: 10.1002/hep.21897. [DOI] [PubMed] [Google Scholar]
- 42.Thompson AJ, Colledge D, Rodgers S, Wilson R, Revill P, Desmond P, Mansell A, Visvanathan K, Locarnini S. Stimulation of the interleukin-1 receptor and Toll-like receptor 2 inhibits hepatitis B virus replication in hepatoma cell lines in vitro. Antivir Ther. 2009;14:797–808. doi: 10.3851/IMP1294. [DOI] [PubMed] [Google Scholar]
- 43.Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R, Rodgers S, Kurtovic J, Chang J, Lewin S, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45:102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, Zhang Q, Wang J, Zhang Z, Shen F, et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400–408. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Chen Z, Hu C, Qian F, Cheng Y, Wu M, Shi B, Chen J, Hu Y, Yuan Z. Hepatitis B virus surface antigen selectively inhibits TLR2 ligand-induced IL-12 production in monocytes/macrophages by interfering with JNK activation. J Immunol. 2013;190:5142–5151. doi: 10.4049/jimmunol.1201625. [DOI] [PubMed] [Google Scholar]
- 46.Lang T, Lo C, Skinner N, Locarnini S, Visvanathan K, Mansell A. The hepatitis B e antigen (HBeAg) targets and suppresses activation of the toll-like receptor signaling pathway. J Hepatol. 2011;55:762–769. doi: 10.1016/j.jhep.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 47.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 48.Horner SM, Liu HM, Park HS, Briley J, Gale M Jr. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc Natl Acad Sci USA. 2011;108:14590–14595. doi: 10.1073/pnas.1110133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Wei C, Ni C, Song T, Liu Y, Yang X, Zheng Z, Jia Y, Yuan Y, Guan K, Xu Y, et al. The hepatitis B virus X protein disrupts innate immunity by downregulating mitochondrial antiviral signaling protein. J Immunol. 2010;185:1158–1168. doi: 10.4049/jimmunol.0903874. [DOI] [PubMed] [Google Scholar]
- 52.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 53.Jegaskanda S, Ahn SH, Skinner N, Thompson AJ, Ngyuen T, Holmes J, De Rose R, Navis M, Winnall WR, Kramski M, et al. Downregulation of interleukin-18-mediated cell signaling and interferon gamma expression by the hepatitis B virus e antigen. J Virol. 2014;88:10412–10420. doi: 10.1128/JVI.00111-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tjwa ET, van Oord GW, Hegmans JP, Janssen HL, Woltman AM. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J Hepatol. 2011;54:209–218. doi: 10.1016/j.jhep.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137:1151–1160, 1160.e1-1160.e7. doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 56.Lunemann S, Malone DF, Hengst J, Port K, Grabowski J, Deterding K, Markova A, Bremer B, Schlaphoff V, Cornberg M, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis. 2014;209:1362–1373. doi: 10.1093/infdis/jit561. [DOI] [PubMed] [Google Scholar]
- 57.Heiberg IL, Pallett LJ, Winther TN, Høgh B, Maini MK, Peppa D. Defective natural killer cell anti-viral capacity in paediatric HBV infection. Clin Exp Immunol. 2015;179:466–476. doi: 10.1111/cei.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh S, Nandi M, Pal S, Mukhopadhyay D, Chakraborty BC, Khatun M, Bhowmick D, Mondal RK, Das S, Das K, et al. Natural killer cells contribute to hepatic injury and help in viral persistence during progression of hepatitis B e-antigen-negative chronic hepatitis B virus infection. Clin Microbiol Infect. 2016;22:733.e9–733.e19. doi: 10.1016/j.cmi.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 59.Li H, Zhai N, Wang Z, Song H, Yang Y, Cui A, Li T, Wang G, Niu J, Crispe IN, et al. Regulatory NK cells mediated between immunosuppressive monocytes and dysfunctional T cells in chronic HBV infection. Gut. 2017;pii:gutjnl–2017-314098. doi: 10.1136/gutjnl-2017-314098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang M, Broering R, Trippler M, Poggenpohl L, Fiedler M, Gerken G, Lu M, Schlaak JF. Toll-like receptor-mediated immune responses are attenuated in the presence of high levels of hepatitis B virus surface antigen. J Viral Hepat. 2014;21:860–872. doi: 10.1111/jvh.12216. [DOI] [PubMed] [Google Scholar]
- 61.Huang YW, Lin SC, Wei SC, Hu JT, Chang HY, Huang SH, Chen DS, Chen PJ, Hsu PN, Yang SS, et al. Reduced Toll-like receptor 3 expression in chronic hepatitis B patients and its restoration by interferon therapy. Antivir Ther. 2013;18:877–884. doi: 10.3851/IMP2630. [DOI] [PubMed] [Google Scholar]
- 62.Real CI, Lu M, Liu J, Huang X, Trippler M, Hossbach M, Deckert J, Jahn-Hofmann K, Ickenstein LM, John MJ, et al. Hepatitis B virus genome replication triggers toll-like receptor 3-dependent interferon responses in the absence of hepatitis B surface antigen. Sci Rep. 2016;6:24865. doi: 10.1038/srep24865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia Y, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, Michler T, Wisskirchen K, Cheng X, Zhang K, et al. Interferon-γ and Tumor Necrosis Factor-α Produced by T Cells Reduce the HBV Persistence Form, cccDNA, Without Cytolysis. Gastroenterology. 2016;150:194–205. doi: 10.1053/j.gastro.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 64.Chan HLY, Ahn SH, Chang TT, Peng CY, Wong D, Coffin CS, Lim SG, Chen PJ, Janssen HLA, Marcellin P, et al. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: A randomized phase 2b study (LIRA-B) J Hepatol. 2016;64:1011–1019. doi: 10.1016/j.jhep.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 65.Isogawa M, Robek MD, Furuichi Y, Chisari FV. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol. 2005;79:7269–7272. doi: 10.1128/JVI.79.11.7269-7272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucifora J, Maadadi S, Floriot O, Daffis S, Fletcher S, Zoulim F, Durantel D. P0535: Direct antiviral effects of various pattern recognition receptor (PRR) agonists in HBV-replicating hepatocytes. J Hepatol. 2015;62:S515–S516. [Google Scholar]
- 67.Janssen HLA, Brunetto MR, Kim YJ, Ferrari C, Massetto B, Nguyen AH, Joshi A, Woo J, Lau AH, Gaggar A, et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J Hepatol. 2018;68:431–440. doi: 10.1016/j.jhep.2017.10.027. [DOI] [PubMed] [Google Scholar]