Abstract
Mucormycoses were difficult-to-manage infections owing to limited diagnostic tools and therapeutic options. We review here advances in pathology understanding, diagnostic tools including computed tomography, and serum polymerase chain reaction and therapeutic options.
Keywords: Mucormycosis, Reverse Halo sign, PCR, Isavuconazole, Liposomal amphotericin B
Introduction
Mucormycoses are life-threatening fungal infections mostly occurring in hematology, solid organ transplant, or diabetic patients, it may also affect immunocompetent patients following a trauma or burn 1. Nosocomial or community outbreaks have been described 2. Mucormycosis is characterized by host tissue infarction and necrosis resulting from vasculature invasion by hyphae starting with a specific interaction with endothelial cells. Most common clinical presentations are rhino-orbito-cerebral and pulmonary. Multicenter and single-center studies have reported an increasing incidence probably due to an increase in the at-risk population and improved diagnostic tools 3, 4. In a French study, mucormycosis incidence increased by 7.3% per year, especially in patients with neutropenia 5.
These infections are difficult to manage for several reasons 6. Firstly, diagnosis is difficult because of clinico-radiological similarities with invasive aspergillosis and historical lack of diagnostic tools. However, new tools in serum and tissue as well as the recognition of highly suggestive radiological signs recently modified diagnostic possibilities. Secondly, treatment is an emergency and combines surgery, which is frequently required owing to the angioinvasive and necrotic character of infection 7, and antifungal treatment. Primary in vitro resistance to several antifungal drugs limits therapeutic options 8. However, recent data enlarge the antifungal armamentarium with the US Food and Drug Administration’s and European Medicines Agency’s approval of the new triazole isavuconazole. However, comparative clinical data are lacking, and the respective places of polyenes and different azoles need to be discussed.
Moving towards improved mucormycosis understanding
Human mucormycoses are caused by a wide range of pathogenic species. Mucormycosis location is linked to the mucorales species; Rhizopus arrhizus ( Figure 1) is present in 85% of rhino-cerebral forms, compared with only 17% of non-rhino-cerebral forms in the French RetroZygo study 9. This finding could be explained by virulence differences between Mucorales species. In experimental studies, ketoacidosis has been found to predispose mice to Rhizopus spp. but not Lichtheimia spp. infection 10, 11. In parallel, corticosteroid treatment enhanced the susceptibility of mice to lung infections caused by Lichtheimia corymbifera or Lichtheimia ramosa 10, 11.
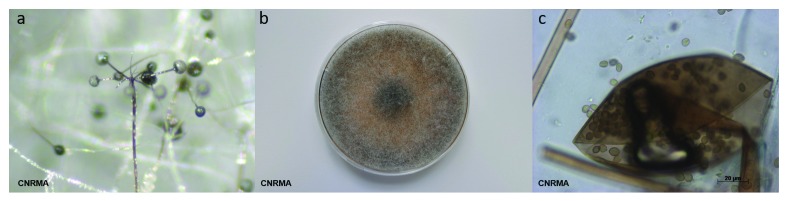
Figure 1. Typical features of Rhizopus arrhizus.
( a) Sporangiophore branching and rhizoids (stereomicroscope); ( b) grey-brownish colony on malt 2% medium; ( c) melanized sporangium and sporangiospores.
Mucormycosis’ clinical presentation is also related to underlying conditions. Rhino-cerebral mucormycosis is the most common form in patients with diabetes mellitus 1, while pulmonary mucormycosis occurs most often in patients with hematological malignancies 12. Radiological findings in patients with pulmonary mucormycosis are also related to immunological status 13. Although unusual, lately there have been diagnoses made in the gastrointestinal system. The stomach is more frequently involved, and then the colon. Symptoms are abdominal pain and gastro-intestinal bleeding 14. Diagnosis is suspected on endoscopic findings with necrotic lesions that can lead to perforation and peritonitis 15.
