ABSTRACT
Background
In previous meta-analyses of prospective observational studies, we investigated the association between food groups and risk of chronic disease.
Objective
The aim of the present network meta-analysis (NMA) was to assess the effects of these food groups on intermediate-disease markers across randomized intervention trials.
Design
Literature searches were performed until January 2018. The following inclusion criteria were defined a priori: 1) randomized trial (≥4 wk duration) comparing ≥2 of the following food groups: refined grains, whole grains, nuts, legumes, fruits and vegetables, eggs, dairy, fish, red meat, and sugar-sweetened beverages (SSBs); 2) LDL cholesterol and triacylglycerol (TG) were defined as primary outcomes; total cholesterol, HDL cholesterol, fasting glucose, glycated hemoglobin, homeostasis model assessment insulin resistance, systolic and diastolic blood pressure, and C-reactive protein were defined as secondary outcomes. For each outcome, a random NMA was performed, and for the ranking, the surface under the cumulative ranking curves (SUCRA) was determined.
Results
A total of 66 randomized trials (86 reports) comparing 10 food groups and enrolling 3595 participants was identified. Nuts were ranked as the best food group at reducing LDL cholesterol (SUCRA: 93%), followed by legumes (85%) and whole grains (70%). For reducing TG, fish (97%) was ranked best, followed by nuts (78%) and red meat (72%). However, these findings are limited by the low quality of the evidence. When combining all 10 outcomes, the highest SUCRA values were found for nuts (66%), legumes (62%), and whole grains (62%), whereas SSBs performed worst (29%).
Conclusion
The present NMA provides evidence that increased intake of nuts, legumes, and whole grains is more effective at improving metabolic health than other food groups. For the credibility of diet-disease relations, high-quality randomized trials focusing on well-established intermediate-disease markers could play an important role. This systematic review was registered at PROSPERO (www.crd.york.ac.uk/PROSPERO) as CRD42018086753.
Keywords: network meta-analysis, food groups, intervention trials, evidence synthesis, intermediate disease markers
INTRODUCTION
According to the Global Burden of Disease study, in 2016 dietary risk factors were accountable for nearly 20% of all deaths worldwide and 10% of all disability-adjusted life years (1). Several dose-response meta-analyses of prospective observational studies recently investigated the association between food groups and risk of various chronic diseases (all-cause mortality, cardiovascular disease, colorectal cancer, type 2 diabetes, and hypertension). Overall, a lower disease risk for food groups of plant origin (whole grains, fruits, vegetables, nuts, legumes) was found, whereas there was a higher disease risk for sugar-sweetened beverages (SSBs) and certain food groups of animal origin (red meat, processed meat, eggs) (2–7).
Prospective observational studies with hard endpoints provide many insights into diet-disease relations and are the most important source to derive dietary recommendations for the primary prevention of chronic diseases (8). The core limitations of prospective observational studies in the nutritional field, such as residual confounding, measurement error, and small effect sizes, need to be considered (9). Therefore, recommendations without evidence from intervention studies should be cautiously applied (10). However, there are many obstacles that preclude dietary randomized controlled trials (RCTs) with hard endpoints, including long-term adherence, difficulty to induce dietary change, ethical considerations, and very long follow-up (11).
A promising approach to close the gap between evidence generated by meta-analyses of prospective observational studies and the often missing evidence from RCTs is the meta-analytical utilization of intervention trials with intermediate disease markers that have used similar dietary exposures as investigated in prospective observational studies. The utilization will be considerably enhanced by network meta-analyses (NMA) methods. The methodology of NMA is an extension of the pairwise meta-analysis that enables a simultaneous comparison of intervention trials. NMA combines direct (i.e., from trials comparing directly two interventions) and indirect evidence (i.e., from a connected root via one more intermediate comparators) in a network of trials. In this way, it enables inference about every possible comparison between a pair of interventions in the network even when some comparisons have never been evaluated in a trial (12–14).
The aim of the present NMA was to investigate the hypothesis that increased intake of foods of plant origin, such as nuts, legumes, whole grains, fruits, and vegetables is more effective at the primary prevention of metabolic disturbances and diseases than intake of other food groups. Therefore we compared the effects of different food groups across randomized intervention studies on established intermediate markers of chronic disease that were previously meta-analyzed using prospective observational studies (2–7).
METHODS
The NMA was registered at PROSPERO International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=86753). The present NMA was planned, conducted, and reported in adherence to standards of quality for reporting NMAs (15, 16).
Search strategy
The literature search was performed using the electronic databases PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and Google Scholar until January 2018, with no restriction of language and calendar date, and with a predefined search strategy (Supplemental Appendix 1, Supplemental Figure 1, and Supplemental Table 1).
Furthermore, reviews and the reference lists from the identified articles were screened to search for additional relevant studies. Searches were conducted by one author (LS), with disagreements being resolved with the involvement of another author (GH).
Eligibility criteria
Studies were included in the review if they met all of the following criteria:
1) Randomized study design (parallel or crossover) comparing at least two of the following food groups: refined grains (grain products that were modified to remove the bran and germ, e.g., refined wheat, spaghetti, cookies, white rice, pretzels, breakfast cereals); whole grains (grain products containing the whole grain, e.g., whole grain oatmeal, whole grain cookies, whole grain pasta, whole grain bread, brown rice); fruits and vegetables (e.g., berries, apples, carrots); nuts (e.g., almonds, hazelnuts, walnuts, pistachio); legumes (e.g., beans, lentils, peas, chickpeas, soy); eggs; dairy (e.g., milk); fish (e.g., sardines, salmon, snook); red meat (e.g., ground beef, pork); and SSBs;
2) Similar energy intake across intervention arms within a randomized trial;
3) Minimum intervention period of 4 wk;
4) Patients with a mean age ≥18 y;
5) Outcomes including LDL cholesterol (mmol/L) and triacylglycerol (TG) (mmol/L) (defined as primary outcomes); total cholesterol (TC), HDL cholesterol, fasting glucose (FG), glycated hemoglobin (HbA1c), HOMA-IR, systolic and diastolic blood pressure (SBP/DBP), and C-reactive protein (CRP) (defined as secondary outcomes).
The following studies were excluded:
1) Randomized trials including pregnant women, children and adolescents, and patients with cancer;
2) Intervention studies solely based on dietary patterns, multiple food groups (>1 of the above-mentioned food groups within a intervention arm), or dietary supplements;
3) Studies with a co-intervention (e.g., lifestyle, drug) that was not applied in all the intervention arms.
Data extraction
After determination of the study selection, two reviewers extracted the following characteristics: name of first author, year of publication, study origin (country), study design (randomized controlled trial: parallel or crossover, washout period), sample size, mean baseline age, mean baseline BMI, study duration, sex, description of the food group arms, type of diet (energy restricted, ad libitum, isocaloric), drop outs, and conflict of interest. Outcome data included postintervention values with corresponding standard deviations.
Risk of bias assessment
Full copies of the studies were assessed by one author (LS) for methodological quality using the risk of bias assessment tool from the Cochrane Collaboration (17). The following sources of bias were assessed: selection bias (random sequence generation and allocation concealment), performance bias (blinding of personnel), attrition bias (incomplete outcome data), reporting bias (selective reporting), and funding bias.
Studies were classified as being at low risk of bias (if ≥4 of a maximum of 6 items were rated as low risk and a maximum of 1 item was rated with a high risk of bias), high risk of bias (if ≥2 out of a maximum of 6 items were rated with a high risk of bias), and moderate or unclear risk (all other studies) (Supplemental Figure 2).
Dealing with missing data
We contacted authors to request missing outcome data (one author was sent additional data). If the postintervention values with the corresponding standard deviations were not available, the change scores with the corresponding standard deviations were used (Supplemental Table 2), according to the guidelines of the Cochrane Handbook (18).
