ABSTRACT
Background
Type 2 resistant starch (RS2) has been shown to improve glycemic control and some cardiovascular endpoints in rodent and human studies.
Objective
The aim of this study was to perform one of the first randomized clinical trials in adults with prediabetes and one of the longest trials to test whether RS2 can improve cardiometabolic health.
Design
68 overweight [body mass index (BMI) ≥27 kg/m2] adults aged 35–75 y with prediabetes were randomized to consume 45 g/d of high-amylose maize (RS2) or an isocaloric amount of the rapidly digestible starch amylopectin (control) for 12 wk. At baseline and postintervention, ectopic fat depots (visceral adipose tissue, intrahepatic lipids, and intramyocellular lipids) were measured by magnetic resonance imaging/spectroscopy, energy metabolism by respiratory chamber, and carbohydrate metabolism by glycated hemoglobin (HbA1c), an intravenous glucose tolerance test, and a meal tolerance test. Cardiovascular risk factors—serum lipids, blood pressure, heart rate, and inflammatory markers (high-sensitivity C-reactive protein [hs-CRP], interleukin-6, and tumor necrosis factor [TNF]-α)—were also measured. The primary endpoints were insulin sensitivity, insulin secretion, ectopic fat, and markers of inflammation. Data were primarily analyzed as treatment effects via a linear mixed model both with and without the addition of covariates.
Results
Relative to the control group, RS2 lowered HbA1c by a clinically insignificant 0.1 ± 0.2% (Δ = −1 ± 2 mmol/mol; P = 0.05) but did not affect insulin secretion, insulin sensitivity, the disposition index, or glucose or insulin areas under the curve relative to baseline (P ≥ 0.23). RS2 decreased heart rate by 5 ± 9 beats/min (P = 0.02) and TNF-α concentrations by 2.1 ± 2.7 pg/mL (P = 0.004), relative to the control group. Ectopic fat, energy expenditure, substrate oxidation, and all other cardiovascular risk factors were unaffected (P ≥ 0.06).
Conclusions
12 wk of supplementation with resistant starch reduced the inflammatory marker TNF-α and heart rate, but it did not significantly improve glycemic control and other cardiovascular disease risk factors, in adults with prediabetes. This trial was registered at clinicaltrials.gov as NCT01708694.
Keywords: prediabetes, resistant starch, glycemic control, intravenous glucose tolerance test, respiratory chamber, ectopic fat, energy expenditure, fat oxidation
INTRODUCTION
More than 84 million, or 1 in 3, American adults have prediabetes (1), and up to 70% of adults with prediabetes will eventually develop type 2 diabetes (2). Intensive lifestyle changes can dramatically reduce the incidence of type 2 diabetes (3). However, since intensive lifestyle interventions can be difficult to maintain over the long term, it is crucial to develop effective, low-cost lifestyle interventions with high adherence rates.
Recently, resistant starch (RS) has emerged as one such potential strategy. RS is any starch that is not enzymatically digested within the stomach and small intestine and therefore passes to the large intestine (4). RS has many of the same physiologic effects as dietary fiber, including slowing of gastric emptying, lowering postprandial glucose concentrations, increasing satiety, and improving incretin secretion. Also similarly to dietary fiber, RS is fermented by the distal gut microbiota and can increase the production of short-chain fatty acids (4–6). There are 5 different types of RS—called Types 1, 2, 3, 4, and 5—that exist within a range of foods, including grains, potatoes, seeds, and legumes (5). Type 2 RS (RS2) encompasses native, uncooked granules like amylose from maize, raw potato, or banana starch that are stable under moderate heat when cooked. Research by us and others has previously demonstrated that RS2 can improve cardiometabolic health in both rodents and humans. For instance, in rodents, RS2 reduces body fat, lowers cholesterol and triglyceride (TG) concentrations, reduces glycemia and insulinemia, improves insulin sensitivity, reduces appetite, improves gut microbiota, and reduces fat accretion (6–9). In humans without diabetes, RS2 improves the gut microbiome (10, 11), increases the production of short-chain fatty acids (12–17), and improves metabolic endpoints, including fasting and postprandial glucose and insulin concentrations, insulin sensitivity, first-phase insulin secretion, and postprandial fat oxidation (12, 14, 16, 18–25).
Although RS2 appears promising for treating metabolic disease, only a handful of studies have examined its effects in adults with type 2 diabetes or prediabetes (26–30). These studies reported improved glycemic control (26–28, 30), lower free fatty acids (FFAs) (26), increased glucagon-like peptide-1 (GLP-1) concentrations (26), and improvements in some inflammatory and oxidative stress markers (26–28, 30). However, total cholesterol (26–28) and pancreatic fat (26) were unchanged, whereas the effects on body weight, ectopic fat, and TGs were mixed (26, 28, 29). To our knowledge, however, there has been no randomized clinical trial testing the effects of RS2 in an exclusively prediabetic population.
We therefore conducted the first randomized, double-blind, placebo-controlled clinical trial (STARCH: NCT01708694) to test whether RS2 supplementation can improve glycemic control and other cardiometabolic disease risk factors in adults with prediabetes. We hypothesized that 12 wk of 45 g/d RS2 supplementation would improve insulin sensitivity, decrease insulin secretion, reduce ectopic fat, and improve inflammatory markers.
METHODS
Study design
The STARCH trial was a randomized, double-blind, placebo-controlled, parallel-arm trial conducted between November 2012 and March 2016 at Pennington Biomedical Research Center (PBRC). Participants with confirmed prediabetes were randomized (1:1) to consume 45 g/d of an RS2 called high-amylose maize (HAM-RS2; Hi-Maize 260 resistant starch provided by Ingredion Incorporated., Westchester, IL) or an isocaloric amount of the placebo Amioca cornstarch (amylopectin; also provided by Ingredion Inc.) for 12 wk. Amylopectin is a highly purified rapidly digestible starch, distinguishing it from slowly digestible and resistant starches. Using amylopectin ensures that there are no contaminating effects from other types of beneficial starches in the control group. Participants were counseled to replace an isocaloric amount of food with RS2 or the placebo and to maintain their body weight throughout the intervention (≤1.5 kg deviation from baseline weight) to determine whether RS2 has effects on cardiometabolic health independent of weight loss. Furthermore, we asked the participants to continue to eat their usual diet to provide generalizability of the data to the broader population with prediabetes.
