Abstract
Background:
Studies comparing standalone real-time continuous glucose monitoring (rtCGM) to self-monitoring of blood glucose (SMBG) in patients with type 1 diabetes mellitus (T1DM) have found that rtCGM is associated with lower glycated hemoglobin (A1C) levels, yet does not increase the risk of severe hypoglycemia. However, little is known about the relationship between rtCGM and health care costs and utilization. The objective of this study was to compare health care spending, hospital admissions, and A1C levels of patients using rtCGM to that of patients not using rtCGM.
Methods:
This retrospective, cross-sectional analysis used a large repository of health plan administrative data to compare average health care costs (excluding durable medical equipment), hospital admissions, and A1C levels of those using rtCGM (N = 1027) versus not using rtCGM (N = 32 583). To control for potentially confounding variables, a propensity score method was used to match patients using rtCGM to those not using rtCGM, based on characteristics such as age, gender, and comorbidities.
Results:
Patients using rtCGM spent an average of approximately $4200 less in total health care costs, when compared to patients not using rtCGM (P < .05). They also experienced fewer hospital admissions (P < .05) and lower A1C (P < .05) during the postindex year.
Conclusions:
Use of rtCGM by patients with T1DM is associated with lower health care costs, fewer hospital admissions, and better glycemic control.
Keywords: type 1 diabetes, rtCGM, continuous glucose monitoring, insulin, A1C, claims analysis
According to a recent report from the Centers for Disease Control, nearly 10% of Americans have diabetes, a chronic condition and the leading cause of kidney failure, nontraumatic lower-limb amputation, and new cases of blindness among adults, as well as a major cause of heart disease and stroke.1 Diabetes and its complications impose a significant economic burden on the health care system, exceeding $245 billion in total direct and indirect costs in 2012.2
Blood glucose monitoring is considered an integral component of effective therapy for diabetes management.1,3 The finger-stick test, also known as self-monitoring of blood glucose (SMBG), is the most commonly used technique. However, SMBG necessitates multiple blood samples over the course of a day and is not typically used to detect nocturnal hypoglycemia and asymptomatic hypoglycemia. Patients with diabetes frequently use SMBG less often than recommended, due to a number of issues, including pain, lifestyle interference, lack of information, and motivational and behavioral barriers.4-6 Up to 75% of hyper- and hypoglycemic episodes can therefore go undetected.7 In addition, SMBG provides only a single, immediate measure without information regarding glucose trends over time, potentially resulting in significant fluctuations that go untreated.8,9
In contrast, real-time continuous glucose monitoring (rtCGM) uses a small subcutaneously inserted sensor to measure glucose levels in the interstitial fluid every few minutes, 24 hours per day. rtCGM provides patients with real-time and trend data, as well as alerts when blood glucose level is rapidly rising or falling, which allows better self-management throughout the day.10
A meta-analysis comparing the effectiveness of rtCGM and SMBG supported the superiority of rtCGM in lowering glycated hemoglobin (A1C) levels in patients with type 1 diabetes mellitus (T1DM), without increasing the risk of severe hypoglycemia.11 More recently, two randomized clinical trials comparing the use of rtCGM and SMBG in adults with T1DM using multiple daily insulin injections found that the former group had greater decreases in A1C, as well as greater time within target glucose range and less glycemic variability.12,13 Although more limited evidence supports the use of rtCGM in patients with type 2 diabetes (T2DM), a 2016 consensus conference statement by the American Association of Clinical Endocrinologists and the American College of Endocrinology notes that rtCGM is likely to be of benefit “whenever intensive insulin therapy is used, regardless of diabetes type.”14 This is echoed by the Endocrine Society’s 2016 Clinical Practice Guideline, which recommends rtCGM for “adult patients with T2DM (not on prandial insulin) who have A1C levels ≥7% and are willing and able to use the device.”15 Significantly greater reductions in A1C have been observed in patients using rtCGM at least 75% of the time, when compared to patients using rtCGM less than 25% of the time.16
Some studies have also compared the cost-effectiveness of rtCGM versus SMBG. The majority use validated models, such as the Center for Outcomes Research (CORE) Diabetes Model17 (a combination of Markov structure and Monte Carlo simulation), to simulate disease progression for a cohort of patients typically drawn from clinical trials or meta-analyses; however, results vary widely, likely due to differences in methodology.18-22 Other concerns regarding much of the published cost-effectiveness research are estimation of costs over a long follow-up period (eg, 30 years to lifetime) and inclusion of both direct and indirect costs, both of which may limit the utility of the findings for payers.23
Most of the modeling studies examined the cost-effectiveness of standalone rtCGM versus SMBG in patients with T1DM and reported incremental cost-effectiveness ratios (ICERs) ranging from $33 789 per quality-adjusted life-year (QALY) gained to $98 679 per QALY.18-20 In contrast, when modeling the cost-effectiveness of rtCGM in patients with T2DM, Fonda et al reported an ICER of $13 030 per QALY, and a modeling study by Roze et al, which focused on patients using an insulin pump in conjunction with rtCGM, reported an ICER of $54 698 per QALY.21,22 A somewhat different type of modeling study used data derived from previously published research to determine the cost-effectiveness of standalone rtCGM in terms of decreasing the rate of severe hypoglycemia and subsequent hospitalization in adults with T1DM.23 The authors found that rtCGM lowered the annual cost of hypoglycemia-related hospitalizations by $946 to $1345 per patient.