Data synthesis
Description of the available data
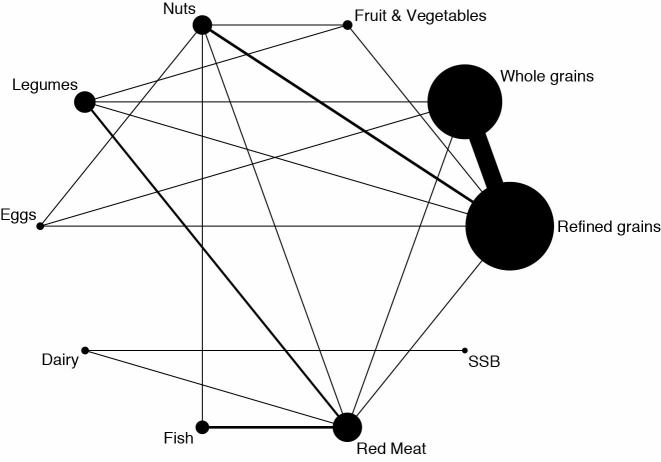
We illustrated the available direct comparisons between different food groups using a network diagram for each outcome (19). The size of the nodes is proportional to the sample size of each dietary intervention and the thickness of the lines is proportional to the number of studies available (Figure 1).
FIGURE 1.
Network diagram for LDL cholesterol. The size of the nodes is proportional to the total number of participants allocated to each food group, and the thickness of the lines is proportional to the number of studies evaluating each direct comparison. SSB, sugar-sweetened beverages.
Assessment of transitivity
To evaluate the assumption of transitivity, we compared the distribution of the potential effect modifiers across the available direct comparisons. We considered the following effect modifiers: age, BMI, length of follow-up, sample size, percentage of female participants (Supplemental Figures 3–7).
Statistical analysis
For each outcome measure of interest, we performed random effects NMA in order to determine the pooled relative effect of each food group against every other food group. The similarity of trials within each direct comparison was assessed. NMA was then used to synthesize the direct and indirect effects (Supplemental Table 3). Compared with the standard pairwise meta-analysis, the method of NMA is an extension and enables a simultaneous comparison of multiple interventions, forming a connected network while preserving the internal randomization of individual trials. We ran random effects NMA for each outcome to estimate all possible pairwise relative effects and to obtain a clinically meaningful relative ranking of the different dietary interventions. The summary mean differences (together with their 95% CIs) are presented in league tables (Tables 1 and 2, Supplemental Tables 4–11). The relative ranking of the different food groups for each outcome were estimated using the distribution of the ranking probabilities and the surface under the cumulative ranking curves (SUCRA) (Table 3) (20). We fitted all analyses described into a frequentist framework in Stata (21) with the network package (22), and produced presentation tools with the network graphs package (23).
TABLE 1.
League table for LDL cholesterol1
| Nuts | |||||||||
| −0.04 (−0.23, 0.14) | Legumes | ||||||||
| −0.12 (−0.24, 0.01) | −0.08 (−0.24, 0.09) | Whole grains | |||||||
| −0.24 (−0.35, −0.13) | −0.19 (−0.36, −0.03) | −0.12 (−0.18, −0.06) | Refined grains | ||||||
| −0.15 (−0.36, 0.07) | −0.10 (−0.31, 0.11) | −0.03 (−0.23, 0.18) | 0.09 (−0.11, 0.29) | Fruits and vegetables | |||||
| −0.25 (−0.45, −0.06) | −0.21 (−0.45, 0.04) | −0.13 (−0.33, 0.06) | −0.02 (−0.21, 0.18) | −0.11 (−0.38, 0.17) | Eggs | ||||
| −0.32 (−0.76, 0.12) | −0.28 (−0.72, 0.16) | −0.20 (−0.64, 0.24) | −0.08 (−0.52, 0.35) | −0.18 (−0.65, 0.29) | −0.07 (−0.54, 0.40) | Dairy | |||
| −0.34 (−0.54, −0.14) | −0.29 (−0.50, −0.08) | −0.22 (−0.42, −0.02) | −0.10 (−0.29, 0.10) | −0.19 (−0.45, 0.07) | −0.08 (−0.35, 0.18) | −0.01 (−0.45, 0.42) | Fish | ||
| −0.34 (−0.50, −0.18) | −0.30 (−0.46, −0.13) | −0.22 (−0.38, −0.06) | −0.10 (−0.26, 0.05) | −0.20 (−0.43, 0.03) | −0.09 (−0.32, 0.15) | −0.02 (−0.43, 0.39) | −0.01 (−0.14, 0.13) | Red meat | |
| −0.35 (−0.91, 0.20) | −0.31 (−0.87, 0.25) | −0.24 (−0.79, 0.32) | −0.12 (−0.67, 0.44) | −0.21 (−0.79, 0.37) | −0.10 (−0.68, 0.48) | −0.03 (−0.37, 0.31) | −0.02 (−0.57, 0.53) | −0.01 (−0.55, 0.52) | SSBs |
1The value below the food groups corresponds to the difference in mean (95% CI) in LDL cholesterol (mmol/L) between the column and the row (e.g. the mean difference in average LDL-cholesterol between nuts and red meat is −0.34 mmol/L). SSB, sugar-sweetened beverage.
TABLE 2.
League table for triacyglycerols1
| Nuts | |||||||||
| −0.05 (−0.18, 0.08) | Legumes | ||||||||
| −0.07 (−0.17, 0.03) | −0.02 (−0.14, 0.11) | Whole grains | |||||||
| −0.15 (−0.23, −0.06) | −0.09 (−0.21, 0.03) | −0.08 (−0.12, −0.03) | Refined grains | ||||||
| −0.12 (−0.26, 0.02) | −0.07 (−0.23, 0.09) | −0.05 (−0.19, 0.09) | 0.03 (−0.11, 0.16) | Fruits and vegetables | |||||
| −0.19 (−0.30, −0.07) | −0.13 (−0.29, 0.02) | −0.12 (−0.24, 0.01) | −0.04 (−0.16, 0.08) | −0.07 (−0.24, 0.10) | Eggs | ||||
| −0.10 (−0.38, 0.17) | −0.05 (−0.35, 0.24) | −0.03 (−0.32, 0.25) | 0.04 (−0.24, 0.32) | 0.01 (−0.29, 0.32) | 0.08 (−0.21, 0.37) | Dairy | |||
| 0.07 (−0.00, 0.14) | 0.12 (−0.02, 0.26) | 0.14 (0.01, 0.27) | 0.22 (0.10, 0.33) | 0.19 (0.03, 0.35) | 0.26 (0.12, 0.39) | 0.17 (−0.10, 0.45) | Fish | ||
| −0.01 (−0.07, 0.04) | 0.04 (−0.09, 0.16) | 0.06 (−0.04, 0.15) | 0.13 (0.04, 0.22) | 0.10 (−0.04, 0.25) | 0.17 (0.05, 0.30) | 0.09 (−0.18, 0.36) | −0.08 (−0.17, −0.00) | Red meat | |
| −0.23 (−0.63, 0.16) | −0.18 (−0.60, 0.23) | −0.16 (−0.57, 0.24) | −0.09 (−0.49, 0.32) | −0.11 (−0.53, 0.31) | −0.05 (−0.46, 0.37) | −0.13 (−0.42, 0.16) | −0.30 (−0.71, 0.10) | −0.22 (−0.61, 0.18) | SSBs |
1The value below the food groups corresponds to the difference in mean (95% CI) in triacylglycerols (mmol/L) between the column and the row (e.g. the mean difference in average triacyglycerols between nuts and red meat is −0.01 mmol/L). SSB, sugar-sweetened beverage.
TABLE 3.
Food group relative ranking for each individual primary and secondary outcome and summary ranking across outcomes1
| Primary outcomes | Secondary outcomes | Summary ranking | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Food group | LDL-C | TG | TC | HDL-C | FG | HbA1c | HOMA-IR | SBP | DBP | CRP | All outcomes combined |
| Nuts | 93 | 78 | 92 | 62 | 84 | 37 | 67 | 32 | 42 | 76 | 66 |
| Legumes | 85 | 58 | 91 | 12 | 51 | 61 | 76 | 69 | 70 | 45 | 62 |
| Whole grains | 70 | 53 | 71 | 44 | 57 | 76 | 86 | 44 | 57 | 61 | 62 |
| Refined grains | 42 | 25 | 42 | 49 | 74 | 70 | 56 | 14 | 30 | 36 | 44 |
| Fruits and vegetables | 63 | 35 | 58 | 49 | 20 | 52 | 43 | 91 | 54 | 26 | 49 |
| Eggs | 40 | 16 | 30 | 58 | NA | NA | 6 | 41 | 41 | 80 | 39 |
| Dairy | 33 | 44 | 33 | 49 | 32 | NA | 21 | NA | NA | 48 | 37 |
| Fish | 23 | 97 | 23 | 91 | NA | NA | 47 | 62 | 33 | 32 | 51 |
| Red meat | 20 | 72 | 28 | 57 | 24 | 5 | NA | 48 | 74 | 46 | 42 |
| SSBs | 30 | 23 | 32 | 30 | 28 | NA | NA | NA | NA | NA | 29 |
1The values represent the SUCRA for all outcomes (e.g, nuts were ranked as the best food group for reducing LDL cholesterol, SUCRA: 93%; fish was ranked as the best food group for reducing triacylglycerol, SUCRA: 97%). CRP, C-reactive protein; DBP, diastolic blood pressure; FG, fasting glucose; HbA1c, glycated hemoglobin; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol NA, not applicable; SBP, systolic blood pressure; SSB, sugar-sweetened beverage; SUCRA, surface under the cumulative ranking curves; TC, total cholesterol; TG, triacyglycerols.