The study design, multi-stage screening process, and enrollment strategies have been described in detail elsewhere (31). In brief, adults with prediabetes aged 35–75 y, with a BMI ≥27 kg/m2 and weight ≤143 kg (the weight limit for the magnetic resonance spectroscopy measurements), and without major chronic disease or medications that affect the study endpoints were eligible to participate. Prediabetes was confirmed by either having impaired fasting glucose (100–125 mg/dL) or elevated glycated hemoglobin (HbA1c; 5.7–6.4%; 39–46 mmol/mol) at screening. All participants provided written informed consent, and the study was approved by the PBRC Institutional Review Board. Additional details on the study design are provided in the Supplemental Materials.
During the 12-wk intervention, participants consumed RS2 or placebo in a combination of yogurts (∼1/3) and packets. All packets were added to the yogurt prior to consumption. In-person behavioral counseling was provided every 2 wk by a study staff member to foster adherence and to ensure weight stability throughout the trial. To foster compliance, participants were required to bring lids/labels of the yogurts and empty packets that they consumed to each counseling session and were questioned about adherence. Metabolic weight, height, waist circumference, hip circumference, and vital signs were measured in the morning following an overnight fast at screening, baseline (wk 0), and postintervention (wk 12). Study endpoints were measured both at baseline (wk 0) and at the end of the trial (wk 12) and included measures of body composition (total and ectopic fat); insulin sensitivity and secretion; energy metabolism; cardiovascular risk factors; and inflammatory markers.
Body composition and ectopic fat
Fat-free mass (FFM), fat mass (FM), % body fat, bone mineral density (BMD), and visceral adipose tissue (VAT) were measured by dual-energy X-ray absorptiometry (Lunar iDXA; General Electric, Milwaukee, WI) and analyzed with the use of enCore software version 13.60.033 (GE Medical Systems, Milwaukee, WI). Extramyocellular lipid (EMCL) and intramyocellular lipid (IMCL) in skeletal muscle (soleus and anterior tibialis), along with intrahepatic lipid (IHL), were measured via 1H magnetic resonance spectroscopy (1H-MRS). Images were acquired with the use of a 3.0-Tesla whole-body imaging and spectroscopy system (GE Medical Systems, Milwaukee, WI), employing the Point Resolved Spectroscopy (PRESS) box technique (32). Lipid peaks were normalized to an external oil phantom (33), and oil-adjusted values for IHL, EMCL, and IMCL were analyzed with the use of the software package jMRUi.
Intravenous glucose tolerance test
An intravenous glucose tolerance test (IVGTT) was performed after an overnight fast to measure insulin sensitivity and secretion. After a baseline blood sample was drawn, a bolus of glucose (300 mg/kg body weight) was injected at time 0 (min), and blood was drawn at 2, 4, 8, and 19 min. Twenty minutes later, a bolus of insulin (0.03 U/kg body weight) was injected, and blood was drawn at 22, 25, 30, 40, 50, 70, 100, 120, and 180 min. Each blood sample was assayed for glucose and insulin. The Minimal Model was then used to calculate the acute insulin response to glucose (AIRg), insulin sensitivity (SI), the disposition index (DI), glucose effectiveness (Sg), and the rates of entry (P3) and removal (P2) of insulin to or from the interstitial space with the use of the MinMod software (MINMOD-PC, R. Bergman) (34).
Standardized meal test
Following an overnight fast, participants consumed a 400-kcal smoothie consisting of raw banana, Greek yogurt, ProCel Whey Protein, unsweetened coconut milk, strawberry cream cheese, Yoplait Original Yogurt, and nonfat, instant, dry milk powder (40% carbohydrate, 40% fat, and 20% protein). Via an intravenous catheter, blood was drawn at −15, 15, 30, 45, 60, 90, 120, and 180 min relative to smoothie ingestion to measure glucose and insulin. Area under the curve (AUC) and peak values were determined for each analyte.
Respiratory chamber
Participants resided in a respiratory chamber for 12 h (1900–0700 h). Upon entering the indirect calorimeter, participants consumed a standardized dinner that constituted 30% of their estimated daily energy requirements, which were calculated as resting energy expenditure (REE) (35) at wk 0 multiplied by an activity factor of 1.5. At 2100 h, participants consumed a snack that constituted 20% of their estimated resting metabolic rate (RMR) × 1.5 at wk 0. The macronutrient composition of both the meal and snack was 50% carbohydrate, 35% fat, and 15% protein. Participants were instructed to sleep from 2230 h to 0630 h the following day. Energy expenditure, respiratory quotient, and substrate oxidation were calculated via standard equations (36). Sleep energy expenditure (SleepEE) was calculated as the mean energy expenditure between 0200 h and 0500 h during all minutes for which activity was less than 1% as measured by radar.
Serum chemistry
Glucose, serum chemistry panels, cholesterol, and TGs were assayed through the use of a DXC600 instrument (Beckman Coulter, Inc.; Brea, CA). FFAs were also measured on a DXC600 instrument (Beckman Coulter, Inc.) but via an enzymatic assay with colorimetric detection (WAKO Chemicals USA, Inc.; Richmond, CA). HDL cholesterol was measured via an immunoinhibition assay (Trinity Biotech USA, Inc.; Jamestown, NY or WAKO Chemicals USA, Inc.), whereas LDL cholesterol was determined through the use of the Friedewald equation. The inflammatory marker TNF-α was measured by immunoassay with fluorescent detection (EMD Millipore Corporation; Billerica, MA) on a Luminex instrument (Luminex Corporation; Austin, TX), whereas high-sensitivity C-reactive protein (hs-CRP) and insulin were measured by chemiluminescent immunoassay via an Immulite 2000 platform (Siemens Corporation; Washington, DC).