Mucorales can gain entry to a susceptible host through inhalation, ingestion of contaminated food, or abraded skin. These routes result in rhino-orbito-cerebral, pulmonary, gastrointestinal, or cutaneous/wound infections. One of the characteristic features of mucormycosis is its angioinvasive property, resulting in vascular thromboses and ultimately tissue necrosis. Ketoacidosis and deferoxamine are known to predispose to mucormycosis, revealing the importance of hyperglycemia, iron, and acidifying ketone bodies in mucorales virulence. Angioinvasion was reported to be related to the interaction between a spore-coating protein family (CotH) on Rhizopus spp. surface and endothelium glucose regulator protein 78 (GRP78) expressed at the surface of endothelial cells. This interaction triggers host cell injury and subsequent fungus hematogenous dissemination 16. Elevated levels of serum glucose, iron, and ketone bodies increase fungal growth and induce the expression of GRP78 and CotH, resulting in increased ability of Rhizopus to invade host tissues and explaining the susceptibility of diabetic and deferoxamine-treated patients to mucormycosis. However, it should be noted that the majority of studies on virulence and the association between ketoacidosis and the occurrence of mucormycosis have been conducted with Rhizopus species 17. Decreased numbers and impaired function of monocytes and neutrophils are important mucormycosis risk factors, since they are known to inhibit Mucorales spore germination. This includes patients with hematological disorders, AIDS, or liver cirrhosis, those who have undergone solid organ transplant, and those being treated with high-dose steroids 18, 19. Finally, victims of natural disaster are also at risk 20 owing to wounds contaminated with water, soil, or debris 21, such as after the 2004 Indian Ocean tsunami 22 or after the 2011 Missouri tornado 23.
Computed tomography
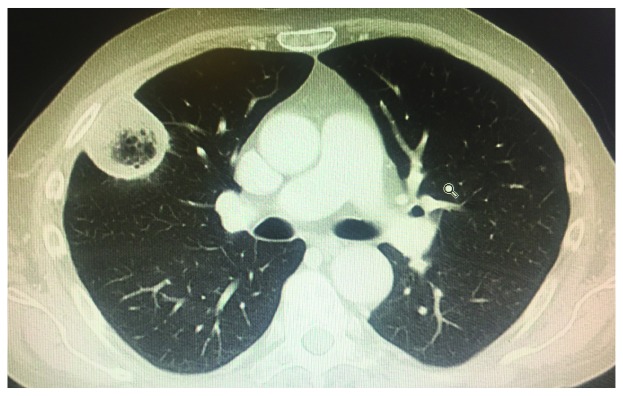
The most common radiological pattern of lung mucormycosis on initial computed tomography (CT) scan is a halo sign and then nodule or mass 13, 24. However, when studied very early and on serial follow-up, sequential morphologic changes could be observed as (i) reversed halo sign ( Figure 2) followed by (ii) consolidation or nodule or mass with halo sign and, finally, (iii) central necrosis and air-crescent sign. For pulmonary mucormycosis, a recent study showed that there was a significant increase in the prevalence of reversed halo sign in neutropenic (79%) and non-neutropenic (31%) patients ( P <0.05) 25.
Figure 2. Inversed halo sign.
Inversed halo sign.
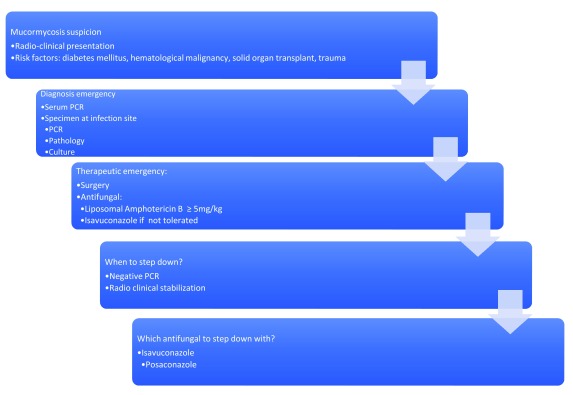
Major steps in mucormycosis: towards earlier diagnosis ( Figure 3)
Mucormycosis diagnosis is challenging, as it is associated with high mortality, especially in hematological patients. Early distinction from invasive aspergillosis is of utmost importance, as antifungal treatment may differ, whereas underlying conditions and clinical presentation are often similar. Until recently, mucormycosis diagnostic tools were based on limited basic microbiology and frequently led to diagnosis delay.
Figure 3. Mucormycosis: from suspicion to treatment.
PCR, polymerase chain reaction.
