Highlights
-
•
Phycocyanin (8.66%) was the main product from the process of the stepwise extraction.
-
•
In addition, 3.55% TFA and 0.72% polysaccharide were coproducts from the Arthrospira residue.
-
•
Arthrospira should contain 15% phycocyanin to ensure a positive NPV of investment.
Keywords: Arthrospira (Spirulina), Phycocyanin, Lipid/TFA, Polysaccharide, Economic feasibility
Abstract
Arthrospira (Spirulina) consists of diverse high-value chemicals, such as phycocyanin, lipids/total fatty acids (TFA), and polysaccharides, which have been used for food, cosmetic and pharmacological applications. This study compared various stepwise extraction processes for these high-value chemicals. Considering the yield and properties of extracts, the most suitable extraction order was phycocyanin, lipid/TFA and polysaccharides. The yield of the main product (food-grade phycocyanin) was 8.66% of the biomass dry weight, whereas the yields of the subsequent lipid/TFA and polysaccharide coproducts were 3.55% and 0.72%, respectively. The economic analysis showed that producing phycocyanin alone was economically feasible, but producing coproducts (lipid/TFA and polysaccharides) was not. The production cost of phycocyanin was US$ 249.70 kg−1, which is an encouraging figure for large-scale production. Moreover, the phycocyanin content of Arthrospira materials utilized for extraction should not be lower than 15% of dry weight to ensure positive the net present value (NPV) of investment.
1. Introduction
Arthrospira (Spirulina) consists of numerous valuable compounds, such as phycocyanin (20–28% of the dry weight), lipids (6–13%) and carbohydrates (15–20%) [1]. The majority of carbohydrates are polysaccharides [2]. These extracts have been used in cosmetic, health food, nutraceutical and pharmacological applications. Currently, the total market value of phycobiliprotein products, including fluorescent agents, is estimated to be over US$ 60 million [3]. The commercial value of phycocyanin is directly related to its degree of purity (food-grade or analytical grade). The market for gamma-linolenic acid (GLA) oil is estimated to exceed US$ 100 million. The GLA product is commonly obtained from evening primrose (8–14% GLA), black currant (15–20% GLA), and borage (20–25% GLA). The price is dependent on the percentage of GLA contained in the crude product. The price of evening primrose oil is around US$ 10–15 kg−1, while borage oil is US$ 30–35 kg−1 [4,5]. With a comparable GLA content of approximately 18–21%, Arthrospira is a promising alternative source of GLA which represents an opportunity for economic profit. In contrast, the value of polysaccharides varies according to their use and purity [6].
Because the downstream processes (extraction, separation and purification) are of great importance for obtaining natural products, many research groups have sought to develop efficient processing methods that maximize yield and quality of Arthrospira products while lowering production costs. A search of literature and patents revealed that most procedures focus on the extraction of single products (phycocyanin, lipid or polysaccharide), a trend that may not be feasible on an industrial scale. Reis et al. [7] studied the production, extraction and purification of phycobiliproteins from Nostoc sp. and proposed the extraction and utilization of byproducts, such as carotenoids in order to offset the high cost of producing phycoerythrin. In previous studies, we investigated the optimal conditions for extraction and separation of lipid/fatty acids, phycocyanin, and polysaccharides from Arthrospira as single products [[8], [9], [10], [11]]. In this study, we aimed to develop a stepwise extraction technique that maximizes the yields and qualities of phycocyanin, lipids, and polysaccharides produced. In addition, the economic feasibility of producing phycocyanin (scenario 1), phycocyanin and lipids (scenario 2), and phycocyanin, lipids, and polysaccharides (scenario 3) were analyzed.
2. Materials and methods
2.1. Materials and extraction process
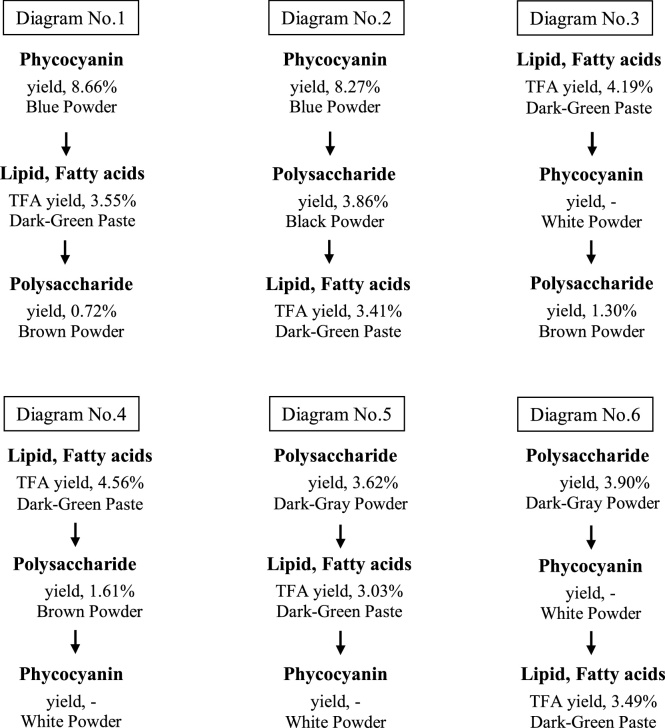
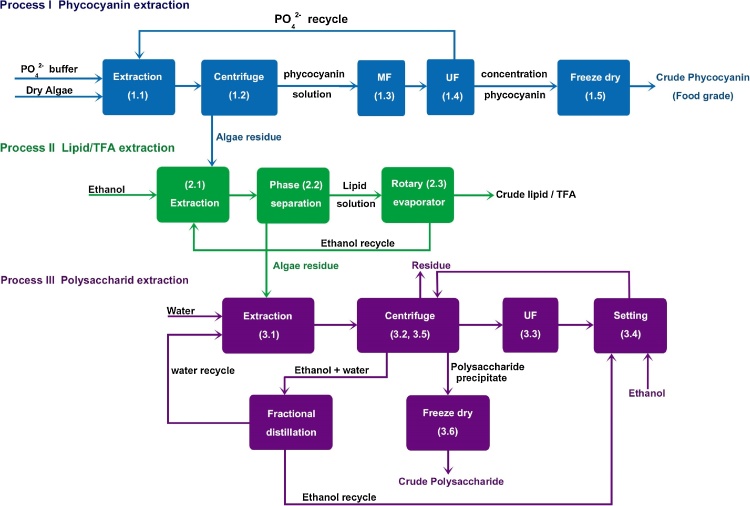
Dried Arthrospira biomass was purchased from the Nathong Spirulina Group (Chachoengsao, Thailand). The phycocyanin, lipid and polysaccharides were extracted from the dried Arthrospira following different extraction sequences (Fig. 1) to determine the best order in terms of product yield and quality. The conditions of extraction and separation for each compound are described below.