Statistical analyses
Analyses were performed with the use of SAS version 9.4 (SAS Institute, Inc.; Cary, NC) as 2-sided with a significance level of α = 0.05. Power calculations revealed that n = 40 completers per group provide 85% and 95% power (2-tailed, α = 0.05) to detect 15% and 18% improvements, respectively, in insulin sensitivity relative to the control group, assuming a within-group SD of 22% (31). Unfortunately, because of slow recruitment rates, the study was ended after n = 30 and n = 29 participants in the control and RS2 groups, respectively, completed the trial. These numbers provided 80% statistical power to detect a 16% improvement in insulin sensitivity, which is equivalent to an effect size of d = 0.74. Data were analyzed as difference scores via linear mixed models, with compound symmetry, adjusting for treatment and time as fixed effects. Additional analyses were performed by including sex and race as fixed effects, by log-transforming the data when necessary, or by nonparametric tests. Similarly, we also assessed whether the addition of various glycemic covariates (HbA1c, fasting glucose, AIRg, SI, DI)—representative of the various prediabetic phenotypes, including impaired fasting glucose and impaired glucose tolerance—affected the study outcomes. None of these extra analyses changed the statistical significance of the study outcomes, so all results reported are the least-squares means ± SDs obtained from linear mixed modeling without adjustment for covariates (which, in the absence of covariates, are equivalent to t tests and are labeled as such in the figure legends) or multiple comparisons. Treatment effects, which represent the change induced in the RS2 group relative to the change in the control group, are denoted with the symbol Δ. Analyses for IHL, soleus IMCL, soleus EMCL, AIRg, SI, and DI are presented with extreme outliers (range: 2.5–5 SDs away from the mean) removed; this materially changed the P value only for soleus EMCL, which became significant, but did not change any of our conclusions concerning the effectiveness of RS2.
RESULTS
Participants
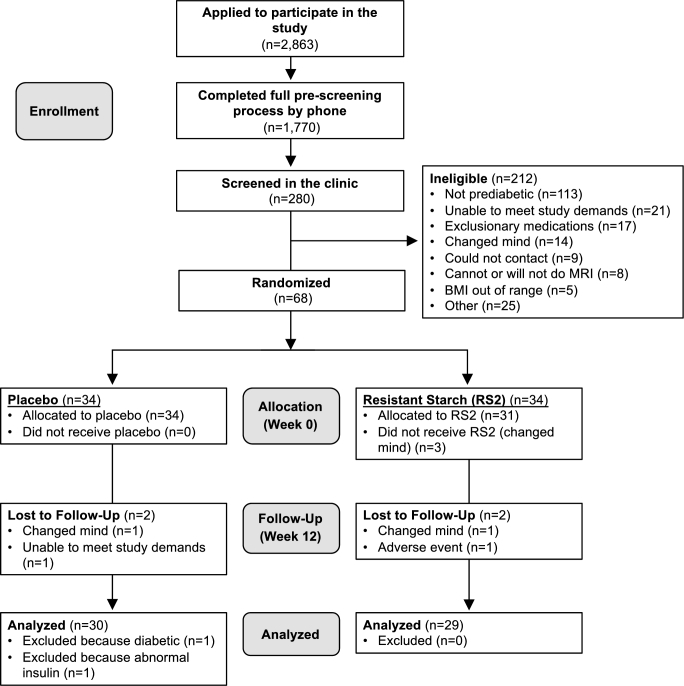
As shown in Figure 1, a total of 2863 individuals applied to participate in the study, and 1770 of them completed the full prescreening process. Of these, 280 adults were eligible for in-clinic screening. Following in-clinic screening, a majority (212 individuals) were excluded for not meeting the diagnostic criteria for prediabetes. Ultimately, 68 adults were randomized, and 61 of these (90%) completed the intervention. Five withdrew from the trial after they changed their mind, 1 withdrew for no longer being able to meet the study demands, and 1 withdrew because of an adverse event. Of those who completed the intervention in the control group, 1 participant displayed exceptionally high insulin concentrations (likely indicative of a congenital condition), and another participant was mistakenly enrolled despite having type 2 diabetes, so neither was included in the analyses. In total, our analyses included data from 59 participants who completed the study (n = 30 in the control group, n = 29 in the RS2 group) and included 20 men and 39 women (30 African Americans, 25 Caucasians, 1 Asian, and 3 who identified as biracial). As shown in Table 1, participants were aged 55 ± 10 y and had a mean BMI of 35.6 ± 4.8 kg/m2. Participants in both groups had a mean HbA1c of 5.7 ± 0.3% (39 ± 3 mmol/mol) at baseline (P = 0.90); fasting glucose concentrations were 104 ± 11 mg/dL and 106 ± 12 mg/dL in the RS2 and control groups, respectively (P = 0.52). Mean blood pressure and lipid concentrations were in the normal ranges and were not different between groups (P ≥ 0.06). Body fat percentage was lower (41.4 ± 7.0% compared with 45.5 ± 6.4%; P = 0.02), whereas height (170.6 ± 8.4 cm compared with 165.9 ± 8.3 cm; P = 0.04) and lean mass (57.1 ± 9.2 kg compared with 50.2 ± 7.1 kg; P = 0.002) were higher in the RS2 group, due to the higher number of males in the RS2 group (52% compared with 17%; P = 0.005). TNF-α was also higher at baseline in the RS2 group (12.7 ± 4.2 pg/mL compared with 10.4 ± 4.3 pg/mL; P = 0.04). There were no other significant differences between groups at baseline. Additional statistical analyses revealed that sex and ethnicity did not affect any of our conclusions.
FIGURE 1.
Participant flow diagram.
TABLE 1.