Other investigators have conducted controlled trials and observational studies to explore the effect of rtCGM on health care utilization. For example, a multicenter, randomized, controlled crossover study examined the effect of a combination of insulin pump therapy and rtCGM on medical resource use. The authors reported that insulin pump use in conjunction with rtCGM was associated with lower A1C, without increases in health care utilization.24 More recently, a multicenter, prospective, observational cohort study found that patients with T1DM using an insulin pump and rtCGM experienced fewer hospital admissions for acute diabetes complications, compared to similar patients using an insulin pump without rtCGM.25
Finally, Parkin et al used health plan administrative data for a cohort of patients with T1DM to evaluate the effect of rtCGM versus SMBG.26 They reported that the former was associated with improvements in A1C, as well as reductions in hospital admissions; however, with the exception of inpatient and emergency room (ER) costs, they did not evaluate health care spending. Our research builds on these findings by including a detailed analysis of spending. More specifically, we used a large repository of health plan administrative data linked to automated lab results to compare annual health care costs/utilization and A1C levels in patients with T1DM using standalone rtCGM (ie, without an insulin pump) to those not using standalone rtCGM.
Methods
Study Design
We conducted a population-based, cross-sectional analysis that compared annual health care costs/utilization and A1C of patients with diabetes who used rtCGM for at least 3 quarters, to those who did not use rtCGM.
Data Source
For this analysis, we utilized the Optum Clinformatics® Data Mart (OptumInsight, Eden Prairie, MN) database of commercially insured and Medicare Advantage subscribers and their dependents. The database contains deidentified, person-specific data, including insurance claims submitted by all providers of medical care or treatments, including durable medical equipment (DME), and claims submitted by pharmacies. In addition, actual lab results/values were available in the database for a subset of all laboratory tests, including the A1C tests used in this analysis. Institutional review board oversight was not necessary, because the database was deidentified in compliance with HIPAA rules.
Sample selection and creation of analytic variables were performed using the Instant Health Data (IHD) platform (BHE, Boston, MA). Statistical analyses were undertaken with SAS software, version 9.4 (SAS Institute Inc, Cary, NC).
Population Selection and Study Period
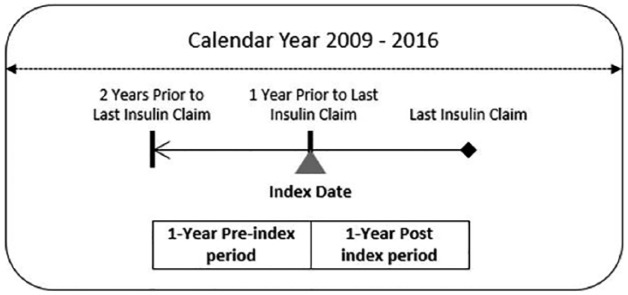
Using the database described above, we began our population selection by identifying individuals with at least one pharmacy claim for insulin between January 1, 2009, and December 31, 2016. Patients were excluded if they used an insulin pump or had gestational or nonclinical diabetes during the same time period. We then defined the index date as the date of service on the last claim for insulin minus one year. Our study period consisted of the 2 years prior to last insulin claim, with 1 year prior to the index date defined as “preindex” period and 1 year following and including the index date as the “postindex” period (Figure 1).