Fig. 1.
Schematic diagram of the stepwise extraction.
2.1.1. Phycocyanin extraction
Fifteen grams of biomass or residue, depending on the order of extraction, was added to 1.5 L of 100 mM phosphate buffer (pH 7.0) in an agitation tank to obtain a mixture ratio of 1:100 (w/v). The mixture was stirred at room temperature for 3–4 h and then was centrifuged to remove the cell residue that was used to extract other compounds. The crude extract was filtered using microfiltration membranes (MF) with pore sizes of 1 and 0.2 μm and an ultrafiltration membrane (UF) with a molecular weight cut-off (MWCO) of 50 kDa. The filtration membranes were purchased from the Pall Corporation. Food-grade phycocyanin powder was obtained after lyophilization.
2.1.2. Lipid and fatty acids extraction
Dried biomass or residue from the preceding extraction was used, and lipid was extracted thrice with ethanol at a ratio of 1:5 (w/v) for 20 min at 60 °C. The sample was centrifuged to separate the lipid solution from the residue. The solvent in the lipid solution was removed with a vacuum rotary evaporator to obtain crude lipid, which contains fatty acids. The defatted/depigmented residue was dried at room temperature and was used for the extraction of polysaccharides accordingly.
2.1.3. Polysaccharide extraction
The polysaccharide was extracted with a biomass or residue-to-water ratio of 1:45 (w/v) for 120 min at 90 °C. The sample was centrifuged to collect the polysaccharide solution. The crude polysaccharide was concentrated to approximately one-fifth of its original volume using a membrane with an MWCO of 30 kDa. Two volumes (v/v) of ethanol were added, and the solution was incubated overnight at 4 °C. The polysaccharide precipitate was then collected by centrifugation and was lyophilized for long-term storage.
2.2. Phycocyanin purification
To obtain high-purity phycocyanin, the solution of phycocyanin extract obtained after partial purification by MF and UF was subjected to further purification using column chromatography. Three types of matrixes (activated charcoal, Sephadex G100 and DEAE Sepharose Fast Flow) were investigated as described below:
The phycocyanin sample was subjected to chromatography on an activated charcoal column (2.5 × 20 cm) of commercial grade at a flow rate of 2 mL/min.
For gel filtration chromatography, the phycocyanin sample (8 mL) was loaded on a Sephadex G100 (40–120 μm) column that was pre-equilibrated and eluted with a 0.005 M sodium-phosphate buffer (pH 7.0).
For ion-exchange chromatography, the phycocyanin sample (8 mL) was loaded onto a DEAE Sepharose Fast Flow (45–165 μm) column that was developed with a linearly increasing ionic concentration gradient of 0–0.25 M sodium chloride as proposed by Patel et al. [12].
Both Sephadex G100 and DEAE Sepharose columns were carried out using a bed height of 20 cm and a column dimension of 2.5 cm. The phycocyanin extract was eluted at a flow rate of 2 mL/min, and fractions of 2 mL were collected. The purity ratio of the resulting phycocyanin extract was determined spectrophotometrically by the A620/A280 ratio, where A620 is the absorbance of phycocyanin at a wavelength of 620 nm, and A280 is the absorbance of the protein at a wavelength of 280 nm.
2.3. Analyses
Proteins were assayed using the method described by Lowry et al. [13], with slight modification. Samples were hydrolyzed with 1 N sodium hydroxide and were heated in a boiling water bath for 20 min. Afterwards, 2.5 mL mixed reagent (50 mL of 5% Na2CO3, 1 mL of 1% of CuSO4.5H2O and 1 mL of 2% NaKC4H6O6. 4H2O) was added to the sample. The mixture was incubated at room temperature for 10 min, and 0.5 mL of Folin-Ciocalteu reagent was added. The absorbance was measured at 750 nm, and the protein content was calculated based on the standard curve of bovine serum albumin (BSA).
A modified method of Dubois et al. [14] was used to determine the total sugar content in regards to polysaccharide. Each sample was analyzed by the phenol-sulfuric acid reaction using distilled water and glucose as the blank and standard solutions, respectively. The absorbance was determined at 490 nm.
The phycocyanin content was determined according to Boussiba & Richmond [15]
The biomass was extracted in phosphate buffer (pH 7). The optical density of the extract was measured at a wavelength of 620 nm.
The total fatty acid (TFA) in the samples was determined following the transesterification protocol proposed by Lapage & Roy [16], with modification. Lipid extract (≥10 mg) was direct transmethylated in 5% HCl in methanol at 85 °C for 1 h using heptadecanoic acid (C17:0; Sigma Co.) as the internal standard. Fatty acid methyl esters were analyzed by gas chromatography (GC 17-A; Shimadzu, Japan) using capillary columns of fused silica glass (60 m × 0.25 mm, BPX70, SGE, USA) with a film thickness of 0.25 μm. The split ratio was 1:50, and the column temperature was initially set at 170 °C and was increased to 205 °C at a rate of 1 °C per minute. The injector and flame ionization detector temperature were 250 °C and 260 °C, respectively. Identification was carried out by cochromatography with authentic standards (Sigma Co.).
The results are expressed as the means ± standard deviation (SD) of triplicate data sets from independent experiments. Statistical analysis was performed through an analysis of variance (ANOVA) with a confidence level of 95% (P < 0.05).
2.4. Calculation
Normally, the yield of the extract is calculated and expressed as a percentage based on the weight of extracts per weight of sample. Because all of the extracts in this work were crude extracts, the equations below were used for the calculation of product yields:
| (1) |
| (2) |
| (3) |
where PC = phycocyanin content, TFA = Total fatty acids.