Participant characteristics at baseline1
| Control group (n = 30) | RS2 group (n = 29) | P | |
|---|---|---|---|
| Anthropometrics | |||
| Age, y | 55 ± 10 | 54 ± 10 | 0.82 |
| Sex, male/female | 5/25 | 15/14 | 0.0052 |
| Race/ethnic group, white/black/other | 14/14/2 | 11/16/2 | 0.513 |
| Weight, kg | 98.1 ± 14.6 | 103.3 ± 13.3 | 0.16 |
| Height, cm | 165.9 ± 8.3 | 170.6 ± 8.4 | 0.042 |
| Waist circumference, cm | 108.3 ± 11.5 | 111.2 ± 11.5 | 0.34 |
| Hip circumference, cm | 117.9 ± 11.4 | 116.1 ± 10.9 | 0.55 |
| Body composition and ectopic fat | |||
| BMI, kg/m2 | 35.7 ± 5.2 | 35.5 ± 4.4 | 0.91 |
| Body fat, % | 45.5 ± 6.4 | 41.4 ± 7.0 | 0.022 |
| Fat mass, kg | 44.9 ± 10.9 | 42.8 ± 9.8 | 0.44 |
| Lean mass, kg | 50.2 ± 7.1 | 57.1 ± 9.2 | 0.0022 |
| Bone mineral density, g/cm3 | 1.24 ± 0.13 | 1.29 ± 0.12 | 0.09 |
| Visceral adipose tissue, kg | 1.87 ± 1.00 | 2.14 ± 1.14 | 0.33 |
| Intrahepatic lipid (IHL), % | 6.13 ± 8.99 | 5.62 ± 7.66 | 0.83 |
| Soleus intramyocellular lipid (IMCL), % | 0.81 ± 0.72 | 1.29 ± 1.28 | 0.10 |
| Tibialis anterior IMCL, % | 0.53 ± 0.69 | 0.58 ± 0.75 | 0.76 |
| Soleus extramyocellular lipid (EMCL), % | 2.56 ± 3.03 | 3.12 ± 3.45 | 0.53 |
| Tibialis anterior EMCL, % | 3.26 ± 3.23 | 3.54 ± 3.60 | 0.76 |
| Glycemic control | |||
| HbA1c, % | 5.7 ± 0.3 | 5.7 ± 0.3 | 0.90 |
| Fasting glucose, mg/dL | 106 ± 12 | 104 ± 11 | 0.52 |
| Fasting insulin, mU/L | 21.0 ± 11.1 | 22.6 ± 9.0 | 0.55 |
| Insulin secretion (AIRg), mU/L × min | 507 ± 392 | 826 ± 876 | 0.09 |
| Insulin sensitivity (SI), (mU/L × min)−1 | 2.02 ± 1.44 | 1.67 ± 1.03 | 0.32 |
| Disposition Index (DI) | 1032 ± 1169 | 1187 ± 870 | 0.60 |
| Glucose effectiveness (Sg), min−1 | 0.0165 ± 0.0062 | 0.0165 ± 0.0075 | 1.00 |
| Glucose AUC, mg/dL × h | 335 ± 39 | 332 ± 50 | 0.78 |
| Insulin AUC, mU/L × h | 158 ± 60 | 180 ± 74 | 0.22 |
| Cardiovascular risk factors | |||
| Systolic blood pressure, mm Hg | 114 ± 10 | 118 ± 13 | 0.19 |
| Diastolic blood pressure, mm Hg | 68 ± 7 | 72 ± 10 | 0.13 |
| Heart rate, beats/min | 69 ± 8 | 71 ± 9 | 0.54 |
| Total cholesterol, mg/dL | 178 ± 26 | 192 ± 35 | 0.10 |
| LDL cholesterol, mg/dL | 105 ± 22 | 118 ± 27 | 0.06 |
| HDL cholesterol, mg/dL | 52.9 ± 11.7 | 49.9 ± 10.8 | 0.32 |
| Triglycerides, mg/dL | 102 ± 52 | 117 ± 93 | 0.44 |
| Free fatty acids, mmol/L | 0.505 ± 0.192 | 0.496 ± 0.187 | 0.85 |
| hs-CRP, mg/L | 9.6 ± 8.7 | 7.1 ± 6.9 | 0.22 |
| TNF-α, pg/mL | 10.4 ± 4.3 | 12.7 ± 4.2 | 0.042 |
1Data were analyzed with the use of an independent-samples t test. Data are mean ± SD. AIRg, acute insulin response; HbA1c, glycated hemoglobin; hs-CRP, high-sensitivity C-reactive protein.
2 P ≤ 0.05.
3 P value is for differences in the number of African American compared with non–African American participants.
Body composition and ectopic fat
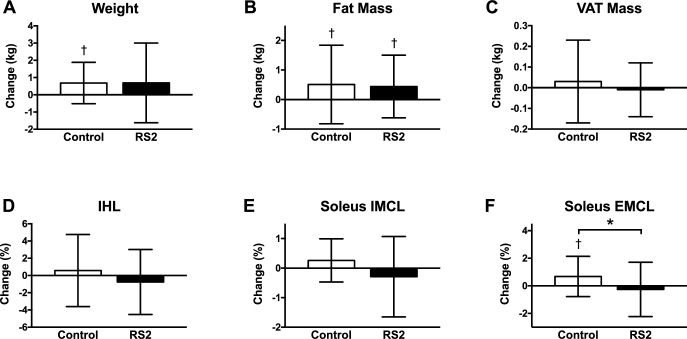
Per the study protocol, participants in both groups maintained their body weight within the required range throughout the trial, with only slight increases by 0.7 ± 2.3 kg in the RS2 group (P = 0.12) and 0.7 ± 1.2 kg in the control group (P = 0.004) but no between-group difference (Δ = 0.0 ± 1.8 kg; P = 0.99; Figure 2). RS2 did not affect % body fat (Δ = −0.1 ± 1.0%; P = 0.74), fat mass (Δ = −0.1 ± 1.2 kg; P = 0.82), lean mass (Δ = 0.1 ± 1.4 kg; P = 0.78), or BMD (Δ = 0.00 ± 0.02 g/cm3; P = 0.64) relative to the control group. Similarly, RS2 did not affect VAT (Δ = −0.04 ± 0.17 kg; P = 0.35), IHL (Δ = −1.34 ± 3.96%; P = 0.23), tibialis anterior IMCL (Δ = −0.23 ± 0.68%; P = 0.20), or tibialis anterior EMCL (Δ = −1.20 ± 3.96%; P = 0.26) relative to the control group. RS2 tended to decrease soleus IMCL (Δ = −0.56 ± 1.10%; P = 0.07) and decreased soleus EMCL (Δ = −0.94 ± 1.75%; P = 0.05) relative to the control group. However, the between-group difference in soleus EMCL was driven by an increase in EMCL in the control group (0.68 ± 1.46%; P = 0.03) rather than an improvement in the RS2 group.