Figure 1.
Study period.
In addition, patients had to have at least one insulin claim in the first 6 months of the postindex period. The sample was then further narrowed to include only those patients who were continuously enrolled with medical and pharmacy benefits during the study period. Additional exclusion criteria included patients who were younger than 14 years or older than 64 years at the index date, and who had no insulin claims during the preindex period. Then, whether a patient had T1DM or T2DM was defined by a combination of diagnosis codes and use of oral antidiabetic medications (OAD). Only commercially insured patients with T1DM were included in the final study sample. These criteria were applied since they most closely match the suggested target population for rtCGM, and very few individuals with T2DM and Medicare Advantage were found to use rtCGM during exploratory analyses.
Our intervention, or the “rtCGM” cohort, was the subset of each of the above samples that included patients with a rtCGM order at least 3 quarters of a year during the postindex period. We chose this cut-off based on previously published research.16 Our comparison group, or the “non-rtCGM” cohort, was the subset of each of the above samples that did not use rtCGM during the postindex period.
Measures
Health Care Costs and Utilization
We computed direct health care costs based on all medical and pharmacy claims, excluding DME (eg, rtCGM and SMBG), for each patient. We then categorized medical claims based on place of service (appendix). Pharmacy claims that were not diabetes-related were combined into an “other prescription” category. The Optum database treats claim dollar amounts as if they were paid by a single payer with a consistent (ie, standard) fee schedule, so that any differences in cost could be attributed to differences in health care utilization.
We defined health care utilization as the number of hospitalizations and the length of stay (LOS) for each hospitalization.
Demographics
Demographic data collected at the index date included age, gender, and geographic location.
Health Status/Comorbidities
We determined a composite measure of overall disease severity based on the Charlson Comorbidity Index (CCI)27 and the presence of select conditions based on ICD-928 and ICD-10 codes,28,29 as well as the presence of macrovascular or microvascular conditions (eg, coronary artery disease and retinopathy, respectively). These data were collected during both the preindex and postindex periods.
A1C
We collected the last A1C measure during both the preindex and postindex periods. A1C values were available for a subset of patients.
Multivariate Analyses
To control for confounding factors in our cost analyses, we used a propensity score method to select equal-sized groups of patients using rtCGM and patients not using rtCGM (ie, 1:1 matching). Prior to matching, we excluded patients with ≥99th percentile total costs (including DME) during the pre- or postindex period. Then, propensity scores were estimated based on logistic regression with preindex predictors of age, gender, macrovascular and microvascular complications, geographic region, presence of inpatient costs, index year, CCI score, use of multiple daily injections (MDI) or oral medications, and total costs. rtCGM users and nonusers were matched if their individual propensity score fell within a set range (caliper). The caliper was set as 0.20 of pooled standard deviation of logit of the propensity score.
This matching procedure was also applied for our A1C analysis, but only the last A1C in the preindex period was used in the propensity score regression. In addition, only patients with at least one A1C measurement in both the preindex and postindex period were included prior to matching.
After matching, a paired or matched t-test was used to test whether postindex costs differed between the rtCGM group and non-rtCGM group.
Results
Selection of the Study Sample
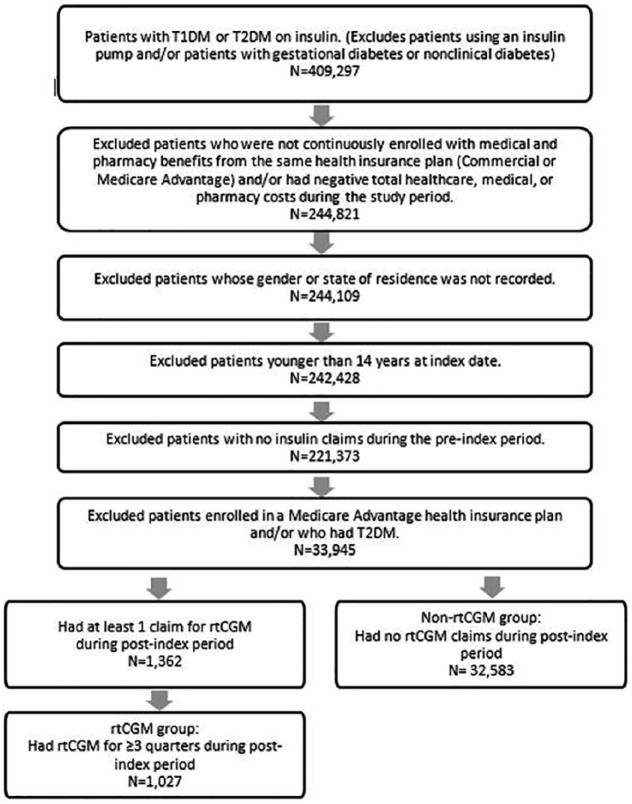
Of the 409 297 patients having at least 1 pharmacy claim for insulin during calendar years 2009 to 2016, 375 687 were excluded from the study based on the factors described in Figure 2, leaving a total of 33 610 patients in the study sample. Of these, 1027 patients used rtCGM and 32 583 patients did not use rtCGM.
