Abstract
Replication-competent retrovirus (RCR) is a safety concern for individuals treated with retroviral gene therapy. RCR detection assays are used to detect RCR in manufactured vector, transduced cell products infused into research subjects, and in the research subjects after treatment. In this study, we reviewed 286 control (n = 4) and transduced cell products (n = 282) screened for RCR in the National Gene Vector Biorepository. The transduced cell samples were submitted from 14 clinical trials. All vector products were previously shown to be negative for RCR prior to use in cell transduction. After transduction, all 282 transduced cell products were negative for RCR. In addition, 241 of the clinical trial participants were also screened for RCR by analyzing peripheral blood at least 1 month after infusion, all of which were also negative for evidence of RCR infection. The majority of vector products used in the clinical trials were generated in the PG13 packaging cell line. The findings suggest that screening of the retroviral vector product generated in PG13 cell line may be sufficient and that further screening of transduced cells does not provide added value.
Keywords: retrovirus, safety testing, replicating virus, lentivirus
Introduction
The use of genetically modified cells containing retroviral vectors is in advanced stages of clinical development. Retroviral-based gene therapy for adenosine deaminase deficiency was approved in Europe in 2016.1 The US Food and Drug Administration approved the first retroviral ex vivo transduced T cell therapy in 2017 for the treatment of non-Hodgkin’s lymphoma.2
Many retroviral vectors used in clinical applications are based on murine gammaretroviruses and are designed to be replication defective (subsequent use of the term retrovirus in this paper refers to gammaretroviruses). A potential risk of retroviral vectors is the development of replication-competent retroviruses (RCRs), which can arise by recombination of viral and cellular components during vector manufacture.3 Unlike lentiviral vectors, which have not been shown to generate replication-competent viruses, retroviral vectors have been associated with RCR development. Most commonly, RCRs arose through recombination of vector and packaging sequences, and decreasing homology between these sequences has been shown to decrease virus formation.4, 5, 6, 7, 8, 9, 10 Some recombinant retroviruses have also been shown to contain vector-packaging sequences and cellular-derived genetic sequeces.11, 12
A major concern with RCR infection is treatment-related malignancy. Like the parent murine viruses, RCR generated during vector production has been shown to cause malignancies in mice and non-human primates.13, 14 Furthermore, retroviral gene therapy has been associated with leukemia development in a limited number of clinical trials.15, 16, 17, 18, 19, 20 While research subjects who developed vector-associated malignancies did not have evidence of RCR, the mechanisms of insertional mutagenesis suggest that exposure to RCR would significantly increase the risk of treatment-related malignancy. This has influenced recommendations from the US FDA regarding RCR testing, which has mandated testing at three points.21 First, RCR testing is a required release criterion for retroviral vector lots used in clinical applications.22 Second, research subjects are to be monitored at various time points after treatment for the presence of RCR. The FDA has also imposed a third assessment: RCR testing must be performed on any cell product cultured ex vivo for more than 4 days. The number of cells to be tested is 1% of the cell product or 108 cells, whichever is less.22, 23 The rationale for testing an ex vivo cell product is to detect a low-level RCR that evaded detection in the vector product.
The similarity between vector and viral particles complicates RCR detection. As both contain viral proteins such as capsid, integrase, and reverse transcriptase, protein-detection methods are generally not helpful. Similarly, assays for reverse transcriptase activity cannot distinguish RCR from vector particles. While replication-defective vectors lack the genetic material used in viral replication (i.e., gag, pol, and env gene regions), the sequences are present in the vector-producing cells. Carryover of cellular or plasmid DNA into the vector product will lead to false-positive molecular assays. To date, culture-based assays provide the highest level of sensitivity for RCR detection.
As a recombinant RCR may have a variety of viral and cellular components, the growth rate of a given RCR will be unknown at the time of analysis. Therefore, regulators have required biologic assays to have a minimum of five passages (approximately 3 weeks) of culture in order to amplify any slow-growing RCR.22 A number of RCR culture assays have been described.14, 24, 25, 26, 27, 28 Most combine an amplification phase allowing a virus to grow to a high titer, followed by a detection method for viral particles.
While current RCR assays are sensitive, the large number of cells that must be tested along with the extended period of culture adds significant cost. Furthermore, it is unknown if screening of ex vivo transduced cells adds additional value when the vector product has been shown to be RCR negative. Revision in existing RCR testing requirements have been called for.29 In this paper, we review the experience of the National Gene Vector Biorepository (NGVB), an NHLBI-funded resource (https://www.NGVBCC.org/), which assists investigators in RCR testing of clinical samples. As none of the products tested to date were found to contain RCR, the experience suggests re-evaluation of current requirements for testing ex vivo transduced products.
