Abstract
At the 2017 10th annual International Conference on Advanced Technologies and Treatments for Diabetes (ATTD) in Paris, France, four speakers presented their perspectives on the roles of continuous glucose monitoring (CGM) and of blood glucose monitoring (BGM) in patient management within one symposium. These presentations included discussions of the differences in the accuracy of CGM and BGM, a clinical perspective on the physiological reasons behind differences in CGM and BGM values, and an overview of the impact of variations in device accuracy on patients with diabetes. Subsequently a short summary of these presentations is given, highlighting the value of good accuracy of BGM or CGM systems and the ongoing need for standardization. The important role of both BGM and CGM in patient management was a theme across all presentations.
Keywords: blood glucose monitoring, continuous glucose monitoring
During the 10th annual International Conference on Advanced Technologies and Treatments for Diabetes (ATTD) in Paris, France, in 2017, four speakers presented their thoughts on the role of continuous glucose monitoring (CGM) versus that of blood glucose monitoring (BGM) within one symposium, which provided new perspectives. The key messages and takeaways from these presentations are summarized here.
Regular BGM usage has been associated with improved glycemic control.1 A higher frequency of measurements is associated with lower glycated hemoglobin (HbA1c) levels.2 While BGM was the standard of care for patients with type 1 diabetes for a number of years, the market introduction of CGM in 2000 has slowly changed this. CGM offers patients continuous information on glucose levels in interstitial fluid (ISF) over the whole day, including real-time (rt) display of glucose levels, trends in glucose levels, alerts for hypo- or hyperglycemic events, and round-the-clock monitoring.3 However, the widespread use of CGM in patients with diabetes has been hindered by a number of obstacles, including concerns about lag times, accuracy, costs, and system errors.3
The assumption of many colleagues is that all market-approved CGM and BGM systems are sufficiently accurate and safe. Differences in accuracy between CGM and BGM are not well understood by health care providers and patients. It is also not widely known that although there is a technical standard for checking the performance of BGM systems, none exists for CGM systems. However, it is of help for patients and physicians to understand which “technology” is better for monitoring patients and making treatment decisions. This symposium was unique as it brought together the clinical relevance of high accuracy of BGM systems and potential implications of insufficient accuracy on patient management.
The essential question raised in this symposium was, will CGM—a newer technology that has improved substantially since its relatively recent market introduction with respect to analytical performance and handling—replace BGM, a mature technology with a long-standing history that has continued to improve over the past several decades. The questions are:
Which technology is better?
Are BGM and CGM accuracies the same?
Will BGM be replaced by CGM?
It is clear that, at least in part, these questions are too black-and-white, and no definite answer was given by the four speakers. However, to our knowledge, the importance and differences between CGM and BGM have not been widely discussed in this format.
Busting the Myth: MARD for CGM versus ISO for BGM
The major topic Guido Freckmann, Ulm, Germany, discussed was the role of accuracy “standards” for CGM and BGM: “Is a 10% CGM MARD comparable to ±10% BGM ISO?” As there is no technological standard for real-time CGM (rtCGM) systems, the mean absolute relative difference (MARD) is often used to characterize their analytical performance. In contrast, the International Organization for Standardization (ISO) 15197 standard for BGM systems was established a number of years ago and is widely accepted. It is worth mentioning that BGM systems are supposed to fulfill this standard for market approval; however, once they are on the market, there is no systematic evaluation of their performance. It must be highlighted that the MARD is estimated very differently from the ISO. The MARD is estimated in clinical studies that vary depending on a number of factors (eg, study design, number and distribution of paired points, and accuracy of the BGM); thus different studies may provide quite different MARD values for the same device.4 The MARD does not differentiate between trueness and precision, which are both required for accuracy estimation.
Freckmann showed data that a MARD of 4% CGM is correlated with 10% ISO; however, no rtCGM system with a 4% MARD exists.5 As a reference, currently available rtCGM systems have MARDs of ~9% to 20%.3 Conversely, a MARD of 10% is correlated with ±25% ISO. Such a BGM system would not meet ISO standards. The major message is that MARD and ISO are quite different, and MARD for rtCGM systems is not directly comparable to ISO of BGM systems. The difference between a MARD of 10% and an ISO of 10% is illustrated in Figure 1. Lower MARD values are indicative of better analytical performance; however, the relationship between MARD and ISO is complex, and defining a threshold MARD that provides sufficient accuracy to satisfy ISO criteria is not possible.6 Thus, the MARD is not the ideal parameter for characterizing the performance of rtCGM systems; additional parameters and standardization are necessary to improve assessment of rtCGM systems, as shown in a recent analysis.7
Figure 1.