Figure 2.
Selection of the study sample.
Preindex Period Characteristics
Table 1 shows the characteristics of the study sample during the preindex period, after propensity score matching for health care cost and utilization. Since 4 patients were excluded as outliers, based on ≥99th percentile total costs during the pre- or postindex period, there were 1023 patients in each group. After matching, no significant differences between the rtCGM and non-rtCGM groups remained on preindex characteristics used in the matching, indicating that the matching was successful.
Table 1.
Preindex Period Characteristics Following Propensity Score Matching for Health Care Costs and Utilization.
| Variable | rtCGM | Non-rtCGM | P value |
|---|---|---|---|
| Na | 1023 | 1023 | |
| Female, n (%) | 402 (39.3) | 410 (40.1) | .7177 |
| Age, mean | 40.7 | 40.7 | 1.0000 |
| Region | .7483 | ||
| Northeast, n (%) | 214 (20.9) | 221 (21.6) | |
| West, n (%) | 133 (13.0) | 126 (12.3) | |
| Midwest, n (%) | 435 (42.5) | 418 (40.9) | |
| South, n (%) | 241 (23.6) | 258 (25.2) | |
| CCI, mean | 1.59 | 1.56 | .5751 |
| Long-term complications | .9517 | ||
| None, n (%) | 523 (51.1) | 520 (50.8) | |
| Microvascular only, n (%) | 27 (2.6) | 31 (3.0) | |
| Macrovascular only, n (%) | 187 (18.3) | 183 (17.9) | |
| Microvascular and macrovascular, n (%) | 286 (28.0) | 289 (28.3) | |
| Index year, n (%) | .4549 | ||
| 2008 | 12 (1.2) | 13 (1.3) | |
| 2009 | 15 (1.5) | 16 (1.6) | |
| 2010 | 29 (2.8) | 27 (2.6) | |
| 2011 | 28 (2.8) | 37 (3.6) | |
| 2012 | 76 (7.4) | 64 (6.3) | |
| 2013 | 93 (9.1) | 89 (8.7) | |
| 2014 | 99 (9.7) | 81 (7.9) | |
| 2015 | 655 (64.0) | 688 (67.3) | |
| 2016 | 16 (1.6) | 8 (0.8) | |
| Had hospitalization, n (%) | 49 (4.8) | 46 (4.5) | .7526 |
| Total costs, mean | $19 983 | $18 123 | .1248 |
T-test was used for continuous variables; chi-square test was used for categorical variables.
4 patients were excluded as outliers, based on ≥99th percentile total costs during the pre- or postindex period.
Table 2 shows the characteristics of the study sample during the preindex period, after propensity score matching for A1C. There were 283 patients in each group and no significant differences between the rtCGM and non-rtCGM groups, indicating that the matching was successful.
Table 2.
Preindex Period Characteristics Following Propensity Score Matching for A1C.
| Variable | rtCGM | Non-rtCGM | P value |
|---|---|---|---|
| N | 283 | 283 | |
| Last A1c, mean | 7.62 | 7.62 | 1.00 |
Note: only a subset of patients had A1C value available in both the preindex and postindex periods.
T-test was used for continuous variables.
Health Care Costs and Utilization
During the postindex period, patients using rtCGM had total health care costs that were more than $4000 lower on average when compared to patients not using rtCGM (P < .05). The majority of this cost difference was in medical costs, compared to pharmacy costs. Within medical costs, about $2200 lower costs were seen in the “outpatient other facility” category, which includes outpatient hospital, outpatient psychiatry, outpatient rehabilitation, and surgical center costs. In addition, almost $2000 lower costs, on average, were seen in the “inpatient hospital” category. These lower inpatient costs are in line with fewer hospital admissions (P < .05) and shorter lengths of stay during the postindex period (P = .08). (Table 3)
Table 3.