FIGURE 2.
Body composition and ectopic fat. Body weight (A), fat mass (B), VAT mass (C), IHL (D), and soleus IMCL (E) were unchanged (P ≥ 0.07). Relative to the control group, RS2 reduced soleus EMCL (F) by Δ = −0.94 ± 1.75% (P = 0.05); however, this was driven by an increase in soleus EMCL in the control group (0.68 ± 1.46%; P = 0.03) rather than an improvement in the RS2 group. n = 30 and n = 29 participants completed the control and RS2 interventions, respectively. Data are displayed as mean ± SD and were analyzed by t tests. * P ≤ 0.05 for the between-group difference, †P ≤ 0.05 for the within-group change. EMCL, extramyocellular lipid; IHL, intrahepatic lipid; IMCL, intramyocellular lipid; RS2, type 2 resistant starch; VAT, visceral adipose tissue.
Glucose metabolism
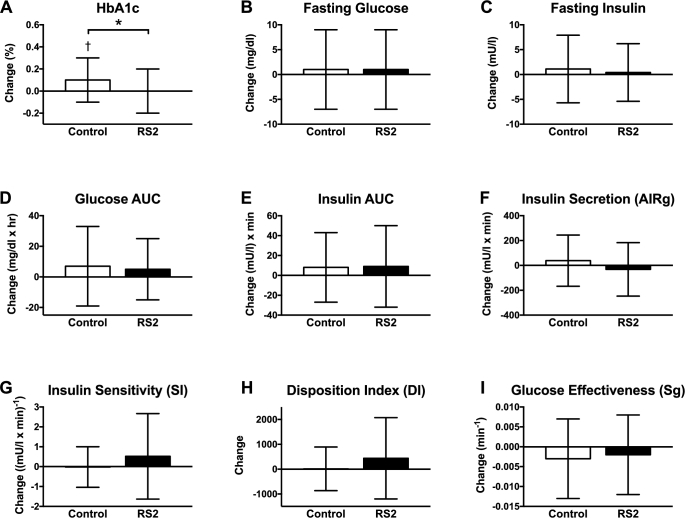
Relative to the control group, RS2 decreased HbA1c concentrations by Δ = −0.1 ± 0.2% (Δ = −1 ± 2 mmol/mol; P = 0.05); however, this value was driven by an increase in HbA1c in the control group (0.1 ± 0.2%, or 1 ± 2 mmol/mol; P = 0.01) rather than an improvement in the RS2 group (Figure 3). RS2 did not affect fasting glucose (Δ = 0 ± 8 mg/dL; P = 0.99), fasting insulin (Δ = −0.7 ± 6.3 mU/L; P = 0.68), the glucose AUC (Δ = –3 ± 25 mg/dL × h; P = 0.61), or the insulin AUC (Δ = 1 ± 40 mU/L × h; P = 0.93) during a 3-h mixed meal tolerance test. RS2 also did not affect insulin secretion as measured by AIRg (Δ = −70 ± 211 mU/L × min; P = 0.23), SI (Δ = 0.55 ± 1.69 (mU/L × min)−1; P = 0.26), the DI (Δ = 425 ± 1314; P = 0.26), or the effectiveness of glucose (Sg; Δ = 0.000 ± 0.009 min−1; P = 0.89) during an IVGTT.
FIGURE 3.
Glucose metabolism: (A) HbA1C, (B) fasting glucose, (C) fasting insulin, (D) glucose AUC, (E) insulin AUC, (F) insulin secretion, (G) insulin sensitivity, (H) disposition index, (I) glucose effectiveness. Relative to the control group, RS2 reduced HbA1c by Δ = −0.1 ± 0.2% (Δ = −1 ± 2 mmol/mol; P = 0.05); however, this result was driven by an increase in HbA1c in the control group (0.1 ± 0.2%; P = 0.01) rather than an improvement in the RS2 group. All other facets of glucose metabolism were unaffected (P ≥ 0.23). n = 30 and n = 29 participants completed the control and RS2 interventions, respectively. Data are displayed as mean ± SD and were analyzed by t tests. * P ≤ 0.05 for the between-group difference, †P ≤ 0.05 for the within-group change. AIRg, acute insulin response; HbA1c, glycated hemoglobin; RS2, type 2 resistant starch.
Cardiovascular disease risk factors
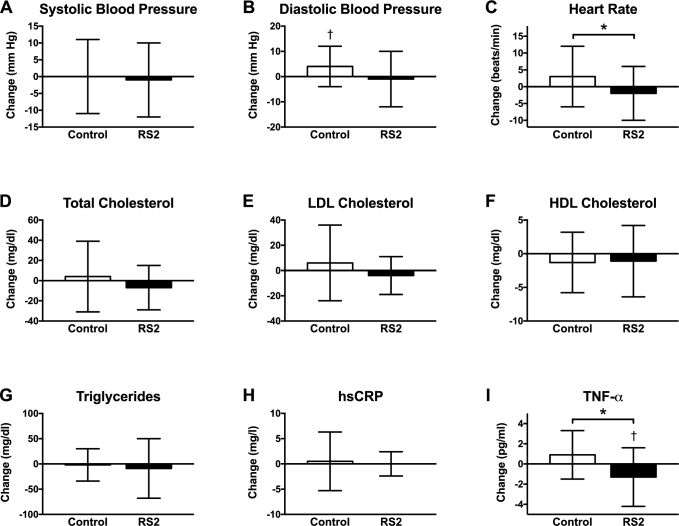
Changes in cardiovascular disease risk factors are shown in Figure 4. RS2 did not affect systolic blood pressure (Δ = −1 ± 11 mm Hg; P = 0.72), but it tended to decrease diastolic blood pressure by Δ = −5 ± 10 mm Hg (P = 0.06) and decreased heart rate by Δ = −5 ± 9 beats/min (P = 0.02) relative to the placebo. However, the trend towards a difference in diastolic blood pressure between the 2 groups was driven by an increase in the control group (4 ± 8 mm Hg; P = 0.01), and there was no change in heart rate in the RS2 group relative to baseline (−2 ± 8 beats/min; P = 0.10). Relative to the control group, RS2 did not affect total (Δ = −11 ± 29 mg/dL; P = 0.15), LDL (Δ = −9 ± 24 mg/dL; P = 0.14), or HDL (Δ = 0.2 ± 4.9 mg/dL; P = 0.88) cholesterol; TGs (Δ = −7 ± 48 mg/dL; P = 0.56); FFAs (Δ = −0.039 ± 0.179 mmol/L; P = 0.41); or hs-CRP (Δ = −0.4 ± 4.4 mg/L; P = 0.71). RS2 did lower the inflammatory marker TNF-α by 1.3 ± 2.9 pg/mL (P = 0.03) relative to baseline and by Δ = −2.1 ± 2.7 pg/mL (P = 0.004) relative to the control group. IL-6 concentrations were undetectably low (<3.4 pg/mL) in a large fraction of participants and thus were not suitable for analysis.