Graphical representation of difference between A. +/–10% BGM ISO and B. 10% CGM MARD.
Figure 1A adapted with permission from Link M, Schmid C, Pleus S, Baumstark A, Rittmeyer D, Haug C, Freckmann G, Journal of Diabetes Science and Technology (Volume 9, Issue 5): pp. 1041-1050. © 2015 Diabetes Technology Society. Adapted by Permission of Sage Publications, Ltd. Figure 1B reproduced with permission from Dr Guido Freckmann, based on a data subset from Freckmann G, Pleus S, Link M, Kamecke U, Haug C. Accuracy assessment of two tissue glucose monitoring devices, presented at the 17th Annual Diabetes Technology Meeting (November 2-4, 2017, Bethesda, Maryland).
Interaction Between BGM Accuracy and CGM Performance: Relationship Between BGM and CGM
Marc Breton, Charlottesville, Virginia, US, addressed the following questions: What is the outcome of using an rtCGM system or BGM system that is not accurate? How does an inaccurate BGM system impact calibration of rtCGM systems and dosing decisions? Would more frequent recalibration with BGM optimize performance of rtCGM systems? As an engineer, he evaluated such questions in a computer model to simulate what happened when a less accurate rtCGM system and/or BGM system was used. The model was based on an adult population of 55 patients who use a sensor-augmented pump system (a combination of an rtCGM system and insulin pump). Different scenarios were evaluated, with BGM or CGM measurement results being used to determine insulin dosing with or without use of rtCGM for hypo-/hyperglycemia alerts, low glucose suspend (LGS), or rate of change (ROC) corrections.8 In this model, no matter which method (BGM or CGM with or without alerts, LGS, or ROC) was used for insulin dosing, the same effect was observed; as the bias of the BGM system increased, HbA1c decreased and frequency of hypoglycemic events increased. This effect was exaggerated with an rtCGM system with low accuracy (MARD 20%) and reduced with one of higher accuracy (MARD 8%; Figure 2). In this model, random error had relatively little impact; however, the effects of random error were increased with less frequent calibration of the rtCGM system (every 1-2 days or less). Only a rtCGM system that is optimally calibrated ensures proper functioning of hypoglycemia alarms, LGS, or closed-loop systems. Along the same line, it is impressive to see to what extent an inaccurate system has a negative effect on patient treatment decisions. Based on these simulations, it is obvious that a BGM system with high accuracy is essential for rtCGM system calibration and the reliable performance of rtCGM in practice.
Figure 2.
Summary of CGM accuracy impact.
Original figure adapted with permission from: Campos-Náñez E, Breton MD. Journal of Diabetes Science and Technology (Volume 11, Issue 6): pp. 1196-1206. © 2017 Diabetes Technology Society. Adapted by Permission of Sage Publications, Ltd.
BGM and CGM: The Clinical Perspective
Thorsten Siegmund, Munich, Germany, addressed clinically relevant aspects: Why do measurement results obtained with rtCGM systems and BGM systems differ? What impact does physiology have on glucose measurements? Based on which result should a patient determine his insulin dose—the BGM or CGM result? Can these two technologies coexist and support each other?