Mean Health Care Costs and by Study Group.
| rtCGM (N = 1023) | Non-rtCGM (N = 1023) | Differencea | P value | |
|---|---|---|---|---|
| Total Cost (w/o DME) | $16 194 | $20 452 | −$4257 | .0010 |
| Medical (w/o DME) | $7749 | $11 583 | −$3834 | .0001 |
| Inpatient | $1116 | $3104 | −$1987 | .0002 |
| Inpatient other facility | $256 | $201 | $56 | .6446 |
| Outpatient office & clinic | $2055 | $1787 | $268 | .0282 |
| Outpatient other facility | $2273 | $4560 | −$2287 | .0002 |
| Emergency room | $869 | $1282 | −$413 | .0180 |
| Postacute care and other location | $1179 | $649 | $530 | .0002 |
| Pharmacy (w/o DME) | $8445 | $8869 | −$424 | .4444 |
| Insulin | $4637 | $4566 | $71 | .5742 |
| OAD | $0 | $1 | −$1 | .3175 |
| Other Rx (nondiabetic) | $3807 | $4301 | −$494 | .3599 |
| Utilization | ||||
| Average hospital admission | 0.06 | 0.13 | −0.07 | .0001 |
| Average LOS per admission | 3.79 | 5.46 | −1.67 | .0788 |
T-test was used for continuous variables. Cost breakdown based on place of service. Details in the appendix.
Rounded to the nearest dollar.
A1C
During the postindex period, patients using rtCGM had significantly lower A1C when compared to patients not using rtCGM (P < .05) (Figure 3).
Figure 3.
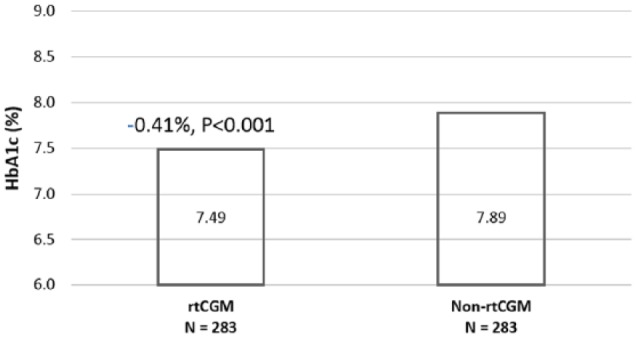
Postindex A1C: rtCGM versus non-rtCGM.
Discussion
A 2016 consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology advocates expanding rtCGM use for patients with diabetes, because it has been found to improve glucose control and reduce the occurrence of hypoglycemic events. The authors note, however, that real-world analyses are needed to confirm potential cost savings.14 Indeed, a number of studies have demonstrated the clinical benefits of rtCGM, but raise questions about its cost-effectiveness.11-13 Our research has addressed these questions by using health plan administrative data to investigate the health care costs, hospital admissions, and glycemic control associated with rtCGM use by commercially insured patients with T1DM.
We found that average annual total health care costs (excluding DME) were $4257 lower for patients using rtCGM for a majority of the time when compared to a similar group of patients who were not using rtCGM. A substantial effect was seen in inpatient costs, with lower costs of almost $2000 seen among patients using rtCGM. Although not analyzed, this may be due to a lower number of diabetes-related admissions, as seen in other analyses.26 The greatest difference (an average of $2287 per year) was seen with respect to expenses related to outpatient treatment that took place outside of the typical office/clinic setting (ie, outpatient psychiatry, outpatient rehabilitation, and surgical centers). Better glucose control may also affect the level of complications seen in other settings, such as outpatient hospitals, resulting in lower costs for these procedures. Patients using rtCGM also experienced fewer hospitalizations than did patients not using rtCGM.