FIGURE 4.
CVD risk factors and inflammatory markers: (A) systolic blood pressure, (B) diastolic blood pressure, (C) heart rate, (D) total cholesterol, (E) LDL cholesterol, (F) HDL cholesterol, (G) triglycerides, (H) hs-CRP, (I) TNF-α. RS2 reduced heart rate by Δ = −5 ± 9 beats/min (P = 0.02) and TNF-α by Δ = −2.1 ± 2.7 pg/mL (P = 0.004). All other CVD risk factors were unaffected (P ≥ 0.06). n = 30 and n = 29 participants completed the control and RS2 interventions, respectively. Data are displayed as mean ± SD and were analyzed by t tests. * P ≤ 0.05 for the between-group difference, †P ≤ 0.05 for the within-group change. CVD, cardiovascular disease; hs-CRP, high-sensitivity C-reactive protein; RS2, type 2 resistant starch.
Energy metabolism
Relative to the control group, RS2 did not affect 12-h overnight (Δ = 13 ± 146 kcal/d; P = 0.74) or sleeping (Δ = 5 ± 135 kcal/d; P = 0.89) energy expenditure, nor did it affect the 12-h (Δ = 0.004 ± 0.045; P = 0.76) or sleeping (Δ = 0.000 ± 0.044; P = 0.98) respiratory quotient (data not shown). As a result, substrate oxidation was unaffected (P ≥ 0.67). With the exception of 12-h energy expenditure, which decreased by 59 ± 114 kcal/d (P = 0.008) in the control group, there were no other within-group changes.
Adverse events
A total of 48 adverse events occurred that were classified as possibly or definitely related to the study intervention, with a little under half of these (23/48) occurring in the RS2 group. The control group reported more instances of constipation than the RS2 group (7 compared with 3), but all other potentially intervention-related adverse events were about equally distributed across the 2 groups. Participants reported instances of gas/flatulence (5 compared with 7), headaches (7 compared with 5), bloating (2 compared with 2), heartburn (0 compared with 3), nausea (2 compared with 0), cramps (0 compared with 2), diarrhea (0 compared with 1), indigestion (1 compared with 0), and swelling (1 compared with 0).
DISCUSSION
RS and other fermentable carbohydrates are currently receiving intense interest as an intervention to improve the health of individuals with obesity and type 2 diabetes, even in the absence of other larger dietary changes such as energy restriction. RS2 has shown particular promise by improving a wide range of metabolic endpoints, including insulin sensitivity and fat accumulation, in rodents (6–9). In humans, several trials report that RS2 improves metabolic endpoints—such as insulin sensitivity, first-phase insulin secretion, and fat oxidation—in both adults without diabetes (12, 14, 16, 18–25) and adults with type 2 diabetes (26–30).
Here, we report results from the first RS2 intervention, to our knowledge, in a rather large group of study participants with prediabetes. Contrary to our hypothesis, we found that 12 wk of supplementation with 45 g/d of high-amylose maize (HAM-RS2) did not improve glycemic control, cardiovascular disease risk factors, ectopic fat, or energy metabolism, relative to baseline. The sole exception was that RS2 decreased circulating concentrations of the inflammatory marker, TNF-α. Although HbA1c, soleus EMCL, and heart rate were also lower in the RS2 group relative to the control group, 2 of these 3 differences were driven mostly by a worsening in the control group. Overall, we conclude that although RS2 may improve some inflammatory markers, it does not improve carbohydrate metabolism, ectopic fat, or cardiovascular disease risk factors in adults with prediabetes.
Our null results for glycemic control are largely at odds with those reported in other human trials. To date, there are a little more than a dozen trials of RS2 supplementation for 4 wk or longer. Of the 14 reporting glycemic outcomes, 10 reported improvements in either HbA1c, glucose concentrations, insulin concentrations, insulin sensitivity, or insulin secretion (14, 18–22, 26–28, 30). Of the remaining trials, 1 reported null results in overweight/obese women but positive results in men (12); 1 studying African Americans at-risk of type 2 diabetes reported a trend towards higher fasting glucose concentrations (37); 1 reported improvements in glycemic control only when a low-carbohydrate diet was consumed (38); and 1 studying individuals at-risk of type 2 diabetes reported mixed results, with postprandial insulin being increased but fasting glucose being decreased (16). One possibility is that the effects of RS2 in individuals with prediabetes are much weaker than would be expected on the basis of prior data; indeed, several of our metabolic endpoints showed modest but not statistically significant improvements.
Another possibility explaining the lack of a beneficial effect of RS2 in our study is the underlying dietary variability among individuals. A recent crossover trial found that RS2 supplementation improved glycemic control when participants followed a low-carbohydrate diet but not a high-carbohydrate diet (38). However, RS2 supplementation also increased trimethylamine N-oxide (TMAO) production when the low-carbohydrate diet was consumed (38). Given that TMAO is linked with increased risks of cancer, heart disease, stroke, and insulin resistance, such divergent effects are intriguing and suggest that the effects of RS2 may depend on the underlying diet composition. Such divergent outcomes are not limited to RS2 but may affect fermentable carbohydrates in general. A recent trial examining supplementation with fructo- and gluco-oligosaccharides found that high doses worsened glucose tolerance in humans (39). Therefore, supplementation with fermentable carbohydrates like RS may need to be tailored by both type and dose to the individual and/or may not be appropriate for all individuals.