Unlike invasive aspergillosis, the detection of circulating antigen such as galactomannan and β-D-1,3-glucan provides no help for mucormycosis diagnosis. Therefore, samples from the infection site are highly required to diagnose mucormycosis based on the microscopic detection of typical hyphae or on a positive culture 26. Recently, the development of molecular biology tools has allowed the non-invasive diagnosis of mucormycosis. Million et al. designed a quantitative multiplex polymerase chain reaction (qPCR)-based 18S rRNA targeting Mucor/Rhizopus, Lichtheimia, and Rhizomucor. This PCR assay was evaluated with the aim to detect Mucorales DNA early in the course of the infection in the blood (serum) 27. The authors were able to detect Mucorales DNA in serum samples from 90% of patients up to three days before mucormycosis diagnosis 27. Negative serum PCR was also associated with better outcome as compared to patients with persistently positive PCR. Furthermore, a study among severely ill burn patients found that circulating Mucorales DNA was detected 11 (4.5–15) days before standard diagnosis for invasive wound mucormycosis 28. Other studies have evaluated the use of real-time PCR targeting Mucorales on tissue or respiratory samples in patients with hematological malignancy suffering from proven and probable mucormycosis 29– 32.
Consequently, it is currently necessary in patients with hematological malignancies to include the value of reverse halo sign on CT combined with serum qPCR targeting Mucorales in the early diagnosis of pulmonary mucormycosis 33.
Several antifungals now available for mucormycosis treatment
Mucormycoses: an indication of emergency surgery
Current guidelines recommend antifungal treatment, surgical debridement, and correction of risk factors 34, 35. Surgical debridement has to be extensive, involving all necrotic areas for rhino-oculo-cerebral infection, and repeated surgical procedures are recommended to achieve local control and improve outcome 36. For pulmonary mucormycosis, the indication and timing of surgical management outside emergency care (hemoptysis) is still unclear 37. In a European series of 230 patients, surgical treatment reduced mortality by 79% 38, leading to discuss surgery when feasible for any localization, however mandatory for rhino-cerebro-oculo-cerebral and post-traumatic mucormycosis 36, 39.
Antifungal treatment
Amphotericin B (Amb) and its lipid formulations and posaconazole were the only antifungal drugs available with in vitro activity against mucorales 40, 41. The antifungal armamentarium recently enlarged with the development of isavuconazole.
The first-line recommended antifungal agent is liposomal Amb (L-Amb) or Amb lipid complex (ABLC) 35. Studies in mice proposed that the efficacy of L-Amb and ABLC was dependent on the dose given and that 10 mg/kg yielded the best outcomes. A prospective French phase II multicenter study (AmBizygo trial) evaluated the efficacy and tolerance of high-dose (10 mg/kg/day) L-Amb in association with surgery when recommended for the treatment of 34 mucormycosis cases. A favorable response was seen in 45% of patients at week 12. However, serum creatinine doubled in 40% of patients, but in 63% of cases, once treatment had ended, creatinine levels normalized after three months 42. According to this study, ECMM/ESCMID and ECIL-6 guidelines recommend the use of L-Amb with a daily dosage of at least 5 mg/kg/day for mucormycosis 34, 35, and dosages at 10 mg/kg/day are strongly supported by ECMM/ESCMID for cerebral infections 35. Moreover, because of better diffusion, L-Amb should be favored in central nervous system infections.
The duration of the first-line antifungal treatment is still a matter of debate and should be determined on an individual basis and adjusted based on the underlying condition. Some authors proposed a lipid Amb treatment for at least three weeks, and, when there is clinical and radiological improvement, a consolidation by posaconazole can be started 43. However, it could possibly be guided by negative PCR and therefore shortened for some patients.
Isavuconazonium sulfate is a water-soluble pro-drug, which is quickly hydrolyzed to the triazole isavuconazole after oral or intravenous administration. Isavuconazole has high oral bioavailability, linear pharmacokinetics, and a broad antifungal spectrum. The in vitro activity of isavuconazole minimum inhibitory concentration (MIC) ranges were 0.125 to 4 mg/L across L. corymbifera, L. ramosa, Rhizomucor pusillus, Rhizomucor microspores, and R. arrhizus but somewhat higher against Mucor circinelloides (1 to 16 mg/L). The MICs were in general one- to three-fold higher than those for posaconazole 44. In the recently published Vital study, 21 patients were treated with isavuconazole as first-line treatment; 42-day response rate was only 14% and week 12 response was 10% (compared to 45% in the AmBizygo study) with 43% deaths 45. The results of this study found a mortality rate at day 42 comparable to that observed in the AmBizygo study 42. In the VITAL study, isavuconazole was well tolerated and toxic effects were an uncommon cause of discontinuation. The place of isavuconazole has not yet been specified in the most recent guidelines 34. Finally, a cost-effectiveness study demonstrated the positive economic impact of the use of isavuconazole compared to Amb in the treatment of mucormycosis 46.
