Abstract
The insulin content in vials/cartridges should be 95 U/ml upon release. No independent confirmation of insulin concentration is available when purchased in the pharmacy (end of the cold supply chain). We quantitatively measured intact insulin in randomly acquired multidose human insulin vials by standard analytical methods. Eighteen 10 ml vials from two manufacturers (M1 and M2) were randomly acquired. The intact insulin concentration ranged from 13.9 to 94.2 U/ml, mean 40.2 U/ml. No vial met the minimum standard of 95 U/ml. These results imply the cold supply chain impacts insulin concentrations to a larger extent than anticipated. Patients are paying high prices for insulin and should expect to receive insulin vials with adequate insulin content in return.
Keywords: insulin, analysis, insulin content, quality assurance, vials
Daily millions of patients with diabetes depend upon subcutaneous insulin replacement therapy. Today insulin is mostly manufactured in large batches by means of biotechnological processes. Details of this production process and the subsequent filling process of the insulin into vials/cartridges are proprietary to each insulin manufacturer. Each manufacturer’s quality assurance program has internal protocols for measuring the final insulin content in the vials/cartridges for sale. In theory all these should contain the USP/FDA required minimum of 95% of intact insulin (= 95 U/ml) upon release.
We previously raised concerns about potential impact of the global cold supply chain on insulin quality and quantity.1 There is a lack of independent confirmation of this (eg, the intact insulin concentration in the vials/cartridges) at the end of the cold supply chain when purchased by patients with diabetes in the pharmacies.
To provide insight into this topic we quantitatively measured the amount of intact insulin in randomly acquired 10 ml multidose human insulin vials by standard analytical methods. To ensure random selection of lots and supply chain sources, the insulin samples were purchased on a variety of calendar dates from various pharmacy locations located in different US states supplied by three different wholesale vendors. To gain insight into potential differences between insulin shipped to the United States from Europe or insulin that was manufactured in the United States, the purchased insulin was from one manufacturer based in the United States and the other from one in Europe.
Methods
Eighteen 10 ml vials representing 16 different insulin lots from two manufacturers (M1 and M2) were randomly purchased from five pharmacies supplied by three different wholesale sources. Four vials of regular insulin and four vials of NPH insulin manufactured by M1 and five vials of regular insulin and five vials of NPH insulin manufactured by M2 were acquired.
All vials were carefully stored under continuously monitored (AmegaView) refrigerated conditions between 2⁰C to 8⁰C after purchasing until analyzed. Lots were cataloged by date of acquisition, pharmacy, wholesale vendor, lot number, and expiration date. Expiration dates for the lots ranged from August 2018 to April 2019; analysis occurred on May 9, 2017.
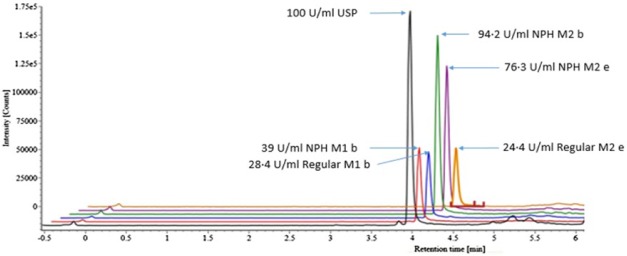
Insulin content was measured using Waters Xevo G2-XS QTOF Quadrapole Time-of-Flight Mass Spectrometer (QTOF) with a Waters Cortecs UPLC C-18, 2.1 × 30 mm ID column by Megan Cooley, PhD and Otis Beverly, BSChem at MRIGlobal per published methods.2 Standards of human insulin USP and bovine pancreas insulin, used as the internal standard, were acquired from Sigma Aldrich. Samples and standards were diluted in 30/10/60 MeOH/CH3COOH/H2O with 0.5% bovine serum albumin (Figure 1).
Figure 1.
Example overlay comparing the USP Insulin Standard intact insulin result to five sample lot intact insulin results using Waters Xevo G2-XS QTOF. All peaks were observed at 4.53 retention time and are offset on the x-axis to allow visualization of differences in concentrations found.
All data and processes were cross-checked by the analytical chemists performing the analysis and confirmed by a third analytical chemist unaffiliated with the study. Per USP/FDA guidance a minimum of 95 U/ml concentration of (intact) insulin, molecular weight 5808, was set as the desired target.