Nonetheless, the rest of our results are largely consistent with those reported in other studies. Nearly all longer-term trials report that RS2 does not affect cholesterol, TGs, FFAs, or blood pressure (14, 16, 18, 20–22, 26, 28, 37). Similarly, all trials report that RS2 does not affect the inflammatory markers hs-CRP and IL-6 (12, 21, 26–28, 30, 37, 40), but 2 out of 3 trials reported a reduction in TNF-α (26, 28, 40). Lastly, 1 trial reported that RS2 increased fat oxidation (25), but the results for ectopic fat accumulation are mixed: 1 trial found that RS2 did not affect ectopic fat depots (21), whereas another reported an increase in soleus IMCL, with no change in IHL (26).
Our trial had a few limitations. First, we did not meet our original enrollment goal, so our post hoc statistical power was slightly less than anticipated. Nonetheless, our statistical power was greater, our intervention duration longer, and our dose of RS2 higher than in almost all published trials on RS2. Only 2 trials had interventions as long as ours, but their sample sizes were significantly smaller. Second, our intervention groups were not balanced by sex, although our statistical analyses revealed that the sex imbalance did not affect the results. Another limitation is that we measured compliance by requiring participants to return the empty RS2 containers rather than directly supervising RS2 consumption. Lastly, participants consumed their habitual diets and supplements and replaced foods of their choosing with the RS2 or placebo, rather than consuming a diet that was matched across arms, which likely increased heterogeneity.
In conclusion, we report that RS2 supplementation does not improve cardiometabolic health in adults with prediabetes, although it does reduce TNF-α concentrations. This lends support to newer evidence that high doses of fermentable carbohydrate supplementation may not always improve cardiometabolic health as so often claimed. Future studies are needed to determine whether there are potential subgroups of individuals—based on their baseline gut microbiota, diet composition, and other biological and environmental factors—who respond better to RS2 supplementation than others. This could lead to a better understanding of the potential beneficial effects of RS supplementation on metabolic health and whether such effects are modulated by diet composition or existing microbial populations in the gut.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—CKM, MLM, RJM, MJK, and ER: designed the research; CMP, CKM, MLM, MJK, KJA, and ER: conducted the research; RAB: analyzed the data; CMP and KLM: wrote the paper; ER: had primary responsibility for final content; and all authors: reviewed the manuscript for critical content and approved it prior to submission. The sponsors had no role in the design, conduct, analysis, or reporting of the trial. MJK and ER have received research funding from Ingredion Incorporated and gifts of their products for use in this research. None of the other authors reported a conflict of interest related to the study.
Notes
Supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK092575 (to ER). This work was also supported by National Center for Advancing Translational Sciences career development grant KL2TR001419 (to CMP); by Nutrition Obesity Research Center (NORC) grant P30DK072476; and by Louisiana Clinical and Translational Science Center (LA CaTS) grant U54GM104940.
Supplemental Materials are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- AIRg
acute insulin response to glucose
- DI
disposition index
- EMCL
extramyocellular lipid
- FFAs
free fatty acids
- HbA1c
glycated hemoglobin
- hs-CRP
high-sensitivity C-reactive protein
- IHL
intrahepatic lipid
- IMCL
intramyocellular lipid
- RS
resistant starch
- RS2
type 2 resistant starch
- Sg
glucose effectiveness
- SI
insulin sensitivity
- TG
triglyceride
REFERENCES
- 1. Centers for Disease Control and Prevention. National diabetes statistics report. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017. [Google Scholar]
- 2. Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B, American Diabetes Association . Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 2007;30(3):753–9. [DOI] [PubMed] [Google Scholar]
- 3. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asp NG, Björck I, Holm J, Nyman M, Siljeström M. Enzyme resistant starch fractions and dietary fibre. Scand J Gastroenterol Suppl 1987;129:29–32. [DOI] [PubMed] [Google Scholar]
- 5. Birt DF, Boylston T, Hendrich S, Jane JL, Hollis J, Li L, McClelland J, Moore S, Phillips GJ, Rowling M, et al.. Resistant starch: promise for improving human health. Adv Nutr 2013;4(6):587–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keenan MJ, Zhou J, Hegsted M, Pelkman C, Durham HA, Coulon DB, Martin RJ. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr 2015;6(2):198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins JA. Resistant starch: metabolic effects and potential health benefits. J AOAC Int 2004;87(3):761–8. [PubMed] [Google Scholar]
- 8. Barouei J, Bendiks Z, Martinic A, Mishchuk D, Heeney D, Hsieh YH, Kieffer D, Zaragoza J, Martin R, Slupsky C, et al.. Microbiota, metabolome, and immune alterations in obese mice fed a high-fat diet containing type 2 resistant starch. Mol Nutr Food Res 2017;61(11):1700184. [DOI] [PubMed] [Google Scholar]
- 9. Tachon S, Zhou J, Keenan M, Martin R, Marco ML. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol Ecol 2013;83(2):299–309. [DOI] [PubMed] [Google Scholar]
- 10. Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PloS One 2010;5(11):e15046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venkataraman A, Sieber JR, Schmidt AW, Waldron C, Theis KR, Schmidt TM. Variable responses of human microbiomes to dietary supplementation with resistant starch. Microbiome 2016;4(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maki KC, Pelkman CL, Finocchiaro ET, Kelley KM, Lawless AL, Schild AL, Rains TM. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr 2012;142(4):717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robertson MD, Currie JM, Morgan LM, Jewell DP, Frayn KN. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia 2003;46(5):659–65. [DOI] [PubMed] [Google Scholar]
- 14. Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr 2005;82:559–67. [DOI] [PubMed] [Google Scholar]