Posaconazole has been shown to have in vitro and in vivo activity against mucorales, but there are no data for the use of first-line posaconazole therapy. Posaconazole, therefore, finds its place in the therapeutic armamentarium for prophylaxis or consolidation after induction treatment with L-Amb. No study on the efficacy of posaconazole intravenous or tablet formulations in mucormycosis treatment were conducted. Finally, mucormycosis cases have been reported in patients undergoing posaconazole prophylaxis despite satisfactory serum concentrations 47.
However, it seems important to note that there are no current validated MIC breakpoints for any of the available antifungals and thus the determination of susceptibility categories is not possible for the agents of mucormycosis.
Immunostimulating drugs
A case report has recently reported the benefit of a treatment with the checkpoint inhibitor nivolumab and interferon-Υ for an immunocompetent patient with extensive abdominal mucormycosis unresponsive to conventional therapy 48.
Conclusion
Mucormycosis is a life-threatening fungal infection characterized by host tissue infarction and necrosis that occurs mostly in immunocompromised patients and is associated with an increasing incidence and mortality despite the availability of therapeutic tools. Determining whether the patient has invasive aspergillosis or mucormycosis could be challenging at the bedside. In this context, new tools of molecular biology have been developed to obtain earlier diagnosis and start optimal medico-surgical treatment. Comparative studies are needed to better optimize induction and consolidation treatment.
Acknowledgements
Thanks to Dea Garcia-Hermoso for reading the manuscript and providing strain photographs.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Immaculata Xess, Department of Microbiology, All India Institute of Medical Sciences, Delhi, India
Arunaloke Chakrabarti, Department of Medical Microbiology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Roden MM, Zaoutis TE, Buchanan WL, et al. : Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 2. Alanio A, Desnos-Ollivier M, Garcia-Hermoso D, et al. : Investigating Clinical Issues by Genotyping of Medically Important Fungi: Why and How? Clin Microbiol Rev. 2017;30(3):671–707. 10.1128/CMR.00043-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bitar D, Van Cauteren D, Lanternier F, et al. : Increasing incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis. 2009;15(9):1395–401. 10.3201/eid1509.090334 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Guinea J, Escribano P, Vena A, et al. : Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS One. 2017;12(6):e0179136. 10.1371/journal.pone.0179136 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Bitar D, Lortholary O, Le Strat Y, et al. : Population-based analysis of invasive fungal infections, France, 2001-2010. Emerg Infect Dis. 2014;20(7):1149–55. 10.3201/eid2007.140087 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Ribes JA, Vanover-Sams CL, Baker DJ: Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236–301. 10.1128/CMR.13.2.236-301.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katragkou A, Walsh TJ, Roilides E: Why is mucormycosis more difficult to cure than more common mycoses? Clin Microbiol Infect. 2014;20 Suppl 6:74–81. 10.1111/1469-0691.12466 [DOI] [PubMed] [Google Scholar]
- 8. Dannaoui E: Antifungal resistance in mucorales. Int J Antimicrob Agents. 2017;50(5):617–21. 10.1016/j.ijantimicag.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 9. Lanternier F, Dannaoui E, Morizot G, et al. : A global analysis of mucormycosis in France: the RetroZygo Study (2005-2007). Clin Infect Dis. 2012;54 Suppl 1:S35–43. 10.1093/cid/cir880 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Ibrahim AS, Spellberg B, Walsh TJ, et al. : Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S16–22. 10.1093/cid/cir865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spellberg B, Edwards J, Jr, Ibrahim A: Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–69. 10.1128/CMR.18.3.556-569.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tedder M, Spratt JA, Anstadt MP, et al. : Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044–50. 10.1016/0003-4975(94)90243-7 [DOI] [PubMed] [Google Scholar]