Assessment of inconsistency
To evaluate the presence of statistical inconsistency, we used a loop-specific approach (24) (Supplemental Figures 8 and 9) to detect loops of evidence that might present important inconsistency, as well as a side-splitting approach (25) to detect comparisons for which direct estimates disagree with indirect evidence from the entire network (Supplemental Tables 12 and 13). We used a design-by-treatment interaction model (26, 27) to investigate the presence of inconsistency jointly from all possible sources in the entire network simultaneously.
Sensitivity analyses
We conducted a sensitivity analysis by excluding studies considered to be at high risk of bias for the two primary outcomes (LDL cholesterol and TG) (Supplemental Tables 14 and 15), excluding trials conducted before the year 2000, and by comparing food groups of plant origin with ones of animal origin.
Small study effects and publication bias
We produced a comparison-adjusted funnel plot (19) to assess the magnitude of funnel plot asymmetry for the primary outcomes (Supplemental Figures 10 and 11).
Quality of evidence
To make inferences about the quality of evidence from the NMA, we used the GRADE system extended for NMA following the approach suggested by Salanti et al. (28) for the primary outcomes (Supplemental Tables 16 and 17).
RESULTS
Out of 15,192 records identified by the literature search, 309 full-text articles were assessed in detail as they reported on one or more of the food groups of interest in the title or abstract (Supplemental Figure 1). Of these, 223 were excluded (Supplemental References). The reasons for exclusion are summarized in Supplemental Table 1.
Overall, 66 trials (86 reports) (29–114) met the eligibility criteria and provided sufficient data to be included in the meta-analysis. The included studies were published between 1979 and 2018, and had enrolled a total of 3595 participants (280 type 2 diabetes patients). Detailed study and participant characteristics are summarized in Supplemental Table 2.
Of the trials, 8 were judged to be at low risk of bias, 11 trials were judged to be at high risk of bias, and 47 trials were classified to be at moderate or unclear risk of bias (Supplemental Figure 2). The most common comparison in the trials was between a whole grains arm and a refined grains arm (n = 30). In the transitivity analyses, we observed differences for the distribution of participant characteristics, BMI, age, and percentage of female participants across the direct comparisons. For the study characteristics, study length, and sample size, the differences between direct comparisons was minor. For several direct comparisons, the number of included trials was too low to appropriately test transitivity (Supplemental Figures 3–7). Study effects came more often from indirect comparisons than from direct comparisons (Supplemental Table 3).
The effect size estimates for the comparison of every food group compared with each other food group on LDL cholesterol and TG (Tables 1 and 2), TC, HDL cholesterol, FG, HOMA-IR, HbA1c, SBP, DBP, and CRP are given in Supplemental Tables 4–11.
Primary outcomes
Nuts were more effective at reducing LDL cholesterol (−0.34 to −0.24 mmol/L) compared with refined grains, eggs, fish, and red meat. Legumes and whole grains were more effective at reducing LDL cholesterol (−0.30 to −0.12 mmol/L) compared with refined grains, fish, and red meat (Table 1). Regarding TG reduction, nuts and whole grains were more effective compared with refined grains (−0.15 to −0.08 mmol/L). Fish was more effective at reducing TG (−0.26 to −0.08 mmol/L) compared with whole grains, refined grains, fruits and vegetables, eggs, and red meat (Table 2).
Nuts had the highest SUCRA value (93%), followed by legumes (85%) and whole grains (70%), for LDL cholesterol reduction, whereas fish (97%) had the highest SUCRA value for TG reduction, followed by nuts (78%) and red meat (72%) (Table 3).
The loop-specific approach showed some inconsistency in the loop formed by refined grains, whole grains, eggs, and red meat for LDL cholesterol and TG (Supplemental Figures 8 and 9). However, the side-splitting approach suggested no significant inconsistency for LDL cholesterol (Supplemental Table 12) or TG (Supplemental Table 13), and also the design-by-treatment model showed no significant inconsistency for LDL cholesterol (P = 0.87) and TG (P = 0.92).
Secondary outcomes
Total cholesterol and HDL cholesterol
Nuts were more effective at reducing TC (−0.39 to −0.30 mmol/L) than were refined grains, eggs, fish, and red meat. Legumes and whole grains were more effective at reducing TC (−0.39 to −0.16 mmol/L) than were refined grains, eggs, fish, and red meat (Supplemental Table 4). Nuts, whole grains, refined grains, fish, and red meat were more effective at increasing HDL cholesterol (0.06 to 0.13 mmol/L) than were legumes (Supplemental Table 5).
Nuts had the highest SUCRA value (92%) for TC reduction, whereas fish (91%) had the highest SUCRA value to improve HDL cholesterol (Table 3).
We observed some important inconsistency (with the side-splitting approach) for TC in the comparisons of refined grains with nuts and nuts with red meat, but not for HDL cholesterol. The design-by-treatment model showed no significant inconsistency for TC (P = 0.36), and HDL cholesterol (P = 0.86).
Glycemic control
Nuts, whole grains, and refined grains were more effective at reducing FG (−0.43 to −0.35 mmol/L) than were fruits and vegetables and red meat (Supplemental Table 6). Whole grains were more effective at reducing HOMA-IR (−0.22; 95% CI: −0.40, −0.05) than were refined grains. Whole grains, nuts, legumes, and refined grains were more effective at reducing HOMA-IR (−1.01 to −0.53) compared with eggs and dairy (Supplemental Table 8). No significant effects were detected for HbA1c (Supplemental Table 7).
Whole grains had the highest SUCRA value for improvements in FG (87%), HbA1c (76%), and HOMA-IR (86%) (Table 3).
Although no significant inconsistency in the side-splitting approach was observed for HbA1c and HOMA-IR, some inconsistency was observed for FG when comparing refined grains with fruits and vegetables, and between nuts and legumes. The design-by-treatment model also showed no significant inconsistency for HbA1c (P = 0.95) or HOMA-IR (P = 0.48), but did for FG (P < 0.01).
Blood pressur
Whole grains were more effective at reducing SBP (−1.79 mm Hg, 95% CI −3.55, −0.03 mm Hg) compared with refined grains, whereas fruits and vegetables were more effective at reducing SBP compared with nuts and refined grains (−8.61 to −7.49 mm Hg) (Supplemental Table 9). No significant effects were observed for DBP (Supplemental Table 10).
Fruits and vegetables had the highest SUCRA value (91%) to improve SBP, whereas red meat (74%) had the highest SUCRA value to improve DBP (Table 3).
The side-splitting approach and design-by-treatment model suggested that there was no significant inconsistency for SBP (P = 0.34) and DBP (P = 0.96).
C-reactive protein
Nuts were more effective at reducing CRP (−0.43 to −0.28 mg/L) compared with refined grains, fish, and red meat, whereas eggs were more effective compared with nuts, refined grains, fish, and red meat (−0.77 mg/L to −0.35 mg/L) (Supplemental Table 11).
Eggs had the highest SUCRA value (80%) for CRP reduction (Table 3).
The side-splitting approach and design-by-treatment model suggested no significant inconsistency.
Summary across outcomes
When combining all 10 outcomes (LDL cholesterol, TG, TC, HDL cholesterol, FG, HbA1c, HOMA-IR, SBP, DBP, and CRP), the highest SUCRA values were found for nuts (66%), legumes (62%), and whole grains (62%). SSBs performed the worst (29%) (Table 3).