These results confirm the findings of a somewhat similar study of patients with T1DM by Parkin et al, which also focused on the effect of rtCGM using data extracted from the Optum database. More specifically, when compared to SMBG, rtCGM was associated with lower inpatient and ER costs (–17.4% and
–15.0%, respectively), as well as fewer inpatient and ER admissions (−42.2% and −17.0%, respectively), however only the results for inpatient admissions reached statistical significance (P = .013).26 Several important methodological differences between the study by Parkin et al and ours include the fact that (1) our study also examined total health care costs, as well as many other cost subcategories, whereas the former study focused only on inpatient and ER costs; (2) our study population excluded patients using an insulin pump, whereas the former study did not; (3) our study defined the index date as 1 year prior to the last insulin claim and defined rtCGM use as having rtCGM orders in at least 3 quarters of a year, whereas the former study defined the index date as the first claim for the initiation of rtCGM and assumed that patients continued using rtCGM during the follow-up period; and (4) our sample size was almost twice as large.
In addition to the positive effect of rtCGM on health care costs and utilization, our study also found that patients with T1DM who used rtCGM for at least 1 year had lower A1C than did similar patients who did not use rtCGM (P < .05). This supports the findings of a number of other studies that have reported a positive association between rtCGM use and lower A1C.11-13,26
Our study had several limitations that must be considered when interpreting the results. First, because the Optum database does not include actual allowed amounts for claims, but instead treats claims as if they were paid by a single payer with a consistent fee schedule, we were unable to determine the actual effect of rtCGM on spending. This, however, does not affect the relative comparison between the rtCGM and non-rtCGM groups, since the standardized pricing affects both groups similarly. Second, the Optum database includes only direct costs, so we were unable to evaluate the impact of rtCGM on indirect costs, such as days missed from work. Third, the sample size for the subgroup of patients for whom A1C data were available was relatively small. This subgroup may have also introduced bias, if characteristics of patients for whom A1C data were available differed from characteristics of patients for whom A1C data were not available, and this, in turn, impacted the outcomes. The fact that the Optum database does not contain information regarding socioeconomic status or mental health benefits is another source of potential bias that we were unable to consider as part of our propensity score matching. Finally, some of the patients in the rtCGM group may have been using rtCGM during both the pre- and postindex periods, however we were able to control for this to some extent by including total health care costs during matching.
Conclusion
Despite its limitations, this study had yielded real-world evidence that rtCGM use by patients with T1DM is associated with improved glucose control, as well as with decreased health care costs and utilization. An international expert panel has recommended that rtCGM, in conjunction with A1C, be considered “for glycemic status assessment and therapy adjustment in all patients with type 1 diabetes and patients with type 2 diabetes treated with intensive insulin therapy who are not achieving glucose targets,” and both the American Association of Clinical Endocrinologists and the American College of Endocrinology note that “expanded CGM coverage by government and private payers is an urgent need.”14,30 Our results should contribute to the evidence required to make such coverage a reality.
Acknowledgments
The authors thank Keren Price, MS, RD, for her assistance with writing this article, and Francine Kaufman, MD, for her assistance with manuscript review.
Appendix
Appendix.
Health Care Locations
| General category | IHD-defined location |
|---|---|
| Inpatient | Inpatient hospital |
| Inpatient, other facility | Inpatient psychiatry Inpatient rehabilitation Inpatient other |
| Outpatient office and clinic | Office Clinic |
| Outpatient, other facility | Outpatient hospital Outpatient psychiatry Outpatient rehabilitation Surgical center Other outpatient |
| Emergency room | Emergency department |
| Postacute care and other location | Home health care Hospice facility Long-term care Other location Pharmacy Skilled nursing facility |
Footnotes
Abbreviations: A1C, glycated hemoglobin; CCI, Charlson Comorbidity Index; CORE, Center for Outcomes Research; DME, durable medical equipment; ER, emergency room; IHD, Instant Health Data; LOS, length of stay; MDI, multiple daily injections; OAD, oral antidiabetic; QALY, quality-adjusted life-year; rtCGM, real-time continuous glucose monitoring; SMBG, self-monitoring of blood glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This research was supported by Medtronic–Diabetes, and all of the authors are employees of Medtronic.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services;2017. [Google Scholar]