- 13. Nam BD, Kim TJ, Lee KS, et al. : Pulmonary mucormycosis: serial morphologic changes on computed tomography correlate with clinical and pathologic findings. Eur Radiol. 2018;28(2):788–95. 10.1007/s00330-017-5007-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Dioverti MV, Cawcutt KA, Abidi M, et al. : Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses. 2015;58(12):714–8. 10.1111/myc.12419 [DOI] [PubMed] [Google Scholar]
- 15. Singh N, Gayowski T, Singh J, et al. : Invasive gastrointestinal zygomycosis in a liver transplant recipient: case report and review of zygomycosis in solid-organ transplant recipients. Clin Infect Dis. 1995;20(3):617–20. 10.1093/clinids/20.3.617 [DOI] [PubMed] [Google Scholar]
- 16. Baldin C, Ibrahim AS: Molecular mechanisms of mucormycosis-The bitter and the sweet. PLoS Pathog. 2017;13(8):e1006408. 10.1371/journal.ppat.1006408 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Spellberg B: Mucormycosis pathogenesis: Beyond Rhizopus. Virulence. 2017;8(8):1481–2. 10.1080/21505594.2017.1364335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xhaard A, Lanternier F, Porcher R, et al. : Mucormycosis after allogeneic haematopoietic stem cell transplantation: a French Multicentre Cohort Study (2003-2008). Clin Microbiol Infect. 2012;18(10):E396–400. 10.1111/j.1469-0691.2012.03908.x [DOI] [PubMed] [Google Scholar]
- 19. Pana ZD, Seidel D, Skiada A, et al. : Invasive mucormycosis in children: an epidemiologic study in European and non-European countries based on two registries. BMC Infect Dis. 2016;16(1):667. 10.1186/s12879-016-2005-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benedict K, Park BJ: Invasive fungal infections after natural disasters. Emerg Infect Dis. 2014;20(3):349–55. 10.3201/eid2003.131230 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Ivers LC, Ryan ET: Infectious diseases of severe weather-related and flood-related natural disasters. Curr Opin Infect Dis. 2006;19(5):408–14. 10.1097/01.qco.0000244044.85393.9e [DOI] [PubMed] [Google Scholar]
- 22. Andresen D, Donaldson A, Choo L, et al. : Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet. 2005;365(9462):876–8. 10.1016/S0140-6736(05)71046-1 [DOI] [PubMed] [Google Scholar]
- 23. Neblett Fanfair R, Benedict K, Bos J, et al. : Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367(23):2214–25. 10.1056/NEJMoa1204781 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Legouge C, Caillot D, Chrétien ML, et al. : The reversed halo sign: pathognomonic pattern of pulmonary mucormycosis in leukemic patients with neutropenia? Clin Infect Dis. 2014;58(5):672–8. 10.1093/cid/cit929 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Bourcier J, Heudes PM, Morio F, et al. : Prevalence of the reversed halo sign in neutropenic patients compared with non-neutropenic patients: Data from a single-centre study involving 27 patients with pulmonary mucormycosis (2003-2016). Mycoses. 2017;60(8):526–33. 10.1111/myc.12624 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Garcia-Hermoso D, Alanio A, Lortholary O, et al. : Agents of Systemic and Subcutaneous Mucormycosis and Entomophthoromycosis. In: Manual of Clinical Microbiology, 11th Pfaller MA, Richter SS, Funke G, Jorgensen JH, Landry ML, Carroll KC, et al.,editors,2015;2087–108. 10.1128/9781555817381.ch121 [DOI] [Google Scholar]
- 27. Millon L, Herbrecht R, Grenouillet F, et al. : Early diagnosis and monitoring of mucormycosis by detection of circulating DNA in serum: retrospective analysis of 44 cases collected through the French Surveillance Network of Invasive Fungal Infections (RESSIF). Clin Microbiol Infect. 2016;22(9):810.e1–810.e8. 10.1016/j.cmi.2015.12.006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Legrand M, Gits-Muselli M, Boutin L, et al. : Detection of Circulating Mucorales DNA in Critically Ill Burn Patients: Preliminary Report of a Screening Strategy for Early Diagnosis and Treatment. Clin Infect Dis. 2016;63(10):1312–7. 10.1093/cid/ciw563 [DOI] [PubMed] [Google Scholar]