2.5. Economic evaluation
The life cycle cost assessment (LCCA) methodology was used to evaluate the economic feasibility of the high-value compounds from Arthrospira (food-grade phycocyanin, lipid/TFA and polysaccharides). The analysis was evaluated with the following assumptions: (1) The processing plant will be situated near the Arthrospira production facility at Mae Wang district, Chiang Mai, Thailand. The plant will have an operating life of 10 years, all processes will be based on batch operations, and the plant will run for 16 h/day, 300 days/year. (2) Arthrospira material contains phycocyanin content with approximately 15% of dry weight, and the extraction allows for 67% phycocyanin recovery. (3) There is an annual production capacity of 600 kg food-grade phycocyanin, based on the information from personal communication with the Arthrospira entrepreneur in Thailand. Generally, 75–80% of Arthrospira biomass are used for health food, 10–15% for feed, and 8–15% for extraction [17]. To produce 600 kg phycocyanin, 6000 kg algae (approximately 8% of total biomass in Thailand) and 600,000 L phosphate buffer will be required. Thus, a phycocyanin extraction volume of 2000 L will be required for the daily operations (1000 L/batch; ×2 batches).
Due to the recovery of Arthrospira residue was a factor that had a significant influence on the production capacity of coproducts. In this study, approximately 70% of the original Arthrospira biomass was recovered (30% was lost) after the extraction of phycocyanin, and approximately 59% of the original biomass remained after the lipid/TFA extraction process. Therefore, the production capacity of 300 and 35 kg/year were used for lipid/TFA and polysaccharides, respectively. The design parameters and values used for the economic analysis, such as production scale, size, and number of units, are summarized in Table 1A.
Table 1A.
Values and design parameters used for economic analysis.
| Items | Cost /units | Required quantity for |
||
|---|---|---|---|---|
| (US$ 1 = THB 32.30) | phycocyanin | 2 productsa | 3 productsb | |
| Equipment: | ||||
| Mixing tanks (500 L) | 4489.16 US$/set | 3 set | 3 set | 3 set |
| Mixing tanks with temp. control | 5417.96 US$/set | – | 1 set | 2 set |
| Continuous centrifuge (5000 L/h) | 160,990.71 US$/set | 1 set | 1 set | 1 set |
| Storage tank (500 L) | 3560.37 US$/unit | 2 unit | 2 unit | 2 unit |
| Cooling system (5000 L/h) | 6191.95 US$/set | 1 set | 1 set | 1 set |
| Freeze Drier (40 kg/day) | 157,894.74 US$/set | 1 set | 1 set | 1 set |
| Rotary evaporator | 139,318.89 US$/set | – | 1 set | 1 set |
| Filtration system: (4000 L/h) | ||||
| MF (1&0.2 μm) and UF 50 kDa | 236,842.11 US$/set | 1 set | 1 set | 1 set |
| UF 30 kDa | 8,359.13 US$/set | – | – | 1 set |
| Plant Assumption: | ||||
| Building (1600 m2) | 13,931.89 US$/unit | 1 unit | 1 unit | 1 unit |
| Plant life | 10 years | |||
| Working day | 300 days/year | |||
| Working hour | 16 hours/day | |||
| Productions capacity: | ||||
| Phycocyanin production (kg/year) | 600 | 600 | 600 | |
| Extraction vol. of phycocyanin (L/year) | 600,000 | |||
| (2000 L/day; 1000 L/batch × 2 batches/day) | ||||
| Arthrospira biomass (kg/year) | 10.84 US$/kg | 6000 | 6000 | 6000 |
| Lipid production (kg/year) | – | 300 | 300 | |
| Polysaccharide production (kg/year) | – | – | 35 | |
| Material assessment: | ||||
| K2HPO4 (ton/year) | 5.26 US$/kg | 6.38 | 6.38 | 6.38 |
| KH2PO4 (ton/year) | 3.87 US$/kg | 3.17 | 3.17 | 3.17 |
| Water (m3/year) | 0.40 US$/m3 | 600 | 600 | 760 |
| Ethanol (m3/year) | 1.15 US$/L | – | 21 | 25.5 |
| Electricity (kW.h/year) | 0.09 US$/kW.h | 26,400 | 30,360 | 34,320 |
| Labor (persons/year) | 12.08 US$/day | 4 | 8 | 8 |
| Utility & Maintenance at 2% of capital cost | ||||
2 products = phycocyanin and lipid.
3 products = phycocyanin, lipid and polysaccharide.
The evaluation was based on the investment and operating costs depicted in the process flow (Fig. 2). The investment costs for each piece of equipment were based on quotations from company contracts (exchange rate THB 32.30 per US$ 1). The depreciation expense on buildings and equipment was calculated using the straight line method and no salvage. The analysis was designed on the basis of a Thailand scenario, as follows: tax rate of 30%, capital investment loan of 10 years, annual loan interest of 7.5%, utility and maintenance cost at 2% of capital cost, and a discount rate of 15%. The labor cost was estimated from the operating labor rate and operating labor hour per shift. The calculation of electricity was based on the energy use and working hours. The operating and management costs (O&M = raw materials, electricity, labor, water, utilities and maintenance) and land rental were assumed an increase of 7% annually, and in the selling price of the products were assumed an annual 3.5% increase. The cost of Arthrospira biomass used as raw material was US$ 10.84 kg−1. However, because the selling price of phycocyanin, lipid/TFA and polysaccharides were not precisely known, estimates of US$ 500 kg−1 for phycocyanin [3] and US$ 30 kg−1 for lipid/TFA were based on the price of GLA oil from borage (US$ 30–35 kg−1). In addition, US$ 250 kg−1 was used for polysaccharide based on price range of US$ 2–990 kg−1 reported online (alibaba.com). The financial assumptions are summarized in Table 1B.
Fig. 2.
Process flow for producing high-value compounds from Arthrospira.
Table 1B.
Financial assumptions.
| Only phycocyanin | 2 products | 3 products | |
|---|---|---|---|
| Revenue: | |||
| Sale revenue (US$/year) | 300,000.00 | 309,000.00 | 317,750.00 |
| Expenses: | |||
| Land rental (US$/year) | 1547.99 | 1547.99 | 1547.99 |
| O&M cost (US$/year) | 140,018.02 | 181,927.52 | 211,946.18 |
| Total investments (US$) | 596,439.63 | 741,176.47 | 754,953.56 |
| (loan 50%) | (298,219.81) | (370,588.24) | (377,476.78) |
| Debit financing (US$/year) | 29,821.98 | 37,058.82 | 37,747.68 |
| Depreciation (US$/year) | 59,643.96 | 74,117.65 | 75,495.36 |
| Replacement cost of membrane (US$/time) | 29,411.76 | 29,411.76 | 29,411.76 |
Economic feasibility study: the net present value (NPV), internal rate of return (IRR) or return on equity (ROE) and payback period are typically key factors for making investment decisions. NPV is the difference between the present value of all cash incomes and the present value of all cash outflows, which can be calculated using the formula:
| (4) |
where Ct = net cash flow during the period t, C0 = total initial investment cost, t = number of time periods (years) and r = discount rate.