- 15. Aryana K, Greenway F, Dhurandhar N, Tulley R, Finley J, Keenan M, Martin R, Pelkman C, Olson D, Zheng J. A resistant-starch enriched yogurt: fermentability, sensory characteristics, and a pilot study in children. F1000Res 2015;4:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bodinham CL, Smith L, Wright J, Frost GS, Robertson MD. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS One 2012;7(7):e40834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jenkins DJ, Vuksan V, Kendall CW, Wursch P, Jeffcoat R, Waring S, Mehling CC, Vidgen E, Augustin LS, Wong E. Physiological effects of resistant starches on fecal bulk, short chain fatty acids, blood lipids and glycemic index. J Am Coll Nutr 1998;17(6):609–16. [DOI] [PubMed] [Google Scholar]
- 18. Gower BA, Bergman R, Stefanovski D, Darnell B, Ovalle F, Fisher G, Sweatt SK, Resuehr HS, Pelkman C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: a randomized, controlled trial. Nutr Metab (Lond) 2016;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maziarz MP, Preisendanz S, Juma S, Imrhan V, Prasad C, Vijayagopal P. Resistant starch lowers postprandial glucose and leptin in overweight adults consuming a moderate-to-high-fat diet: a randomized-controlled trial. Nutr J 2017;16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robertson MD, Wright JW, Loizon E, Debard C, Vidal H, Shojaee-Moradie F, Russell-Jones D, Umpleby AM. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab 2012;97(9):3326–32. [DOI] [PubMed] [Google Scholar]
- 21. Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med 2010;27(4):391–7. [DOI] [PubMed] [Google Scholar]
- 22. Dainty SA, Klingel SL, Pilkey SE, McDonald E, McKeown B, Emes MJ, Duncan AM. Resistant starch bagels reduce fasting and postprandial insulin in adults at risk of type 2 diabetes. J Nutr 2016;146(11):2252–9. [DOI] [PubMed] [Google Scholar]
- 23. Jiménez-Dominguez G, Ble-Castillo JL, Aparicio-Trápala MA, Juárez-Rojop IE, Tovilla-Zárate CA, Ble-Castillo DJ, García-Vázquez C, Olvera-Hernández V, Pérez-Pimienta B, Diaz-Zagoya JC, et al.. Effects of acute ingestion of native banana starch on glycemic response evaluated by continuous glucose monitoring in obese and lean subjects. Int J Environ Res Public Health 2015;12(7):7491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wutzke KD, Schmidek KV. The effect of resistant starches on fat oxidation in healthy adults as measured by a 13CO2-breath test. Isotopes Environ Health Stud 2017;53(6):553–62. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JA, Higbee DR, Donahoo WT, Brown IL, Bell ML, Bessesen DH. Resistant starch consumption promotes lipid oxidation. Nutr Metab (Lond) 2004;1(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bodinham CL, Smith L, Thomas EL, Bell JD, Swann JR, Costabile A, Russell-Jones D, Umpleby AM, Robertson MD. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr Connect 2014;3(2):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karimi P, Farhangi MA, Sarmadi B, Gargari BP, Zare Javid A, Pouraghaei M, Dehghan P. The therapeutic potential of resistant starch in modulation of insulin resistance, endotoxemia, oxidative stress and antioxidant biomarkers in women with type 2 diabetes: a randomized controlled clinical trial. Ann Nutr Metab 2016;68(2):85–93. [DOI] [PubMed] [Google Scholar]
- 28. Gargari BP, Namazi N, Khalili M, Sarmadi B, Jafarabadi MA, Dehghan P. Is there any place for resistant starch, as alimentary prebiotic, for patients with type 2 diabetes? Complement Ther Med 2015;23(6):810–15. [DOI] [PubMed] [Google Scholar]
- 29. Ble-Castillo JL, Aparicio-Trápala MA, Francisco-Luria MU, Córdova-Uscanga R, Rodríguez-Hernández A, Méndez JD, Díaz-Zagoya JC. Effects of native banana starch supplementation on body weight and insulin sensitivity in obese type 2 diabetics. Int J Environ Res Public Health 2010;7(5):1953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kwak JH, Paik JK, Kim HI, Kim OY, Shin DY, Kim HJ, Lee JH, Lee JH. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis 2012;224(2):457–64. [DOI] [PubMed] [Google Scholar]
- 31. Marlatt KL, White UA, Beyl RA, Peterson CM, Martin CK, Marco ML, Keenan MJ, Martin RJ, Aryana KJ, Ravussin E. role of resistant starch on diabetes risk factors in people with prediabetes: design, conduct, and baseline results of the STARCH trial. Contemp Clin Trials 2018;65:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Larson-Meyer DE, Newcomer BR, Hunter GR. Influence of endurance running and recovery diet on intramyocellular lipid content in women: a 1H NMR study. Am J Physiol Endocrinol Metab 2002;282(1):E95–E106. [DOI] [PubMed] [Google Scholar]
- 33. Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, et al.. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 1999;48(8):1600–6. [DOI] [PubMed] [Google Scholar]
- 34. Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol 1979;236(6):E667–77. [DOI] [PubMed] [Google Scholar]
- 35. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51(2):241–7. [DOI] [PubMed] [Google Scholar]
- 36. Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Ann Rev Nutr 1987;7:187–208. [DOI] [PubMed] [Google Scholar]
- 37. Penn-Marshall M, Holtzman GI, Barbeau WE. African Americans may have to consume more than 12 grams a day of resistant starch to lower their risk for type 2 diabetes. J Med Food 2010;13(4):999–1004. [DOI] [PubMed] [Google Scholar]
- 38. Bergeron N, Williams PT, Lamendella R, Faghihnia N, Grube A, Li X, Wang Z, Knight R, Jansson JK, Hazen SL, et al.. Diets high in resistant starch increase plasma levels of trimethylamine-N-oxide, a gut microbiome metabolite associated with CVD risk. Br J Nutr 2016;116(12):2020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu F, Li P, Chen M, Luo Y, Prabhakar M, Zheng H, He Y, Qi Q, Long H, Zhang Y, et al.. Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci Rep 2017;7(1):11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Worthley DL, Le Leu RK, Whitehall VL, Conlon M, Christophersen C, Belobrajdic D, Mallitt KA, Hu Y, Irahara N, Ogino S, et al.. A human, double-blind, placebo-controlled, crossover trial of prebiotic, probiotic, and synbiotic supplementation: effects on luminal, inflammatory, epigenetic, and epithelial biomarkers of colorectal cancer. Am J Clin Nutr 2009;90(3):578–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.