Results
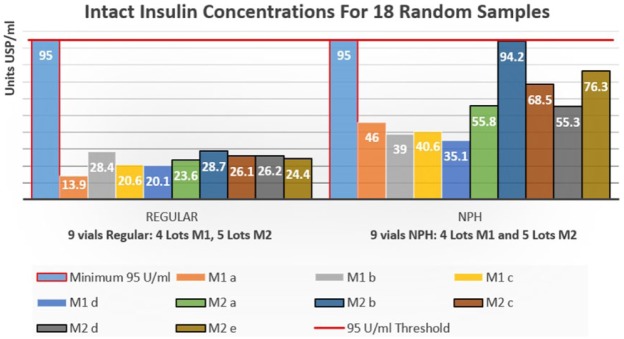
The intact insulin concentration in the 18 vials analyzed ranged from 13.9 to 94.2 U/ml, with a mean value of 40.2 U/ml (Figure 2). No correlation of insulin levels to country of origin, supply source, or cost was observed. Variance between the four lots of regular insulin from M1 was 14.5% and 5.1% for the five lots of regular insulin from M2. With the NPH insulin samples a variance of 10.9% for the four lots from M1 was found and one of 38.9% for the five lots from M2. The within lot variance for duplicate lots of regular insulin was 8.3% for M1 and 2.6% for M2.
Figure 2.
The 95 U/ml minimum outlined in red and the 95 U/ml threshold red line represent the smallest concentration of intact insulin allowed by the FDA for release by the manufacturer. N = 18, range = 13.9-94.2 U/ml, mean = 40.2 U/ml. To deidentify manufacturer source samples are labeled REGULAR M1 (a-d) and REGULAR M2 (a-e); NPH M1 (a-d) and NPH M2 (a-e).
Discussion
To our surprise, the insulin concentration in none of the vials met the minimum labeled concentration standard of 95 U/ml at time of testing. M2 had a lot-to-lot concentration variance of <±5% (10% aggregate) for regular insulin but fell <30 U/ml of labeled concentrations.
To avoid a decline in insulin concentrations after purchasing the vials, all samples were stored together under 24/7 monitored refrigeration conditions until testing was performed. The measurement results and the analytical process were cross-checked by experienced analytical chemists and internally peer reviewed by a third party unaffiliated with the study.
Given the random acquisition of lots from a variety of pharmacy locations and wholesalers, and assuming the vials contained the specified insulin concentration when manufactured, our interpretation of these results is the cold supply chain negatively impacts insulin concentrations in the vials to a much larger extent than we anticipated.
The cold supply chain should ensure the consumer receives insulin meeting acceptable quality standards. This is the combined responsibility of the manufacturer, regulatory agencies, wholesalers and pharmacies as generally outlined in the Drug Quality and Security Act enacted by the US Congress on November 27, 2013. Under Title II, Drug Supply Chain Security, manufacturers, wholesale distributors, dispensers, or repackagers are required to implement systems to:
investigate suspect products
handle illegitimate products, including through quarantine, disposal, and appropriate notice to the Secretary and, as necessary, trading partners.3
Quality of a critical care biologic product, such as insulin, is difficult to determine prior to dispensing by the pharmacist or at home before administration by the patient. If the results presented here can be repeated by others (with additional analysis of insulin analogs/biosimilar insulins, and other biologics shipped through the cold supply chain), we see the need to evaluate during which step in the cold chain the observed massive reductions in insulin concentration are taking place and how this can be avoided. Patients are paying high prices for insulin and should expect to receive insulin vials with adequate insulin content in return.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AWC is employed by MRIGlobal as the pharmacist manager and principal investigator for NIH-NINDS and NCATS Drug Manufacturing Development Program contracts. LH is a consultant for a number of companies that develop novel diagnostic and therapeutic options for the treatment of patients with diabetes. He owns shares of the Profil Institut für Stoffwechselforschung, Neuss, Germany and ProSciento, San Diego, CA, USA.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MRIGlobal is a not-for-profit research institute that provides unrestricted internal research grants to encourage novel independent study that may provide unique insights. A grant of $49 925 was provided to cover costs of insulin standards, purchase of insulin samples and laboratory supplies, cover instrument operation expenses, and provide expert analyst labor necessary to conduct the study. Oversight consists of quarterly review of progress and all results are expected to be compiled and submitted to appropriate journals for peer review and publication consideration.
References
- 1. Carter AW, Heinemann L, Klonoff DC. Quality control of insulins and biosimilar insulins: what do we know? J Diabetes Sci Technol. 2016;10(4):811-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chambers EE, Legido-Quigley C, Smith N, Fountain KJ. Development of a fast method for direct analysis of intact synthetic insulins in human plasma: the large peptide challenge. Bioanalysis. 2013;5(1):65-81. [DOI] [PubMed] [Google Scholar]
- 3. Congress.gov. H.R.3204—Drug Quality and Security Act. Available at: https://www.congress.gov/bill/113th-congress/house-bill/3204. Accessed September 18, 2017.