The IRR is a discount rate that makes the NPV of all cash flows from a project or investment equal to zero. The calculation of IRR relies on the same formula as NPV:
| (5) |
If the IRR is greater than the minimum acceptable rate of return, the investment may be considered desirable. If it is below the required rate of return, the investment should be rejected.
The discounted payback period is a capital budgeting procedure used to determine the profitability of a project. A discounted payback period gives the number of years it takes to break even from undertaking the initial expenditure, by discounting future cash flows and recognizing the time value of money. It is calculated using the formula:
| Discounted payback period = A+ (B/C) | (6) |
where A = the last period with a negative discounted cumulative cash flow, B = absolute value of discounted cumulative cash flow at the end of the period A, and C = discounted cash flow during the period after C.
3. Results and discussion
3.1. Suitable order of stepwise extraction
The average protein, phycocyanin, and polysaccharide (based on total sugar) contents in the Arthrospira raw material utilized in this work were 49.48%, 12.91%, and 16.93% of dry weight, respectively. Arthrospira also contained 10.48% lipid, of which total fatty acids (TFA) comprise half of the lipid (5.09% of dry weight; Table 2). The remaining 10% of dry weight was ash and moisture. Dry Arthrospira powder normally contains moisture of approximately 3–7% of dry weight [18,19].
Table 2.
Chemical composition of the Arthrospira raw material.
| Composition | Protein | Phycocyanin | TFA | Total sugar | Ash | Moisture |
|---|---|---|---|---|---|---|
| Content (% dw) | 49.48 ± 2.05 | 12.91 ± 1.25 | 5.09 ± 0.24 | 16.93 ± 1.98 | 5.89 ± 0.76 | 4.78 ± 0.86 |
dw= dry weight.
Overall, high phycocyanin yields of 8.66% and 8.27% on a dry weight basis were obtained when it was extracted as the first product (Fig. 1 No. 1 and No. 2, respectively). Phycocyanin is a biliprotein; thus, it could be degraded by the extraction solvent and the temperature. The critical temperature for the stability of phycocyanin is 47 °C [20], whereas in this study, lipid/TFA and polysaccharide extractions were carried out at 60 °C and 90 °C, respectively. When phycocyanin was extracted at the second or third product, its blue color degraded and turned white, and a phycocyanin absorbance value of less than 0.1 was observed (Fig. 1 No. 3, No. 4, No. 5 and No. 6). These results, therefore, highlight the importance of extracting phycocyanin as the first product.
The purity ratio of the phycocyanin obtained from diagram No. 1 and No. 2 was 0.9. Soluble proteins and/or some low-molecular-weight polysaccharides might have dissolved in the phosphate buffer, thereby resulting in the rather low purity ratio. The analysis of crude phycocyanin showed protein and total sugar contents of 45.79–47.52% and 11.58–14.90% (% wt. phycocyanin), respectively (Table 3A).
Table 3A.
Composition characterization of phycocyanin extracts.
| Diagram no. | Purity | Protein | Total sugar |
|---|---|---|---|
| (% wt. phycocyanin) | |||
| No.1 | 0.968 | 45.79 ± 0.95 | 11.58 ± 0.28 |
| No.2 | 0.921 | 47.52 ± 2.45 | 14.90 ± 1.64 |
For the lipid/TFA and polysaccharide extraction steps (Fig. 1), the results showed that biomass or algal residue should be defatted or depigmented (chlorophyll and carotenoids) before extracting polysaccharide. Although the yields of polysaccharide extracts from materials that were neither defatted nor depigmented (No. 2, 3.86%; No. 5, 3.62%; and No. 6, 3.90% of dry weight) were higher than yields from defatted or depigmented material (No. 1, 0.72%; No. 3, 1.30%; and No. 4, 1.61%), the total sugar contents of crude polysaccharide extracts from these materials were lower than 26.7% of the crude polysaccharide dry weight compared with the total sugar content of 36.5–45.3% obtained when polysaccharide was extracted from defatted or depigmented materials (Table 3B). Moreover, the colors of the extracts from defatted or depigmented materials were brown or light brown, while the colors of extracts from materials that were neither defatted nor depigmented were dark gray or black, which was probably caused by impurities or pigments.
Table 3B.
Total sugar in crude polysaccharide extracts.
| Content | Diagram no. | |||||
|---|---|---|---|---|---|---|
| No.1 | No.3 | No.4 | No.2 | No.5 | No.6 | |
| (after depigmented/defatted) | (no depigmented/defatted) | |||||
| Total sugar (% wt. polysaccharide) |
37.6 ± 2.1 | 36.5 ± 2.4 | 45.3 ± 1.6 | 17.7 ± 0.8 | 26.4 ± 0.7 | 26.7 ± 1.9 |
In addition, the results showed that extracting polysaccharide at the third step (No. 1 and No. 3) affected the yield and total sugar in crude polysaccharide. Some polysaccharides (based on total sugar content) were dissolved during phycocyanin extraction, resulting in a 23–25% decrease in the initial polysaccharides content (data not shown).
For lipid/TFA extraction, ethanol was used as the extraction solvent, because it is safe for human consumption, and the results of our previous study showed that the yield of lipid/TFA when extracted with ethanol was not significantly different from that obtained when using a solvent mixture (chloroform-methanol; CHCl3-MeOH) (unpublished data). When lipid/TFA was extracted at the first step, the TFA yield was 4.19–4.56% of dry weight, (82–89% recovery of initial value, 5.09%). The TFA yield decreased to 3.03–3.55% of dry weight when it was extracted at the second or third step (Fig. 1). However, there was no difference in the fatty acid composition of the crude lipid (Table 3C).
Table 3C.