Sensitivity analyses
The results of the main analyses were confirmed in the sensitivity analyses excluding high risk of bias trials (n = 11) for the primary outcomes (LDL cholesterol and TG) (Supplemental Tables 14–15). The comparison of foods of plant origin with foods of animal origin showed that there was a more pronounced reduction in LDL cholesterol for the food groups of plant origin (−0.22 mmol/L; 95% CI: −0.33, −0.12 mmol/L), but that there was no difference in TG (0.03 mmol/L; 95% CI: −0.05, 0.10 mmol/L). Moreover, the sensitivity analysis excluding trials conducted prior to the year 2000 also confirmed the findings of the main analysis.
Small study effects
The comparison-adjusted funnel plots for both primary outcomes appear slightly asymmetric in all trials (Supplemental Figures 10–11).
Quality of evidence
The credibility of the evidence for LDL cholesterol and TG was rated very low or low, as was the evidence for all comparisons between the different food groups (Supplemental Tables 16–17). The reason for the low and very low quality of evidence ratings were mainly driven by the small number of trials, the risk of bias, the imprecision, and the indirectness of several comparisons. This implies that further research is needed to provide more evidence on which to base judgments.
DISCUSSION
In the present NMA, we ranked 10 major food groups (refined grains, whole grains, fruits and vegetables, nuts, legumes, eggs, dairy, red meat, fish, and SSBs) according to their effects on cardiometabolic outcomes. Nuts showed the highest SUCRA value for LDL cholesterol and TC reduction; whole grains was the most effective food group at improving glycemic control (FG, HbA1c, and HOMA-IR); fish was ranked best at improving TG and HDL cholesterol; fruits and vegetables were ranked best for SBP reduction; and red meat was ranked best for DBP reduction. However, red meat was the worst at LDL cholesterol reduction and eggs were the worst at TG reduction, respectively.
Comparison with published pairwise meta-analyses
Our results are in congruence with previous pairwise meta-analyses of intervention trials, although most of them did not investigate all of the intermediate disease markers. One meta-analysis showed that consumption of whole-grain diets reduces LDL cholesterol and TC (115), while no such effects were reported with regard to either HDL cholesterol, FG, or SBP (116, 117). A Cochrane Review of 10 RCTs focusing on interventions to increase consumption of fruits and vegetables showed reductions in DBP, SBP, and LDL cholesterol (118). Meta-analyses investigating the effects of nut intake reported reductions in TC, LDL cholesterol, TG, DBP, FG, and HbA1c (119–121), but no effects on HDL cholesterol, SBP, or CRP (120, 122, 123). Regarding legumes, a meta-analysis of 10 RCTs indicated improvements in TC and LDL cholesterol levels (124), and others reported reductions in CRP, SBP, and FG (125, 126). Higher consumption of eggs increased TC, LDL cholesterol, and HDL cholesterol, but not TG, compared with control diets (127). Discrepancies between the present NMA and past meta-analyses were observed for egg consumption, which ranked worst for TG and best for CRP in our analyses. Synthesizing available data of RCTs showed that higher dairy intake has no significant effect on SBP (128). Additionally, neither high nor low fat dairy products seem to affect cardiovascular risk factors (129). Consumption of fatty fish resulted in significant improvements in TG and HDL cholesterol, whereas no effects were observed for TC, LDL cholesterol, DBP, SBP, FG, and CRP (130). Regarding red meat intake, a systematic review suggested that consumption of ≥0.5 servings/d of total red meat has no detrimental effect on blood lipids or blood pressure compared with lower red meat intakes (131). In contrast to previous findings, in the present NMA, red meat performed best for improvement in DBP and third best for TG.
Possible explanations of our findings
The LDL cholesterol–lowering effects of nuts might be mediated by the decreased (re)absorption and increased excretion of cholesterol and bile acid owing to their high content of phytosterols (132) and higher LDL-receptor activity (133). Moreover, the LDL cholesterol and TC-lowering effects provide critical mechanistic evidence to support a potential causal link between nut intake and lower cardiovascular risk (134). In addition, nuts are rich in MUFA and PUFA, both of which might trigger antioxidative as well as anti-inflammatory effects, leading to decreased levels of CRP (135). Soluble fiber may contribute to the cholesterol-lowering effects of legumes; in particular, it binds to bile acids in the intestines and prevents reabsorption. Consequently, an increase in the production of bile acids decreases the liver pool of cholesterol and increases uptake of serum cholesterol by the liver, thereby decreasing circulating cholesterol in the blood (136). Compared with refined grains, whole grains were more effective at reducing LDL cholesterol, TG, TC, HOMA-IR, and SBP. Whole grains, like legumes, might reduce cholesterol concentrations through soluble fiber, and they might exert antioxidant and anti-inflammatory properties owing to the presence of polyphenols and other phytonutrients. Whole grains might also modulate blood glucose and insulin responses, as well as improve vascular function, blood pressure, and weight control (137). Several components may contribute to the blood pressure–lowering effect of fruits and vegetables; for example, potassium, magnesium, vitamin C, folic acid, flavonoids, and carotenoids have all been postulated to lower blood pressure by improving endothelial function, modulating baroreflex sensitivity, or causing vasodilation (138, 139). The beneficial effects of fish on TG and HDL cholesterol are biologically plausible through effects of long-chain n–3 PUFA, which have been associated with antiatherosclerotic and antithrombotic effects (140).
Comparison with observational evidence
A recent dose-response meta-analysis of 123 cohort studies investigating the association between major food groups and risk of cardiovascular disease showed that each daily serving of whole grains, fruits, vegetables, nuts, legumes, and fish was associated with reduced risk of coronary heart disease (CHD). In contrast, each additional daily serving of red meat, processed meat, and SSBs was positively associated with CHD, whereas no associations were observed for eggs, dairy, and refined grains (2). In the present NMA of intervention trials, we were able to confirm the beneficial effects of nuts, legumes, and whole grains and the detrimental effect of red meat on LDL cholesterol, as well as the favorable effects of fish, nuts, and whole grains on TG levels. Both LDL cholesterol and TG are considered to be causal risk factors for CHD (141). Interestingly, the detrimental associations between SSB consumption and CHD risk observed in meta-analyses of prospective observational studies (2) could not be confirmed in the present NMA with respect to any of the risk factors assessed, most likely owing to the low number of trials (n = 2). However, it should be noted that a proper comparison between results from randomized trials and observational studies needs to take into account whether the studies referred to isocaloric trial arms respective of observational substitution models or ad libitum arms and models that investigated the addition of intake (142). In our dose-response meta-analysis, the low number of published substitution models prevented a direct comparison (2).
Strengths and limitations
The main strengths include the application of the novel methodology of NMA to compare the effects of different food groups across randomized intervention studies on established intermediate markers of chronic disease that were previously meta-analyzed using prospective observational studies. Other strengths are the large number of included trials, food groups, and outcomes, the a priori published protocol, and the assessment of both risk of bias and quality of evidence. Nevertheless, important limitations of the present NMA should also be considered. First, only 12% of all trials were judged to be in the low risk of bias category; only 8% of the included studies indicated a low risk of bias for allocation concealment and 15% for blinding of personnel, whereas 68% of the included trials reported a potential conflict of interest. Concerns have been reported about industry benefit bias in nutrition research (143). Second, the credibility of evidence was rated very low or low for the primary outcomes, which indicates that the evidence is very limited and uncertain, and further research will likely change the effect estimate. Third, the comparability of our findings with previous pairwise meta-analyses is limited owing to the fact that food groups were mostly compared with control diets or groups rather than directly with other food groups. Fourth, the similarity across the included trials was only modest, which limits the generalizability of our findings.
Conclusion
In conclusion, the present network meta-analysis with intermediate metabolic health markers supports the hypothesis that increased intake of nuts, legumes, and whole grains is more effective at primary prevention of metabolic disturbances and diseases than other food groups. However, findings of the NMA were rated as being of low and very low quality of evidence. For the future, NMAs with high-quality isocaloric randomized trials are needed to confirm the results of observational studies presenting the study results primarily as substitution models. To improve the quality of evidence for future NMAs, RCTs should improve dietary adherence by direct observations of study participants in experimental in-house settings. By applying adequate methods of sequence generation, allocation concealment, blinding, conducting intention-to-treat analysis, reporting funding sources, increasing sample sizes, and measuring diet carefully, risk of bias can be reduced. Moreover, new randomized study designs like large simple trials, registry-based design, or n-of-1 trials may play an important role in the future of nutrition research.