- 29. Alanio A, Garcia-Hermoso D, Mercier-Delarue S, et al. : Molecular identification of Mucorales in human tissues: contribution of PCR electrospray-ionization mass spectrometry. Clin Microbiol Infect. 2015;21(6):594.e1–5. 10.1016/j.cmi.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 30. Springer J, White PL, Kessel J, et al. : A Comparison of Aspergillus and Mucorales PCR Testing of Different Bronchoalveolar Lavage Fluid Fractions from Patients with Suspected Invasive Pulmonary Fungal Disease. J Clin Microbiol. 2018;56(2): pii: e01655-17. 10.1128/JCM.01655-17 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Springer J, Lackner M, Ensinger C, et al. : Clinical evaluation of a Mucorales-specific real-time PCR assay in tissue and serum samples. J Med Microbiol. 2016;65(12):1414–21. 10.1099/jmm.0.000375 [DOI] [PubMed] [Google Scholar]
- 32. Zaman K, Rudramurthy SM, Das A, et al. : Molecular diagnosis of rhino-orbito-cerebral mucormycosis from fresh tissue samples. J Med Microbiol. 2017;66(8):1124–9. 10.1099/jmm.0.000560 [DOI] [PubMed] [Google Scholar]
- 33. Caillot D, Valot S, Lafon I, et al. : Is It Time to Include CT "Reverse Halo Sign" and qPCR Targeting Mucorales in Serum to EORTC-MSG Criteria for the Diagnosis of Pulmonary Mucormycosis in Leukemia Patients? Open Forum Infect Dis. 2016;3(4):ofw190. 10.1093/ofid/ofw190 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Tissot F, Agrawal S, Pagano L, et al. : ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102(3):433–44. 10.3324/haematol.2016.152900 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. : ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20 Suppl 3:5–26. 10.1111/1469-0691.12371 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Vironneau P, Kania R, Morizot G, et al. : Local control of rhino-orbito-cerebral mucormycosis dramatically impacts survival. Clin Microbiol Infect. 2014;20(5):O336–9. 10.1111/1469-0691.12408 [DOI] [PubMed] [Google Scholar]
- 37. Chretien ML, Legouge C, Pagès PB, et al. : Emergency and elective pulmonary surgical resection in haematological patients with invasive fungal infections: a report of 50 cases in a single centre. Clin Microbiol Infect. 2016;22(9):782–7. 10.1016/j.cmi.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 38. Skiada A, Pagano L, Groll A, et al. : Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–67. 10.1111/j.1469-0691.2010.03456.x [DOI] [PubMed] [Google Scholar]
- 39. Lelievre L, Garcia-Hermoso D, Abdoul H, et al. : Posttraumatic mucormycosis: a nationwide study in France and review of the literature. Medicine (Baltimore). 2014;93(24):395–404. 10.1097/MD.0000000000000221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sabatelli F, Patel R, Mann PA, et al. : In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob Agents Chemother. 2006;50(6):2009–15. 10.1128/AAC.00163-06 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Almyroudis NG, Sutton DA, Fothergill AW, et al. : In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob Agents Chemother. 2007;51(7):2587–90. 10.1128/AAC.00452-07 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Lanternier F, Poiree S, Elie C, et al. : Prospective pilot study of high-dose (10 mg/kg/day) liposomal amphotericin B (L-AMB) for the initial treatment of mucormycosis. J Antimicrob Chemother. 2015;70(11):3116–23. 10.1093/jac/dkv236 [DOI] [PubMed] [Google Scholar]
- 43. Kontoyiannis DP, Lewis RE: How I treat mucormycosis. Blood. 2011;118(5):1216–24. 10.1182/blood-2011-03-316430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lamoth F, Chung SJ, Damonti L, et al. : Changing Epidemiology of Invasive Mold Infections in Patients Receiving Azole Prophylaxis. Clin Infect Dis. 2017;64(11):1619–21. 10.1093/cid/cix130 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. : Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–37. 10.1016/S1473-3099(16)00071-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Bagshaw E, Kuessner D, Posthumus J, et al. : The cost of treating mucormycosis with isavuconazole compared with standard therapy in the UK. Future Microbiol. 2017;12(6):515–25. 10.2217/fmb-2016-0231 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Kang SH, Kim HS, Bae MN, et al. : Fatal Breakthrough Mucormycosis in an Acute Myelogenous Leukemia Patient while on Posaconazole Prophylaxis. Infect Chemother. 2015;47(1):49–54. 10.3947/ic.2015.47.1.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grimaldi D, Pradier O, Hotchkiss RS, et al. : Nivolumab plus interferon-γ in the treatment of intractable mucormycosis. Lancet Infect Dis. 2017;17(1):18. 10.1016/S1473-3099(16)30541-2 [DOI] [PubMed] [Google Scholar]