Fatty acids composition of crude lipid extracts.
| Diagram | Fatty acids composition (% of total fatty acid) |
TFA | GLA (C18:3) | |||||
|---|---|---|---|---|---|---|---|---|
| C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | (% wt. lipid) | ||
| No.1 | 56.5 | 3.1 | 1.7 | 2.7 | 16.9 | 19.2 | 37.7 ± 2.8 | 7.24 ± 0.54 |
| No.2 | 56.0 | 3.1 | 1.8 | 2.8 | 16.8 | 19.5 | 41.3 ± 0.6 | 8.05 ± 1.10 |
| No.3 | 56.6 | 3.1 | 1.9 | 2.7 | 16.6 | 19.0 | 35.3 ± 2.7 | 6.75 ± 0.65 |
| No.4 | 58.3 | 2.9 | 2.0 | 3.0 | 17.2 | 16.7 | 41.4 ± 0.7 | 6.91 ± 0.34 |
| No.5 | 56.7 | 2.9 | 2.0 | 2.9 | 18.1 | 17.4 | 39.9 ± 0.9 | 6.94 ± 0.50 |
| No.6 | 57.0 | 3.3 | 1.7 | 2.6 | 16.4 | 19.0 | 38.5 ± 1.6 | 7.31 ± 0.56 |
Note: SD of fatty acids composition was less than 10%.
Based on the yield and the property of products, the suitable order of stepwise extraction was phycocyanin, lipid/TFA and polysaccharide, respectively (Fig. 1 No. 1).
Furthermore, the results also showed that the quality of raw material affects the yield and quality of extract. The yield and purity ratio of phycocyanin extracts increased as the quantity of phycocyanin in the materials increased. Phycocyanin recovery increased to 68% (yield 8.53% of dry weight), and a purity ratio of approximately 1.0 was obtained when the phycocyanin content in the biomass was higher than 10% of dry weight (Table 4), whereas only 0.7 of the purity ratio and 60% of phycocyanin recovery (4.10% of the yield) were obtained when phycocyanin was extracted from biomass group 1, which contained only 6.75% of dry weight phycocyanin. An increase in the yield of the phycocyanin extracts resulted in a decrease in the amount of Arthrospira biomass needed for the process. For production, phycocyanin 1 kg will use a lower amount of biomass from groups 2 and 3 than the amount of biomass from group 1 by approximately 52% and 72%, respectively, which will affect the production cost. Therefore, this factor will be taken into account in the sensitivity analysis.
Table 4.
Effect of the quality of Arthrospira material on phycocyanin extracts.
| Sample | Phycocyanin content in | Yield of | Purity ratio | Amount of biomass (kg) | |
|---|---|---|---|---|---|
| biomass (% dw.) |
crude extracts (% wt. phyco.) |
phycocyanin (% dw.) |
(A620/A280) | for 1 kg of phycocyanin | |
| Group 1 | 6.75 ± 1.30 | 21.28 ± 1.81 | 4.10 ± 0.49 | 0.70 ± 0.11 | 24.39 |
| Group 2 | 12.46 ± 0.99 | 35.87 ± 1.95 | 8.53 ± 0.73 | 1.06 ± 0.09 | 11.72 |
| Group 3 | 21.31 ± 2.26 | 45.00 ± 2.30 | 14.98 ± 0.87 | 1.22 ± 0.21 | 6.67 |
3.2. Purification of phycocyanin extracts
The price of phycocyanin products depends on the purity ratio, which is defined as the relationship between the absorbance of phycocyanin and protein (A620/A280). The price of food-grade phycocyanin (purity >0.7) is approximately US$ 500 kg−1, whereas the price of the analytical grade (purity ≥4.0) can be as high as US$ 15 /mg−1 [21]. Therefore, food-grade phycocyanin extract was subjected to further purification using column chromatography. The purity ratio of phycocyanin extracts increased by approximately 10% compared with the initial value (1.22) after purification using an activated charcoal column (Table 5). A similar result has been reported by Herrera et al. [22], who found that the purity ratio of phycocyanin increased from 0.72 to 0.77 after passing through the charcoal column as activated charcoal adsorption cleaned the phycocyanin extracts of low molecular weight proteins. The purification of phycocyanin through ultrafiltration and an activated charcoal column resulted in purity ratios of 1.22 and 1.34, respectively, which are consistent with the quality for food- and cosmetic-grade phycocyanin.
Table 5.
Purity ratio of phycocyanin after different purification steps.
| Purification step | Purity ratio (A620/A280) |
Phycocyanin conc. (mg/mL) | Phycocyanin recovery (%) |
|---|---|---|---|
| Crude phycocyanin extract | 0.60 ± 0.14 | 0.83 ± 0.19 | |
| Microfiltration | 0.63 ± 0.09 | 0.66 ± 0.05 | |
| Ultrafiltration | 1.22 ± 0.18 | 6.43 ± 0.53 | 100 |
| One-step chromatography column: | |||
| Activated charcoal | 1.34 ± 0.09 | 5.72 ± 0.23 | 88.9 |
| Sephadex G100 | 2.77 ± 0.38 | 3.34 ± 0.37 | 51.9 |
| DEAE Sepharose Fast Flow | 3.25 ± 0.29 | 3.51 ± 0.36 | 48.2 |
| Two-step chromatography columns: | |||
| Sephadex G100 and DEAE Sepharose | 3.74 ± 0.18 | 1.40 ± 0.02 | 21.8 |
| DEAE Sepharose and Sephadex G100 | 3.82 ± 0.16 | 1.63 ± 0.04 | 25.3 |
Purification using Sephadex G100 or DEAE Sepharose Fast Flow columns increased the purity ratio of phycocyanin by 2.3- and 2.7-folds with 51.9% and 48.2% recovery of the initial value, respectively. High-purity phycocyanin (3.74–3.82) was obtained after further purification using a combination of Sephadex G100 and DEAE Sepharose Fast Flow columns, either before or after the other, but the overall recovery in these processes decreased to approximately 21–25% of the initial value (6.43 mg/mL). A disadvantage of repeated steps or combined chromatography is the low recovery observed after these operations [23,24]. Compared with the phycocyanin recovery of 51.9% for Sephadex G100 and 48.2% for DEAE Sepharose Fast Flow (one-step chromatography), Patel et al. [12] reported 45.6% recovery of the phycocyanin extract from Spirulina sp. using DEAE-Sepharose CL-6B, and Soni et al. [25] recovered 68.57% of the phycocyanin in the extract of Oscillatoria quadripunctulata using ion exchange chromatography.