Supplementary Material
Acknowledgements
We thank Peter Clifton for sending us additional data for the corresponding meta-analysis.
The authors’ responsibilities were as follows—LS, GH, KI, CS, and HB: contributed to the conception and design of the systematic review and meta-analysis; LS, GH, and HB: were involved in the acquisition and analysis of the data; and all authors: interpreted the results, drafted the manuscript, provided critical revisions of the meta-analysis, and approved submission of the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by NutriAct – Competence Cluster Nutrition Research Berlin-Potsdam funded by the German Federal Ministry of Education andResearch (FKZ: 01EA1408A-G). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplemental Appendix 1, Supplemental Tables 1–17, Supplemental Figures 1–11, and Supplemental References are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CHD, coronary heart disease; CRP, C-reactive protein; DBP, diastolic blood pressure; FG, fasting glucose; HbA1c, glycated hemoglobin; NMA, network meta-analysis; SSB, sugar-sweetened beverage; SBP, systolic blood pressure; SUCRA, surface under the cumulative ranking curves; TG, triacylglycerol.
REFERENCES
- 1. GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, Henauw SD, Michels N, Devleesschauwer B, Schlesinger S et al.. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2017. Oct 17 (Epub ahead of print; DOI: 10.1080/10408398.2017.1392288). [DOI] [PubMed] [Google Scholar]
- 3. Schwingshackl L, Hoffmann G, Lampousi AM, Knuppel S, Iqbal K, Schwedhelm C, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwingshackl L, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, Andriolo V, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–73. [DOI] [PubMed] [Google Scholar]
- 6. Schwingshackl L, Schwedhelm C, Hoffmann G, Knuppel S, Laure Preterre A, Iqbal K, Bechthold A, De Henauw S, Michels N, Devleesschauwer B et al.. Food groups and risk of colorectal cancer. Int J Cancer. 2018;142(9):1748–58. [DOI] [PubMed] [Google Scholar]
- 7. Schwingshackl L, Schlesinger S, Devleesschauwer B, Hoffmann G, Bechthold A, Schwedhelm C, Iqbal K, Knuppel S, Boeing H. Generating the evidence for risk reduction: a contribution to the future of food-based dietary guidelines. Proc Nutr Soc. 2018. Apr 30(Epub ahead of print; DOI: 10.1017/S0029665118000125). [DOI] [PubMed] [Google Scholar]
- 8. USDA. USDoA. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. http://www.health.gov/dietaryguidelines/2015-scientific-report/ 2015. [Google Scholar]
- 9. Schwingshackl L, Knuppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopantelis E, Iqbal K et al.. Perspective: NutriGrade:a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7(6):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ioannidis JP. Implausible results in human nutrition research. Bmj. 2013;347:f6698. [DOI] [PubMed] [Google Scholar]
- 11. Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr. 2015;6(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. [DOI] [PubMed] [Google Scholar]
- 13. Schwingshackl L, Chaimani A, Schwedhelm C, Toledo E, Punsch M, Hoffmann G, Boeing H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: a systematic review and network meta-analysis. Crit Rev Food Sci Nutr. 2018. May 2 (Epub ahead of print; DOI: 10.1080/10408398.2018.1463967). [DOI] [PubMed] [Google Scholar]
- 14. Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol. 2018;33(2):157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP et al.. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 16. Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. J Clin Epidemiol. 2017;83:65–74. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benetou V, Trichopoulou A, Orfanos P, Naska A, Lagiou P, Boffetta P, Trichopoulos D. Conformity to traditional Mediterranean diet and cancer incidence: the Greek EPIC cohort. Br J Cancer. 2008;99(1):191–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. [DOI] [PubMed] [Google Scholar]
- 21. StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 22. White I. Network meta-analysis. Stata Journal. 2015;15(4):951–85. [Google Scholar]
- 23. Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal. 2015;15(4):905–50. [Google Scholar]
- 24. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–91. [DOI] [PubMed] [Google Scholar]
- 25. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7-8):932–44. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson D, Barrett JK, Rice S, White IR, Higgins JP. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med. 2014;33(21):3639–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agebratt C, Strom E, Romu T, Dahlqvist-Leinhard O, Borga M, Leandersson P, Nystrom FH. A randomized study of the effects of additional fruit and nuts consumption on hepatic fat content, cardiovascular risk factors and basal metabolic rate. PLoS One. 2016;11(1):e0147149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ampatzoglou A, Williams CL, Atwal KK, Maidens CM, Ross AB, Thielecke F, Jonnalagadda SS, Kennedy OB, Yaqoob P. Effects of increased wholegrain consumption on immune and inflammatory markers in healthy low habitual wholegrain consumers. Eur J Nutr. 2016;55(1):183–95. [DOI] [PubMed] [Google Scholar]
- 31. Ampatzoglou A, Atwal KK, Maidens CM, Williams CL, Ross AB, Thielecke F, Jonnalagadda SS, Kennedy OB, Yaqoob P. Increased whole grain consumption does not affect blood biochemistry, body composition, or gut microbiology in healthy, low-habitual whole grain consumers. J Nutr. 2015;145(2):215–21. [DOI] [PubMed] [Google Scholar]
- 32. Andersson A, Tengblad S, Karlstrom B, Kamal-Eldin A, Landberg R, Basu S, Aman P, Vessby B. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J Nutr. 2007;137(6):1401–7. [DOI] [PubMed] [Google Scholar]
- 33. Ashton E, Ball M. Effects of soy as tofu vs meat on lipoprotein concentrations. Eur J Clin Nutr. 2000;54(1):14–9. [DOI] [PubMed] [Google Scholar]
- 34. Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, Willett WC. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr. 2007;85(3):735–41. [DOI] [PubMed] [Google Scholar]
- 35. Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Soy consumption, markers of inflammation, and endothelial function: a cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care. 2007;30(4):967–73. [DOI] [PubMed] [Google Scholar]
- 36. Beauchesne-Rondeau E, Gascon A, Bergeron J, Jacques H. Plasma lipids and lipoproteins in hypercholesterolemic men fed a lipid-lowering diet containing lean beef, lean fish, or poultry. Am J Clin Nutr. 2003;77(3):587–93. [DOI] [PubMed] [Google Scholar]
- 37. Berryman CE, West SG, Fleming JA, Bordi PL, Kris-Etherton PM. Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL cholesterol: a randomized controlled trial. J Am Heart Assoc. 2015;4(1):e000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burns-Whitmore B, Haddad E, Sabate J, Rajaram S. Effects of supplementing n-3 fatty acid enriched eggs and walnuts on cardiovascular disease risk markers in healthy free-living lacto-ovo-vegetarians: a randomized, crossover, free-living intervention study. Nutr J. 2014;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen J, He J, Wildman RP, Reynolds K, Streiffer RH, Whelton PK. A randomized controlled trial of dietary fiber intake on serum lipids. Eur J Clin Nutr. 2006;60(1):62–8. [DOI] [PubMed] [Google Scholar]
- 40. Cobiac L, McArthur R, Nestel PJ. Can eating baked beans lower plasma cholesterol?. Eur J Clin Nutr. 1990;44(11):819–22. [PubMed] [Google Scholar]
- 41. Daly RM, O'Connell SL, Mundell NL, Grimes CA, Dunstan DW, Nowson CA. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial. Am J Clin Nutr. 2014;99(4):899–910. [DOI] [PubMed] [Google Scholar]