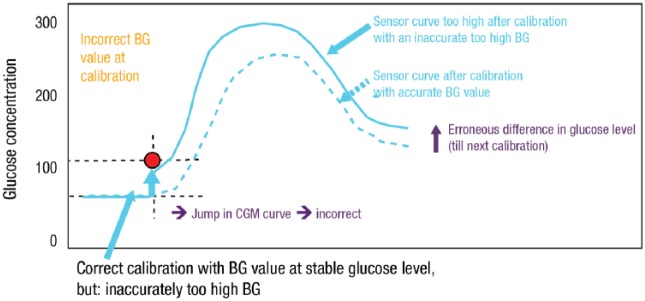
It is important to acknowledge that both glucose measurements are performed in two different compartments. Blood and ISF are clearly closely connected; nevertheless, glucose levels in these are not identical under all physiological conditions. ISF glucose levels compartments are delayed compared with blood glucose (“time lag”) as glucose is transported from the blood to target cells. If glucose levels in one compartment change rapidly (eg, during exercise), it takes some time until the same change can be measured in the other compartment due to slow diffusion of glucose. Thus, the time lag is mainly present in times of glucose variability, that is, when the blood glucose level is changing (or has recently been changing and is not yet at equilibrium) (eg, during/after meals, during/after physical activity, during/after hypoglycemia).9 Measurement differences between the BGM system and the rtCGM system under such conditions are not measurement errors. Reliable therapeutic decisions can be made based on rtCGM system measurements if the MARD is <10%. A technical lag-time (related to the glucose measurement itself) is also present and exaggerates the physiological lag. Accuracy of the BGM system used for calibration is essential for reliable rtCGM system calibration. If an incorrect BGM measurement is used to calibrate the rtCGM system, the glucose values provided are shifted up- or downward until the next calibration, resulting in incorrect measurement of glucose levels by the CGM in the interim (Figure 3).9
Figure 3.
Practical implications of BGM accuracy for CGM.
Original figure adapted with permission from: Unimed Science Verlag Bremen, from Continuous glucose monitoring (CGM) und sensor-augmented pump therapy (SAP). Siegmund T, Kolassa R, Thomas A. © 2012 Unimed Science Verlag Bremen.
Both BGM and CGM are relevant for patient care and provide differing, complementary information that provides a more complete insight into the glucose regulatory situation of a given patient. ISF glucose values reflect more glucose consumption/production of the biological system, while BGM values reflect the glucose concentrations in the body’s transport system. BGM is a static measurement that provides an accurate instant glucose level but does not provide information about changes in glucose levels over time. In contrast, CGM is a dynamic measurement that provides information about both the current value and changes in glucose levels over time.
Usage of rtCGM systems with a high accuracy will help one to understand which physiological and therapeutic factors drive glucose homeostasis in the human body. Accurate BGM readings are essential to ensure that rtCGM readings are reliable and can be used to inform treatment decisions. Thus, both technologies should coexist and support each other for patient care.
Wrapping It All Up!
Jill Weissberg-Benchell, Chicago, Illinois, US, not only presented a different view, but also provided information about the impact of variations in accuracy of BGM or rtCGM systems on patients with diabetes. In principle, accuracy of the devices they use for glucose measurement should not be a concern for patients; all devices should provide accurate readings all the time. This is what many patients (and also physicians and nurses) believe anyway.
It is worthwhile to consider to what extent the accuracy of these diagnostic products impact daily therapy decisions and clinical outcomes. What does information on accuracy mean to the patient? In this context, patient behavioral and emotional aspects related to glucose data they receive are also of relevance. If patients distrust the accuracy of their glucose monitoring system, is this associated with an increased emotional burden? When patients get different results from parallel measurements with their BGM system and rtCGM system, what do they do? Which system do they rely on for their dosing decisions?
In one study by Pickup and colleagues10 that evaluated 100 narratives from patients who used rtCGM systems, the majority of patients expressed concern about the reliability of their readings, while 32% expressed concern about sensor inaccuracy or failure and incorrect alarms. In separate studies, 21% to 34% of patients discontinued use of rtCGM systems due to perceived inaccuracy.11,12 Survey data from the online “GLU community” supported the findings of these studies, showing that approximately 44% of patients corrected a low glucose value based only on an rtCGM system reading (without BGM verification) most or nearly all the time and 41% corrected a high glucose value based only on an rtCGM system reading most or nearly all the time (Figure 4). Several studies have shown that when patients trust CGM accuracy, they are more likely to respond to the numbers and to make more aggressive treatment decisions to achieve better glycemic control.12-14
Figure 4.
Patient responses about correcting based on unverified CGM readings; A. Low readings; B. High readings.
Original figure adapted with permission from: Glu, from Power to the People! How the Voice of People with T1D Can Influence Regulatory Decisions, Christine Frost, https://myglu.org/articles/power-to-the-people-how-the-voice-of-people-with-t1d-can-influence-regulatory-decisions. © 2017 Glu.
Thus, patients need to be able to rely on the results they receive from their rtCGM system or BGM system to ensure that they are making correct dosing decisions, and “convenient” does not always mean “safe.” Clinicians caring for patients with diabetes should consider a number of tips for improving patient care (Table 1).