3.3. Economic feasibility study
According to diagram No. 1 (Fig. 1) and Fig. 2, phycocyanin was the main product from the process of the stepwise extraction, and crude lipid/TFA and polysaccharides were coproducts that were obtained from the Arthrospira residue. Therefore, the economic evaluation was conducted for three scenarios: 1) producing only phycocyanin 2) producing 2 products (phycocyanin and lipid/TFA) and 3) producing 3 products (phycocyanin, lipid/TFA and polysaccharide). The analysis was carried out based on an annual production capacity of 600 kg of food-grade phycocyanin. The market size of food-grade phycocyanin may be higher than 600 kg/year, but the exact demand is not truly known. This work did not consider the economic feasibility of analytical grade phycocyanin, because the results showed a low percentage of phycocyanin recovery, and its purity ratio was not high. For coproducts, the production capacities of 300 kg and 35 kg for lipid/TFA and polysaccharides, respectively, were based on the algal residue recovered.
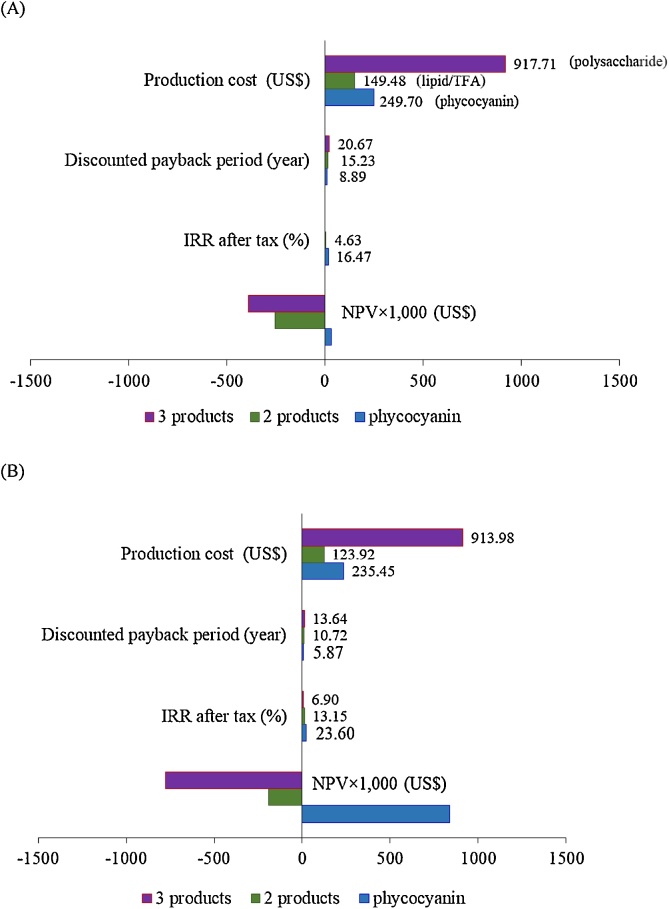
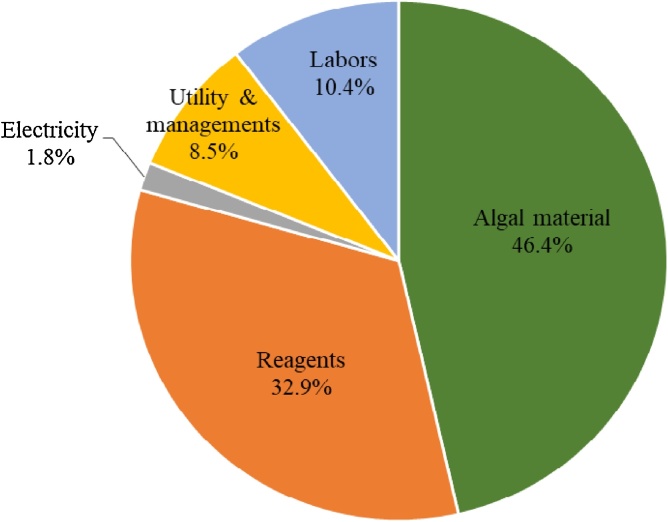
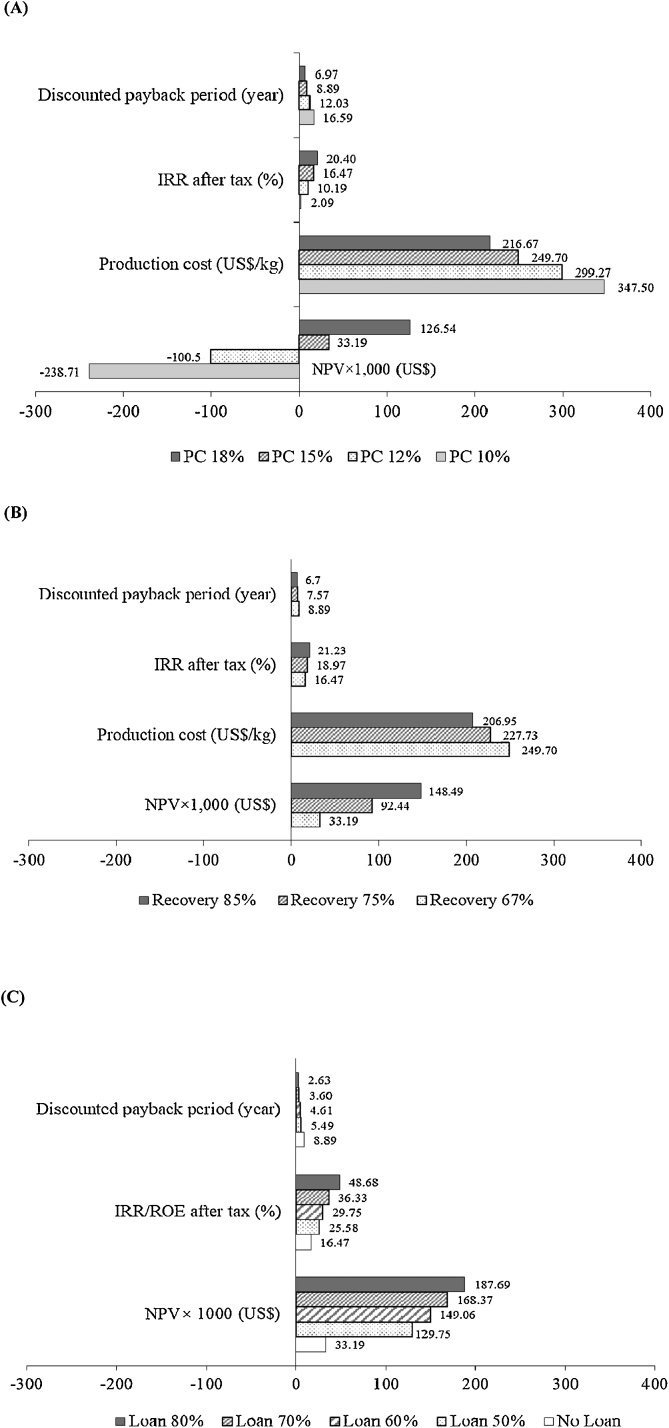
The results of economic analysis using the LCCA methodology showed a positive NPV for phycocyanin production, which is an indication that the investment would be profitable. The NPV of this project was US$ 33,193.27 with an IRR of 16.47% and a discounted payback period of 8.89 years at a 15% discount rate (Fig. 3A). The production cost for phycocyanin was approximately US$ 249.70 kg−1, excluding the cost of marketing and packaging. However, the costs of marketing and packaging can increase the production cost by 10% [26]. Regarding the operating costs for phycocyanin production, it was found that the cost of algae biomass accounts for approximately 46.4% of the total operating costs, followed by the cost of reagents (32.9%), utility and management (8.5%), labors (10.4%), and electricity (1.8%) (Fig. 4).