- 42. Engel S, Tholstrup T, Bruun JM, Astrup A, Richelsen B, Raben A. Effect of high milk and sugar-sweetened and non-caloric soft drink intake on insulin sensitivity after 6 months in overweight and obese adults: a randomized controlled trial. Eur J Clin Nutr. 2017;72:358–66. [DOI] [PubMed] [Google Scholar]
- 43. Bruun JM, Maersk M, Belza A, Astrup A, Richelsen B. Consumption of sucrose-sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: a 6-month randomised controlled trial. Eur J Clin Nutr. 2015;69(8):949–53. [DOI] [PubMed] [Google Scholar]
- 44. Maersk M, Belza A, Stodkilde-Jorgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283–9. [DOI] [PubMed] [Google Scholar]
- 45. Foerster J, Maskarinec G, Reichardt N, Tett A, Narbad A, Blaut M, Boeing H. The influence of whole grain products and red meat on intestinal microbiota composition in normal weight adults: a randomized crossover intervention trial. PLoS One. 2014;9(10):e109606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Freese R, Alfthan G, Jauhiainen M, Basu S, Erlund I, Salminen I, Aro A, Mutanen M. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. Am J Clin Nutr. 2002;76(5):950–60. [DOI] [PubMed] [Google Scholar]
- 47. Gebauer SK, West SG, Kay CD, Alaupovic P, Bagshaw D, Kris-Etherton PM. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose-response study. Am J Clin Nutr. 2008;88(3):651–9. [DOI] [PubMed] [Google Scholar]
- 48. Holligan SD, West SG, Gebauer SK, Kay CD, Kris-Etherton PM. A moderate-fat diet containing pistachios improves emerging markers of cardiometabolic syndrome in healthy adults with elevated LDL levels. Br J Nutr. 2014;112(5):744–52. [DOI] [PubMed] [Google Scholar]
- 49. Giacco R, Lappi J, Costabile G, Kolehmainen M, Schwab U, Landberg R, Uusitupa M, Poutanen K, Pacini G, Rivellese AA et al.. Effects of rye and whole wheat versus refined cereal foods on metabolic risk factors: a randomised controlled two-centre intervention study. Clin Nutr. 2013;32(6):941–9. [DOI] [PubMed] [Google Scholar]
- 50. Giacco R, Costabile G, Della Pepa G, Anniballi G, Griffo E, Mangione A, Cipriano P, Viscovo D, Clemente G, Landberg R et al.. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr Metab Cardiovasc Dis. 2014;24(8):837–44. [DOI] [PubMed] [Google Scholar]
- 51. Vetrani C, Costabile G, Luongo D, Naviglio D, Rivellese AA, Riccardi G, Giacco R. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition (Burbank, Los Angeles County, Calif). 2016;32(2):217–21. [DOI] [PubMed] [Google Scholar]
- 52. Grieger JA, Miller MD, Cobiac L. 2014. Investigation of the effects of a high fish diet on inflammatory cytokines, blood pressure, and lipids in healthy older Australians. Food Nutr Res [Internet]. [cited 2018 Apr 6];58. DOI: 10.3402/fnr.v58.20369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gormley TR, Kevany J, O'Donnell B, McFarlane R. Effect of peas on serum cholesterol levels in humans. Irish J Food Sci Technol. 1979;3(2):101–9. [Google Scholar]
- 54. Harris Jackson K, West SG, Vanden Heuvel JP, Jonnalagadda SS, Ross AB, Hill AM, Grieger JA, Lemieux SK, Kris-Etherton PM. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled-feeding trial. Am J Clin Nutr. 2014;100(2):577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haub MD, Wells AM, Campbell WW. Beef and soy-based food supplements differentially affect serum lipoprotein-lipid profiles because of changes in carbohydrate intake and novel nutrient intake ratios in older men who resistive-train. Metabolism. 2005;54(6):769–74. [DOI] [PubMed] [Google Scholar]
- 56. Hodgson JM, Burke V, Beilin LJ, Puddey IB. Partial substitution of carbohydrate intake with protein intake from lean red meat lowers blood pressure in hypertensive persons. Am J Clin Nutr. 2006;83(4):780–7. [DOI] [PubMed] [Google Scholar]
- 57. Hodgson JM, Ward NC, Burke V, Beilin LJ, Puddey IB. Increased lean red meat intake does not elevate markers of oxidative stress and inflammation in humans. J Nutr. 2007;137(2):363–7. [DOI] [PubMed] [Google Scholar]
- 58. Hosseinpour-Niazi S, Mirmiran P, Fallah-Ghohroudi A, Azizi F. Non-soya legume-based therapeutic lifestyle change diet reduces inflammatory status in diabetic patients: a randomised cross-over clinical trial. Br J Nutr. 2015;114(2):213–9. [DOI] [PubMed] [Google Scholar]
- 59. Hosseinpour-Niazi S, Mirmiran P, Hedayati M, Azizi F. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: a cross-over randomized clinical trial. Eur J Clin Nutr. 2015;69(5):592–7. [DOI] [PubMed] [Google Scholar]
- 60. Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, Haight JS, Faulkner D, Vidgen E, Lapsley KG et al.. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106(11):1327–32. [DOI] [PubMed] [Google Scholar]
- 61. Jenkins DJ, Kendall CW, Marchie A, Josse AR, Nguyen TH, Faulkner DA, Lapsley KG, Blumberg J. Almonds reduce biomarkers of lipid peroxidation in older hyperlipidemic subjects. J Nutr. 2008;138(5):908–13. [DOI] [PubMed] [Google Scholar]
- 62. Jenkins DJ, Kendall CW, Marchie A, Josse AR, Nguyen TH, Faulkner DA, Lapsley KG, Singer W. Effect of almonds on insulin secretion and insulin resistance in nondiabetic hyperlipidemic subjects: a randomized controlled crossover trial. Metabolism. 2008;57(7):882–7. [DOI] [PubMed] [Google Scholar]
- 63. Jenkins DJ, Kendall CW, Augustin LS, Martini MC, Axelsen M, Faulkner D, Vidgen E, Parker T, Lau H, Connelly PW et al.. Effect of wheat bran on glycemic control and risk factors for cardiovascular disease in type 2 diabetes. Diabetes Care. 2002;25(9):1522–8. [DOI] [PubMed] [Google Scholar]
- 64. Jenkins DJ, Kendall CW, Augustin LS, Mitchell S, Sahye-Pudaruth S, Blanco Mejia S, Chiavaroli L, Mirrahimi A, Ireland C, Bashyam B et al.. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med. 2012;172(21):1653–60. [DOI] [PubMed] [Google Scholar]
- 65. Juntunen KS, Laaksonen DE, Poutanen KS, Niskanen LK, Mykkanen HM. High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am J Clin Nutr. 2003;77(2):385–91. [DOI] [PubMed] [Google Scholar]
- 66. Karl JP, Meydani M, Barnett JB, Vanegas SM, Goldin B, Kane A, Rasmussen H, Saltzman E, Vangay P, Knights D et al.. Substituting whole grains for refined grains in a 6-wk randomized trial favorably affects energy-balance metrics in healthy men and postmenopausal women. Am J Clin Nutr. 2017;105(3):589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Karmally W, Montez MG, Palmas W, Martinez W, Branstetter A, Ramakrishnan R, Holleran SF, Haffner SM, Ginsberg HN. Cholesterol-lowering benefits of oat-containing cereal in Hispanic americans. J Am Diet Assoc. 2005;105(6):967–70. [DOI] [PubMed] [Google Scholar]
- 68. Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008;87(1):79–90. [DOI] [PubMed] [Google Scholar]
- 69. Katz DL, Evans MA, Nawaz H, Njike VY, Chan W, Comerford BP, Hoxley ML. Egg consumption and endothelial function: a randomized controlled crossover trial. Int J Cardiol. 2005;99(1):65–70. [DOI] [PubMed] [Google Scholar]
- 70. Katz DL, Gnanaraj J, Treu JA, Ma Y, Kavak Y, Njike VY. Effects of egg ingestion on endothelial function in adults with coronary artery disease: a randomized, controlled, crossover trial. Am Heart J. 2015;169(1):162–9. [DOI] [PubMed] [Google Scholar]
- 71. Kirwan JP, Malin SK, Scelsi AR, Kullman EL, Navaneethan SD, Pagadala MR, Haus JM, Filion J, Godin JP, Kochhar S et al.. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. J Nutr. 2016;146(11):2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Malin SK, Kullman EL, Scelsi AR, Haus JM, Filion J, Pagadala MR, Godin JP, Kochhar S, Ross AB, Kirwan JP. A whole-grain diet reduces peripheral insulin resistance and improvesg lucose kinetics in obese adults: a randomized-controlled trial. Metabolism. 2018;82:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kondo K, Morino K, Nishio Y, Ishikado A, Arima H, Nakao K, Nakagawa F, Nikami F, Sekine O, Nemoto KI et al.. Fiber-rich diet with brown rice improves endothelial function in type 2 diabetes mellitus: a randomized controlled trial. PLoS One. 2017;12(6):e0179869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bugel S, Tetens I, Astrup A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr. 2012;142(4):710–6. [DOI] [PubMed] [Google Scholar]