Table 1.
Suggestions for Physician Behavior to Help Patients With Diabetes.
| • Never forget how tough daily diabetes care is |
| • Praise your patients for every single thing they do to keep themselves healthy |
| • Recognize that our tools are far from perfect and can lead to frustration unless expectations are appropriate |
| • Remind the patients you care for about the disconnect between laboratory testing of accuracy and real-life use accuracy |
| • Teach that inaccuracy is part of daily life with diabetes |
| • Teach strategies for coping with this fact |
| • Teach when to calibrate |
| • Remind the patients you care for that there are all sorts of things they may be doing/not doing that can lead to inaccurate numbers |
Reproduced with permission from Prof Dr Jill Weissberg-Benchell.
Summary
The presentations in this symposium provided a good awareness regarding the high value of good accuracy of either the BGM system or the rtCGM system and also highlighted the need for standardization. It is worth acknowledging that there is value in both diagnostic technologies (ie, there is an ongoing role for both BGM and CGM in patient management), a sentiment that was echoed in all presentations. BGM and rtCGM systems also support patients and make them more confident in dosing decisions. Usage of the most accurate BGM system also helps optimize rtCGM performance in an artificial pancreas system. So, BGM systems and rtCGM systems both offer valuable and complementary data on glucose levels. The tandem use of these technologies, while managing patient expectations, should lead to better patient care.
Footnotes
Abbreviations: ATTD, Advanced Technologies and Treatments for Diabetes; BGM, blood glucose monitoring; CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin; ISF, interstitial fluid; ISO, International Organization for Standardization; LGS, low glucose suspend; MARD, mean absolute relative difference; ROC, rate of change; rtCGM, real-time continuous glucose monitoring.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The symposium described in this commentary and editorial support for this article were funded by Ascensia Diabetes Care.
References
- 1. Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ. 1999;319:83-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36:2009-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18(suppl 2):S3-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reiterer F, Polterauer P, Schoemaker M, et al. Significance and reliability of MARD for the accuracy of CGM systems. J Diabetes Sci Technol. 2017;11:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breton MD, Kovatchev BP. Impact of blood glucose self-monitoring errors on glucose variability, risk for hypoglycemia, and average glucose control in type 1 diabetes: an in silico study. J Diabetes Sci Technol. 2010;4:562-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pardo S, Simmons DA. The quantitative relationship between ISO 15197 accuracy criteria and mean absolute relative difference (MARD) in the evaluation of analytical performance of self-monitoring of blood glucose (SMBG) systems. J Diabetes Sci Technol. 2016;10:1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pardo S, Simmons D, Zhuplatov S, Breton MD. A new method to evaluate analytic performance of CGM devices [abstract]. Diabetes. 2017;66(suppl 1A):111-LB. [Google Scholar]
- 8. Campos-Náñez E, Fortwaengler K, Breton MD. Clinical impact of blood glucose monitoring accuracy: an in-silico study. J Diabetes Sci Technol. 2017;11:1187-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siegmund T, Kolassa R, Thomas A. Continuous Glucose Monitoring (CGM) and Sensor-Augmented Pump Therapy (SAP). London, UK: Unimed Science Verlag Bremen; 2012. [Google Scholar]
- 10. Pickup JC, Ford HM, Samsi K. Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care. 2015;38:544-550. [DOI] [PubMed] [Google Scholar]
- 11. Ramchandani N, Arya S, Ten S, Bhandari S. Real-life utilization of real-time continuous glucose monitoring: the complete picture. J Diabetes Sci Technol. 2011;5:860-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polonsky WH, Hessler D. Perceived accuracy in continuous glucose monitoring: understanding the impact on patients. J Diabetes Sci Technol. 2015;9:339-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pettus J, Price DA, Edelman SV. How patients with type 1 diabetes translate continuous glucose monitoring data into diabetes management decisions. Endocr Pract. 2015;21:613-620. [DOI] [PubMed] [Google Scholar]
- 14. Joubert M, Reznik Y. Personal continuous glucose monitoring (CGM) in diabetes management: review of the literature and implementation for practical use. Diabetes Res Clin Pract. 2012;96:294-305. [DOI] [PubMed] [Google Scholar]