Fig. 3.
Economic analysis of high-value chemicals from Arthrospira at a production capacity of 600 kg/year (A) and 3000 kg/year (B).
Fig. 4.
Contribution of the operating costs of the phycocyanin extracts.
Borowitzka et al. [27] reported that the price of utilizing microalgal residue as animal feed (protein source) is only about US$ 1000 ton−1 (US$ 1 kg−1), at best. Theoretically, producing lipid/TFA and polysaccharides as coproducts may be an alternative way to add more value to the algae residue. However, the economic analysis showed that the investment for coproducts (lipid/TFA and polysaccharide) was infeasible, since their NPV values were negative (Fig. 3A). However, no additional cost was incurred for algae material, the production of lipid/TFA and polysaccharide, as coproducts would require additional expenditure on equipment, labor, electricity, and water. In addition, ethanol was used as a solvent for lipid/TFA extraction and for the precipitation of polysaccharide. Utilizing ethanol to extract lipid/TFA and polysaccharides increased the percentage of reagents from 32.9% –phycocyanin only, to 38.6% – phycocyanin and lipid/TFA, and 47.0% – when phycocyanin, lipid/TFA and polysaccharides were coproduced (data not shown).
Normally, the production costs decrease as the extraction capacity increases; therefore, the economic feasibility of the increasing plant production capacity by 5 times was analyzed. At this scale, a phycocyanin extraction volume of 10,000 L will be performed for the daily operation, and 30 tons of Arthrospira biomass (40% of total biomass) will be used for extracting 3000 kg of phycocyanin, 1510 kg of lipid/TFA, and 175 kg of polysaccharide per year. Although the increasing extraction capacity resulted in an increased IRR and a decreased production cost of coproduct (lipid/TFA) from US$ 149.48 to US$ 123.92 kg−1, producing the coproduct (lipid/TFA) was still not be economic feasibility (Fig. 3B). The obstacles to investment in producing coproducts may be caused by two reasons: 1) The selling price of crude lipid/TFA could be estimated at only US$ 30 kg−1 because the prices of GLA oils from plants (evening primrose and borage oil) were between US$ 10–35 kg−1 [4,5]. However, the production costs of lipid/TFA in this work were US$ 149.48 kg−1 (at a production scale 300 kg/year) and US$ 123.92 kg−1 (at 1510 kg/year), which were higher than the selling price of competitive product. 2) The yield of polysaccharides in this work was low (∼ 1.0% of dry weight), because some of the polysaccharides were dissolved in the phycocyanin extraction process and some of the algal residue was lost during the phycocyanin and lipid/TFA extraction steps, which resulted in a small volume of the production capacity. Moreover, the precipitation of polysaccharide required two volumes of ethanol, which caused the high production cost of polysaccharide.
As shown in Table 4, the phycocyanin content in algal material is an important factor that affects the production cost; thus, a sensitivity analysis of this factor was investigated on the basis of 67% phycocyanin recovery. To produce 600 kg of phycocyanin using algal materials containing 10%, 12% 15% and 18% phycocyanin on a dry weight basis, 8955.22 kg, 7500 kg, 6000 kg, and 5000 kg of materials would be required, respectively. A reduction in the amount algal material used in the extraction process led to a decrease in the production cost from US$ 347.50 kg−1 (10% phycocyanin) to US$ 216.67 kg−1 (18% phycocyanin). Moreover, 10% and 12% phycocyanin content showed a negative NPV and a very low IRR with a payback period of more than 10 years (Fig. 5A). Based on the NPV, IRR, and payback period, the phycocyanin content of the raw material should be approximately 15% of the dry weight.
Fig. 5.
Sensitivity analysis of phycocyanin content (PC) of algal material (A), percentage of phycocyanin recovery (B) and the investment by loan (C).
In this work, the yield of phycocyanin was calculated following Eq. (1), which might have caused the low phycocyanin recovery (67%). Calculating the yield of phycocyanin on the basis of weight of extracts per weight of the sample will result in a higher phycocyanin recovery, and a lower amount of algae material will be needed. In the case of algae containing phycocyanin at 15% of the dry weight, an increase in the percentage of phycocyanin recovery resulted in an increased IRR and a decreased production cost and payback period (Fig. 5B).
Since the results showed that phycocyanin production is economically feasible, the return rates of the investment with and without a loan were analyzed. The results showed that the return rate of the investment with a loan was higher than that of the investment without loan. The highest return to equity (ROE), which was 48.68%, and the shortest discounted payback period, which was 2.63 years, were obtained when 80% of the investment was financed by a loan (Fig. 5C). In addition, the minimum loan that gave a discounted payback period of less than 5 years was 60%. Therefore, the project of phycocyanin production could be invested in by using a loan, because the ROE was greater than the minimum acceptable rate of the return (15%).
4. Conclusion
This work demonstrates the potential of the stepwise extraction of high-value chemicals from Arthrospira. Phycocyanin was the main product from the extraction process, and approximately 67% of the original phycocyanin content in raw materials could be recovered. The difference between the production cost of phycocyanin (US$ 249.70 kg−1) and its likely selling price (US$ 500 kg−1) indicated that its large-scale production is economic feasible. At a discount rate of 15%, the discounted payback period of the investment for phycocyanin production with an 80% loan was 2.63 years with a 48.68% ROE. Crude lipid/TFA and polysaccharide extracts as coproducts can add value to algal residue; thus, the raw material could be fully utilized. However, the investment will be more attractive if the yields and selling prices are increased.