- 75. Lankinen M, Kolehmainen M, Jaaskelainen T, Paananen J, Joukamo L, Kangas AJ, Soininen P, Poutanen K, Mykkanen H, Gylling H et al.. Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: a randomized trial (Sysdimet). PLoS One. 2014;9(2):e90352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lankinen M, Schwab U, Kolehmainen M, Paananen J, Poutanen K, Mykkanen H, Seppanen-Laakso T, Gylling H, Uusitupa M, Oresic M. Whole grain products, fish and bilberries alter glucose and lipid metabolism in a randomized, controlled trial: the Sysdimet study. PLoS One. 2011;6(8):e22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Leaf DA, Hatcher L. The effect of lean fish consumption on triglyceride levels. Phys Sportsmed. 2009;37(1):37–43. [DOI] [PubMed] [Google Scholar]
- 78. Leinonen KS, Poutanen KS, Mykkanen HM. Rye bread decreases serum total and LDL cholesterol in men with moderately elevated serum cholesterol. J Nutr. 2000;130(2):164–70. [DOI] [PubMed] [Google Scholar]
- 79. Li J, Kaneko T, Qin LQ, Wang J, Wang Y. Effects of barley intake on glucose tolerance, lipid metabolism, and bowel function in women. Nutrition. 2003;19(11-12):926–9. [DOI] [PubMed] [Google Scholar]
- 80. Li Z, Song R, Nguyen C, Zerlin A, Karp H, Naowamondhol K, Thames G, Gao K, Li L, Tseng CH et al.. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J Am Coll Nutr. 2010;29(3):198–203. [DOI] [PubMed] [Google Scholar]
- 81. Li J, Armstrong CL, Campbell WW. Effects of dietary protein source and quantity during weight loss on appetite, energy expenditure, and cardio-metabolic responses. Nutrients. 2016;8(2):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Maki KC, Beiseigel JM, Jonnalagadda SS, Gugger CK, Reeves MS, Farmer MV, Kaden VN, Rains TM. Whole-grain ready-to-eat oat cereal, as part of a dietary program for weight loss, reduces low-density lipoprotein cholesterol in adults with overweight and obesity more than a dietary program including low-fiber control foods. J Am Diet Assoc. 2010;110(2):205–14. [DOI] [PubMed] [Google Scholar]
- 83. Maki KC, Nieman KM, Schild AL, Kaden VN, Lawless AL, Kelley KM, Rains TM. Sugar-sweetened product consumption alters glucose homeostasis compared with dairy product consumption in men and women at risk of type 2 diabetes mellitus. J Nutr. 2015;145(3):459–66. [DOI] [PubMed] [Google Scholar]
- 84. McIntosh GH, Noakes M, Royle PJ, Foster PR. Whole-grain rye and wheat foods and markers of bowel health in overweight middle-aged men. Am J Clin Nutr. 2003;77(4):967–74. [DOI] [PubMed] [Google Scholar]
- 85. Navas-Carretero S, Perez-Granados AM, Schoppen S, Vaquero MP. An oily fish diet increases insulin sensitivity compared to a red meat diet in young iron-deficient women. Br J Nutr. 2009;102(4):546–53. [DOI] [PubMed] [Google Scholar]
- 86. Pereira MA, Jacobs DR Jr., Pins JJ, Raatz SK, Gross MD, Slavin JL, Seaquist ER. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am J Clin Nutr. 2002;75(5):848–55. [DOI] [PubMed] [Google Scholar]
- 87. Pins JJ, Geleva D, Keenan JM, Frazel C, O'Connor PJ, Cherney LM. Do whole-grain oat cereals reduce the need for antihypertensive medications and improve blood pressure control?. J Fam Pract. 2002;51(4):353–9. [PubMed] [Google Scholar]
- 88. Nestel P, Cehun M, Chronopoulos A. Effects of long-term consumption and single meals of chickpeas on plasma glucose, insulin, and triacylglycerol concentrations. Am J Clin Nutr. 2004;79(3):390–5. [DOI] [PubMed] [Google Scholar]
- 89. Pittaway JK, Ahuja KD, Cehun M, Chronopoulos A, Robertson IK, Nestel PJ, Ball MJ. Dietary supplementation with chickpeas for at least 5 weeks results in small but significant reductions in serum total and low-density lipoprotein cholesterols in adult women and men. Ann Nutr Metab. 2006;50(6):512–8. [DOI] [PubMed] [Google Scholar]
- 90. Pittaway JK, Ahuja KD, Robertson IK, Ball MJ. Effects of a controlled diet supplemented with chickpeas on serum lipids, glucose tolerance, satiety and bowel function. J Am Coll Nutr. 2007;26(4):334–40. [DOI] [PubMed] [Google Scholar]
- 91. Rajaram S, Haddad EH, Mejia A, Sabate J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr. 2009;89(5):1657s–63s. [DOI] [PubMed] [Google Scholar]
- 92. Chiang YL, Haddad E, Rajaram S, Shavlik D, Sabate J. The effect of dietary walnuts compared to fatty fish on eicosanoids, cytokines, soluble endothelial adhesion molecules and lymphocyte subsets: a randomized, controlled crossover trial. Prostaglandins Leukot Essent Fatty Acids. 2012;87(4-5):111–7. [DOI] [PubMed] [Google Scholar]
- 93. Reynolds HR, Quiter E, Hunninghake DB. Whole grain oat cereal lowers serum lipids. Top Clin Nutr. 2000;15(4):74–83. [Google Scholar]
- 94. Roager HM, Vogt JK, Kristensen M, Hansen LBS, Ibrugger S, Maerkedahl RB, Bahl MI, Lind MV, Nielsen RL, Frokiaer H et al.. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2017. Nov 1 (Epub ahead of print; DOI: 10.1136/gutjnl-2017-314786). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Saltzman E, Das SK, Lichtenstein AH, Dallal GE, Corrales A, Schaefer EJ, Greenberg AS, Roberts SB. An oat-containing hypocaloric diet reduces systolic blood pressure and improves lipid profile beyond effects of weight loss in men and women. J Nutr. 2001;131(5):1465–70. [DOI] [PubMed] [Google Scholar]
- 96. Sauder KA, McCrea CE, Ulbrecht JS, Kris-Etherton PM, West SG. Pistachio nut consumption modifies systemic hemodynamics, increases heart rate variability, and reduces ambulatory blood pressure in well-controlled type 2 diabetes: a randomized trial. J Am Heart Assoc. 2014;3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sauder KA, McCrea CE, Ulbrecht JS, Kris-Etherton PM, West SG. Effects of pistachios on the lipid/lipoprotein profile, glycemic control, inflammation, and endothelial function in type 2 diabetes:a randomized trial. Metabolism. 2015;64(11):1521–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shimabukuro M, Higa M, Kinjo R, Yamakawa K, Tanaka H, Kozuka C, Yabiku K, Taira S, Sata M, Masuzaki H. Effects of the brown rice diet on visceral obesity and endothelial function: the BRAVO study. Br J Nutr. 2014;111(2):310–20. [DOI] [PubMed] [Google Scholar]
- 99. Thongoun P, Pavadhgul P, Bumrungpert A, Satitvipawee P, Harjani Y, Kurilich A. Effect of oat consumption on lipid profiles in hypercholesterolemic adults. J Med Assoc Thai. 2013;96 Suppl 5:S25–32. [PubMed] [Google Scholar]
- 100. Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr. 2010;92(4):733–40. [DOI] [PubMed] [Google Scholar]
- 101. MacKay KA, Tucker AJ, Duncan AM, Graham TE, Robinson LE. Whole grain wheat sourdough bread does not affect plasminogen activator inhibitor-1 in adults with normal or impaired carbohydrate metabolism. Nutr Metab Cardiovasc Dis. 2012;22(9):704–11. [DOI] [PubMed] [Google Scholar]
- 102. Tucker AJ, Mackay KA, Robinson LE, Graham TE, Bakovic M, Duncan AM. The effect of whole grain wheat sourdough bread consumption on serum lipids in healthy normoglycemic/normoinsulinemic and hyperglycemic/hyperinsulinemic adults depends on presence of the APOE E3/E3 genotype: a randomized controlled trial. Nutr Metab (Lond). 2010;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Turner KM, Keogh JB, Clifton PM. Red meat, dairy, and insulin sensitivity: a randomized crossover intervention study. Am J Clin Nutr. 2015;101(6):1173–9. [DOI] [PubMed] [Google Scholar]
- 104. Turner KM, Keogh JB, Meikle PJ, Clifton PM. Changes in lipids and inflammatory markers after consuming diets high in red meat or dairy for four weeks. Nutrients. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Turpeinen AM, Juntunen K, Mutanen M, Mykkanen H. Similar responses in hemostatic factors after consumption of wholemeal rye bread and low-fiber wheat bread. Eur J Clin Nutr. 2000;54(5):418–23. [DOI] [PubMed] [Google Scholar]