- 2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boutati EI, Raptis SA. Self-monitoring of blood glucose as part of the integral care of type 2 diabetes. Diabetes Care. 2009;32(suppl 2):s205-s210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hansen MV, Pedersen-Bjergaard U, Heller SR, et al. Frequency and motives of blood glucose self-monitoring in type 1 diabetes. Diabetes Res Clin Pract. 2009;85:183-188. [DOI] [PubMed] [Google Scholar]
- 5. Vincze G, Barner JC, Lopez D. Factors associated with adherence to self-monitoring of blood glucose among persons with diabetes. Diabetes Educ. 2004;30:112-125. [DOI] [PubMed] [Google Scholar]
- 6. Fisher WA, Cornman DH, Kohut T, et al. What primary care providers can do to address barriers to self-monitoring of blood glucose. Clin Diabetes. 2013;31:34-42. [Google Scholar]
- 7. Kaufman FR, Gibson LC, Halvorson M, et al. A pilot study of the continuous glucose monitoring system: clinical decisions and glycemic control after its use in pediatric type 1 diabetic subjects. Diabetes Care. 2001;24:2030-2034. [DOI] [PubMed] [Google Scholar]
- 8. Boland E, Monsod T, Delucia M, et al. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care. 2001;24:1858-1862. [DOI] [PubMed] [Google Scholar]
- 9. McGowan K, Thomas W, Moran A. Spurious reporting of nocturnal hypoglycemia by CGMS in patients with tightly controlled type 1 diabetes. Diabetes Care. 2002;25:1499-1503. [DOI] [PubMed] [Google Scholar]
- 10. Slattery D, Choudhary P. Clinical use of continuous glucose monitoring in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:S55-S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157:336-347. [DOI] [PubMed] [Google Scholar]
- 12. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371-378. [DOI] [PubMed] [Google Scholar]
- 13. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317:379-387. [DOI] [PubMed] [Google Scholar]
- 14. Fonseca VA, Grunberger G, Anhalt H, et al. Continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22:1008-1021. [DOI] [PubMed] [Google Scholar]
- 15. Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:3922-3937. [DOI] [PubMed] [Google Scholar]
- 16. Battelino T, Liabat S, Veeze HJ, et al. Routine use of continuous glucose monitoring in 10 501 people with diabetes mellitus. Diabet Med. 2015;32:1568-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandle M, Herman WH. The CORE diabetes model. Curr Med Res Opin. 2004;20(suppl 1):s1-s3. [DOI] [PubMed] [Google Scholar]
- 18. Huang ES, O’Grady M, Basu A, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2010;33:1269-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McQueen RB, Ellis SL, Campbell JD, et al. Cost-effectiveness of continuous glucose monitoring and intensive insulin therapy for type 1 diabetes. Cost Eff Resour Alloc. 2011;9:13-7547-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaugule S, Graham C. Cost-effectiveness of G5 Mobile continuous glucose monitoring device compared to self-monitoring of blood glucose alone for people with type 1 diabetes from the Canadian societal perspective. J Med Econ. 2017;20:1128-1135. [DOI] [PubMed] [Google Scholar]
- 21. Fonda SJ, Graham C, Munakata J, et al. The cost-effectiveness of real-time continuous glucose monitoring (RT-CGM) in type 2 diabetes. J Diabetes Sci Technol. 2016;10:898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roze S, Saunders R, Brandt AS, et al. Health-economic analysis of real-time continuous glucose monitoring in people with Type 1 diabetes. Diabet Med. 2015;32:618-626. [DOI] [PubMed] [Google Scholar]
- 23. Bronstone A, Graham C. The potential cost implications of averting severe hypoglycemic events requiring hospitalization in high-risk adults with type 1 diabetes using real-time continuous glucose monitoring. J Diabetes Sci Technol. 2016;10:905-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hommel E, Olsen B, Battelino T, et al. Impact of continuous glucose monitoring on quality of life, treatment satisfaction, and use of medical care resources: analyses from the SWITCH study. Acta Diabetol. 2014;51:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charleer S, Mathieu C, Nobels F, et al. Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a real-world study. J Clin Endocrinol Metab. 2018;103:1224-1232. [DOI] [PubMed] [Google Scholar]
- 26. Parkin CG, Graham C, Smolskis J. Continuous glucose monitoring use in type 1 diabetes: longitudinal analysis demonstrates meaningful improvements in HbA1c and reductions in health care utilization. J Diabetes Sci Technol. 2017;11:522-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [DOI] [PubMed] [Google Scholar]
- 28. Menzin J, Korn JR, Cohen J, et al. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. J Manag Care Pharm. 2010;16:264-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anonymous 2016 ICD-10-CM and GEMs. Available at: https://www.cms.gov/Medicare/Coding/ICD10/2016-ICD-10-CM-and-GEMs.html Accessed May 1, 2018.
- 30. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]