Acknowledgements
This work is supported by King Mongkut’s University of Technology Thonburi, Bangkok, Thailand. The authors thank Assoc. Prof. Warunee Tia for advice in the economic analysis.
References
- 1.Cohen Z. The chemical of Spirulina. In: Vonshak A., editor. A Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology. Taylor & Francis Inc; Philadelphia: 1997. pp. 175–204. [Google Scholar]
- 2.Plaza M., Herrero M., Cifuentes A., Ibáñez E. Innovative natural functional ingredients from microalgae. J. Agric. Food Chem. 2009;57:7159–7170. doi: 10.1021/jf901070g. [DOI] [PubMed] [Google Scholar]
- 3.Borowitzka M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013;25:743–756. [Google Scholar]
- 4.Lindemann J., Merolli A. Rodman Publ. 2006. GLA: the ‘good’ omega-6: recent research reveals interesting consumer perceptions of GLA-containing oils.http://www.nutraceuticalsworld.com/contents/view/13526 Available at. (Accessed September 2009) [Google Scholar]
- 5.Alonso D.L., Maroto F.G. Plant as chemical factories for the production of polyunsaturated fatty acids. Biotechnol. Adv. 2000;18:481–497. doi: 10.1016/s0734-9750(00)00048-3. [DOI] [PubMed] [Google Scholar]
- 6.Caswell M., Zilberman D. Oregon State University; Corvallis, Oregon: 2000. An Economic Analysis of Algoculture, Paper Presented at the Tenth Biennial Conference of the International Institute of Fisheries Economics& Trade, Department of Agricultural and Resource Economics.http://www.orst.edu/dept/IIFET/2000/speakersz.html July 10-14, (2000) [Google Scholar]
- 7.Reis A., Mendes A., Lobo-Fernandes H., Empis J.A., Maggiolly Novais J. Production, extraction and purification of phycobiliproteins from Nostoc sp. Bioresour. Technol. 1998;66:181–187. [Google Scholar]
- 8.Chaiklahan R., Chirasuwan N., Loha V., Bunnag B. Lipid and fatty acids extraction from the cyanobacterium Spirulina. ScienceAsia. 2008;34(3):299–305. [Google Scholar]
- 9.Chaiklahan R., Chirasuwan N., Loha V., Tia S., Bunnag B. Separation and purification of phycocyanin from Spirulina sp. using a membrane process. Bioresour. Technol. 2011;102:7159–7164. doi: 10.1016/j.biortech.2011.04.067. [DOI] [PubMed] [Google Scholar]
- 10.Chaiklahan R., Chirasuwan N., Triratana P., Loha V., Tia S., Bunnag B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. Int. J. Biol. Macromol. 2013;58:73–78. doi: 10.1016/j.ijbiomac.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 11.Chaiklahan R., Chirasuwan N., Triratana P., Tia S., Bunnag B. Effect of extraction temperature on the diffusion coefficient of polysaccharides from Spirulina and the optimal separation method. Biotechnol. Bioprocess Eng. 2014;19:369–377. [Google Scholar]
- 12.Patel A., Mishra S., Pawar R., Ghosh P.K. Purification and characterization of c-phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005;40:248–255. doi: 10.1016/j.pep.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Dubois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 15.Boussiba S., Richmond A. Isolation and purification of phycocyanins from the blue-green alga Spirulina platensis. Arch. Microbiol. 1979;120:155–159. [Google Scholar]
- 16.Lapage G., Roy C.C. Improved recovery of fatty acid through direct transesterification without prior extraction of purification. J. Lipid Res. 1984;25:1391–1396. [PubMed] [Google Scholar]
- 17.Vonshak A. Appendix V- the main commercial producers of spirulina. In: Vonshak A., editor. A Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology. Taylor & Francis Inc; Philadelphia: 1997. pp 221–224. [Google Scholar]
- 18.Belay A. Mass culture of Spirulina outdoors-the earthrise farms experience. In: Vonshak A., editor. A Spirulina platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology. Taylor & Francis Inc; Philadelphia: 1997. pp. 175–204. [Google Scholar]
- 19.Oliveira E.G., Rosa G.S., Moraes M.A., Pinto L.A.A. Characterization of thin layer drying of Spirulina platensis utilizing perpendicular air flow. Bioresour. Technol. 2009;100:1297–1303. doi: 10.1016/j.biortech.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Chaiklahan R., Chirasuwan N., Bunnag B. Stability of phycocyanin extracted from Spirulina sp.: influence of temperature, pH and preservatives. Process Biochem. 2012;47:659–664. [Google Scholar]
- 21.Cisneros M., Rito-Palomares M. A simplified strategy for the release and primary recovery of c-phycocyanin produced by Spirulina maxima. Chem. Biochem. Eng. Q. 2004;18:385–390. [Google Scholar]
- 22.Herrera A., Boussiba S., Napoleone V., Hohlberg A. Recovery of c-phycocyanin from the cyanobacterium Spirulina maxima. J. Appl. Phycol. 1989;1:325–331. [Google Scholar]
- 23.Moraes C.C., Kalil S.J. Strategy for a protein purification design using c-phycocyanin extract. Bioresour. Technol. 2009;100:5312–5317. doi: 10.1016/j.biortech.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Liu L., Chen X., Zhang X., Zhang Y., Zhou B. One-step chromatography method for efficient separation and purification of R- phycoerythrin from Polysiphonia urceolata. J. Biotechnol. 2005;116:91–100. doi: 10.1016/j.jbiotec.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Soni B., Kalavadia B., Trivedi U., Madamwar D. Extraction, purification and characterization of phycocyanin from Oscillatoria quadripunctulata-isotated from the rocky shores of Bet-Dwarka, Gujarat, India. Process Biochem. 2006;41:2017–2023. [Google Scholar]
- 26.Borowitzka M.A. Algal biotechnology productions and processes-matching science and economics. J. Appl. Phycol. 1992;4:267–279. [Google Scholar]
- 27.Borowitzka M.A. Techno-economic modeling for biofuels from microalgase. In: Borowitzka M.A., Moheimani N.R., editors. Algae for Biofuels and Energy. Springer; Dordrecht: 2013. pp. 255–264. [Google Scholar]