- 106. Vitaglione P, Mennella I, Ferracane R, Rivellese AA, Giacco R, Ercolini D, Gibbons SM, La Storia A, Gilbert JA, Jonnalagadda S et al.. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber. Am J Clin Nutr. 2015;101(2):251–61. [DOI] [PubMed] [Google Scholar]
- 107. Winham DM, Hutchins AM. Baked bean consumption reduces serum cholesterol in hypercholesterolemic adults. Nutr Res. 2007;27(7):380–6. [Google Scholar]
- 108. Winham DM, Hutchins AM, Johnston CS. Pinto bean consumption reduces biomarkers for heart disease risk. J Am Coll Nutr. 2007;26(3):243–9. [DOI] [PubMed] [Google Scholar]
- 109. Wolmarans P, Benade AJ, Kotze TJ, Daubitzer AK, Marais MP, Laubscher R. Plasma lipoprotein response to substituting fish for red meat in the diet. Am J Clin Nutr. 1991;53(5):1171–6. [DOI] [PubMed] [Google Scholar]
- 110. Zhang G, Pan A, Zong G, Yu Z, Wu H, Chen X, Tang L, Feng Y, Zhou H, Chen X et al.. Substituting white rice with brown rice for 16 weeks does not substantially affect metabolic risk factors in middle-aged Chinese men and women with diabetes or a high risk for diabetes. J Nutr. 2011;141(9):1685–90. [DOI] [PubMed] [Google Scholar]
- 111. Zhang J, Li L, Song P, Wang C, Man Q, Meng L, Cai J, Kurilich A. Randomized controlled trial of oatmeal consumption versus noodle consumption on blood lipids of urban Chinese adults with hypercholesterolemia. Nutr J. 2012;11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Johnston L, Reiss-Reynolds H, Hunninghake D, Schultz K, Westereng B. Cholesterol-lowering benefits of a whole grain oat ready-to-eat cereal. Nutr Clin Care. 1998;1:6–12. [Google Scholar]
- 113. Freese R, Vaarala O, Turpeinen AM, Mutanen M. No difference in platelet activation or inflammation markers after diets rich or poor in vegetables, berries and apple in healthy subjects. Eur J Nutr. 2004;43(3):175–82. [DOI] [PubMed] [Google Scholar]
- 114. Cooper DN, Kable ME, Marco ML, De Leon A, Rust B, Baker JE, Horn W, Burnett D, Keim NL. The effects of moderate whole grain consumption on fasting glucose and lipids, gastrointestinal symptoms, and microbiota. Nutrients. 2017;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hollaender PL, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2015;102(3):556–72. [DOI] [PubMed] [Google Scholar]
- 116. Ye EQ, Chacko SA, Chou EL, Kugizaki M, Liu S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J Nutr. 2012;142(7):1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2013;98(4):872–84. [DOI] [PubMed] [Google Scholar]
- 118. Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, Stranges S, Hooper L, Rees K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev. 2013(6):Cd009874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Musa-Veloso K, Paulionis L, Poon T, Lee HY. The effects of almond consumption on fasting blood lipid levels: a systematic review and meta-analysis of randomised controlled trials. J Nutr Sci. 2016;5: e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mohammadifard N, Salehi-Abargouei A, Salas-Salvado J, Guasch-Ferre M, Humphries K, Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Am J Clin Nutr. 2015;101(5):966–82. [DOI] [PubMed] [Google Scholar]
- 121. Viguiliouk E, Kendall CW, Blanco Mejia S, Cozma AI, Ha V, Mirrahimi A, Jayalath VH, Augustin LS, Chiavaroli L, Leiter LA et al.. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS One. 2014;9(7):e103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Flores-Mateo G, Rojas-Rueda D, Basora J, Ros E, Salas-Salvado J. Nut intake and adiposity: meta-analysis of clinical trials. Am J Clin Nutr. 2013;97(6):1346–55. [DOI] [PubMed] [Google Scholar]
- 123. Neale EP, Tapsell LC, Guan V, Batterham MJ. The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(11):e016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bazzano LA, Thompson AM, Tees MT, Nguyen CH, Winham DM. Non-soy legume consumption lowers cholesterol levels: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2011;21(2):94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Viguiliouk E, Blanco Mejia S, Kendall CW, Sievenpiper JL. Can pulses play a role in improving cardiometabolic health? Evidence from systematic reviews and meta-analyses. Ann N Y Acad Sci. 2017;1392(1):43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Salehi-Abargouei A, Saraf-Bank S, Bellissimo N, Azadbakht L. Effects of non-soy legume consumption on C-reactive protein: a systematic review and meta-analysis. Nutrition. 2015;31(5):631–9. [DOI] [PubMed] [Google Scholar]
- 127. Rouhani MH, Rashidi-Pourfard N, Salehi-Abargouei A, Karimi M, Haghighatdoost F. Effects of egg consumption on blood lipids: a systematic review and meta-analysis of randomized clinical trials. J Am Coll Nutr. 2017:1–12. [DOI] [PubMed] [Google Scholar]
- 128. Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ. 2017;356:j1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Benatar JR, Sidhu K, Stewart RA. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLoS One. 2013;8(10):e76480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Alhassan A, Young J, Lean MEJ, Lara J. Consumption of fish and vascular risk factors: a systematic review and meta-analysis of intervention studies. Atherosclerosis. 2017;266:87–94. [DOI] [PubMed] [Google Scholar]
- 131. O'Connor LE, Kim JE, Campbell WW. Total red meat intake of >/=0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials. Am J Clin Nutr. 2017;105(1):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Segura R, Javierre C, Lizarraga MA, Ros E. Other relevant components of nuts: phytosterols, folate and minerals. Br J Nutr. 2006;96 Suppl 2:S36–44. [DOI] [PubMed] [Google Scholar]
- 133. Berryman CE, Preston AG, Karmally W, Deckelbaum RJ, Kris-Etherton PM. Effects of almond consumption on the reduction of LDL cholesterol: a discussion of potential mechanisms and future research directions. Nutr Rev. 2011;69(4):171–85. [DOI] [PubMed] [Google Scholar]
- 134. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J et al.. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90. [DOI] [PubMed] [Google Scholar]
- 135. Schwingshackl L, Hoffmann G, Missbach B, Stelmach-Mardas M, Boeing H. An umbrella review of nuts intake and risk of cardiovascular disease. Curr Pharm Des. 2017;23(7):1016–27. [DOI] [PubMed] [Google Scholar]
- 136. Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. 2008;19(2):71–84. [DOI] [PubMed] [Google Scholar]
- 137. Jonnalagadda SS, Harnack L, Liu RH, McKeown N, Seal C, Liu S, Fahey GC. Putting the whole grain puzzle together: health benefits associated with whole grains—summary of American Society for Nutrition 2010 Satellite Symposium. J Nutr. 2011;141(5):1011s–22s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70(3 Suppl):475s–90s. [DOI] [PubMed] [Google Scholar]
- 139. Macready AL, George TW, Chong MF, Alimbetov DS, Jin Y, Vidal A, Spencer JP, Kennedy OB, Tuohy KM, Minihane AM et al.. Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease—FLAVURS: a randomized controlled trial. Am J Clin Nutr. 2014;99(3):479–89. [DOI] [PubMed] [Google Scholar]
- 140. Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet. 2010;376(9740):540–50. [DOI] [PubMed] [Google Scholar]
- 141. Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI et al.. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Song M, Giovannucci E. Substitution analysis in nutritional epidemiology: proceed with caution. Eur J Epidemiol. 2018;33(2):137–40. [DOI] [PubMed] [Google Scholar]
- 143. Bes-Rastrollo M, Schulze MB, Ruiz-Canela M, Martinez-Gonzalez MA. Financial conflicts of interest and reporting bias regarding the association between sugar-sweetened beverages and weight gain: a systematic review of systematic reviews. PLoS Med. 2013;10(12):e1001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.