Results
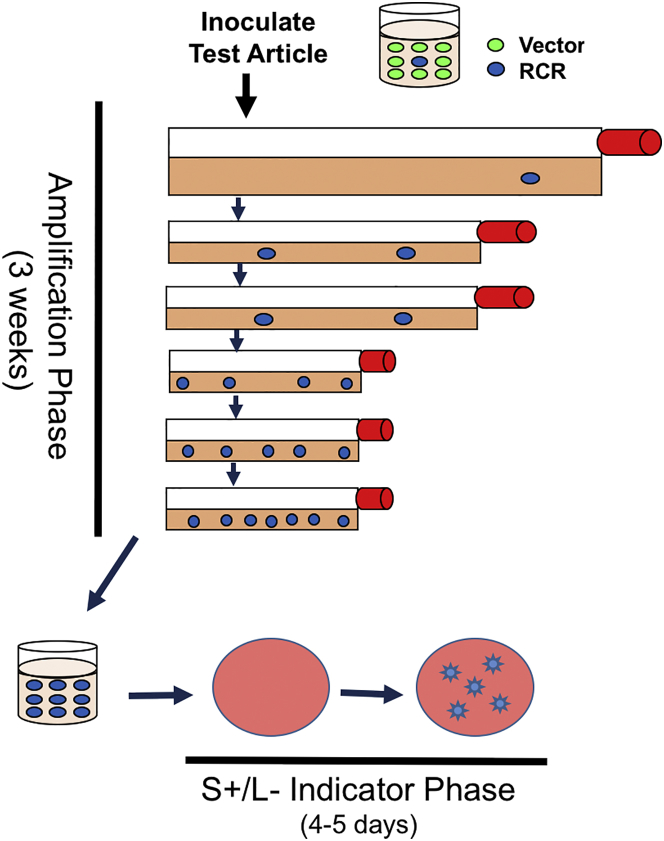
The RCR assay utilized in this study involves incubating test article (cells or culture media) with HEK293 cells (Figure 1). This cell line is highly infectible by retroviral vectors pseudotyped with gibbon ape leukemia virus (GALV) and RD114 envelopes and amplifies RCR to high titer.27, 28 After the amplification phase, media is then inoculated into cultures of PG4 cells. The PG4 line is an S+/L− line (sarcoma positive/leukemia negative) cell line, which assumes a transformed phenotype in the presence of murine leukemia viruses (including RCR).30 If an RCR is present in the test material, foci of transformed cells are evident in the culture. Any test article with foci detected in the indicator phase is considered positive for RCR.
Figure 1.
Schematic Representation of the Extended RCR Assay
The amplification phase allows any existing virus to increase. The presence of RCR in the culture media is confirmed by introducing cell-free media into the S+/L− assay and analyzing for foci of transformed cells 4–5 days after inoculation.
Sample Analysis
This analysis reviewed RCR testing of cellular products from 14 clinical trials (Table 1). While the NGVB offers testing for any vector pseudotype, all the investigators for this study had independently selected the GALV envelope for their vector. All vectors were generated in the PG13 cell line,10 except for one study that utilized the Phoenix-GALV cell line.31 The majority of samples tested were T cells utilized in cancer immunotherapy trials. Autologous CD34+ hematopoietic progenitor cells and autologous keratinocytes were also evaluated. All vector products utilized in the clinical trials reported here tested negative for RCR prior to ex vivo transduction of target cells.
Table 1.
Clinical Trial Samples Evaluated for RCR
| IU VPF Study Number | ClinicalTrial.Gov Study Number | Principle Investigator | Initial Assay Initiated | Final Assay Completed | Vector Generation Cell Line | Indication | Transduced Cell Type |
|---|---|---|---|---|---|---|---|
| 10-8 | NCT01723306, NCT00664196 | Junghans | 8/11/10 | 9/14/10 | PG13 | CAR-T | T cell |
| 11-13 | NCT01593696 | Mackall | 8/13/12 | 7/30/16 | PG13 | CAR-T | T cell |
| 12-8 | NCT00924326 | Rosenberg | 8/27/12 | 1/17/17 | PG13 | CAR-T | T cell |
| 13-4 | NCT01860937 | Curran | 10/23/13 | 9/19/19 | PG13 | CAR-T | T cell |
| 13-7 | NCT01044069 | Davila | 8/22/13 | 5/10/17 | PG13 | CAR-T | T cell |
| 13-8 | NCT01723306 | Junghans | 5/26/16 | 7/18/06 | PG13 | CAR-T | T cell |
| 13-32 | NCT01263379 | Tang | 11/4/13 | 5/10/17 | PG13 | epidermolysis bullosa | keratinocytes |
| 14-4 | NCT01593696 | Mackall | 4/30/14 | 11/6/16 | PG13 | CAR-T | T cell |
| 14-5 | NCT00669669 | Kiem | 1/14/16 | 9/16/16 | Phoenix-GALV | glioblastoma multiforma | CD34+ |
| 15-9 | NCT01723306 | Junghans | 5/26/16 | 7/18/16 | PG13 | CAR-T | T cell |
| 15-10 | NCT02215967 | Kochenderfer | 10/26/15 | 1/21/16 | PG13 | CAR-T | T cell |
| 15-14 | NCT02664363 | Archer | 1/7/16 | 2/12/16 | PG13 | CAR-T | T cell |
| 15-15 | NCT02498912 | O’Cearbhaill | 12/16/15 | 11/30/17 | PG13 | CAR-T | T cell |
As shown in Table 2, there were 286 samples analyzed in this study. Four were control samples submitted by investigators (one cell sample and three supernatants). Of the remaining 282 samples, 266 were transduced cell samples and a total of 4.53 × 109 cells were analyzed. The median number of transduced cells per sample was 1.35 × 107. In addition, 16 supernatant test articles were submitted from transduced cell cultures. The total volume of media tested was 266 mL. All control and transduced test articles were negative for RCR.
Table 2.
Number and Size of RCR Samples Analyzed by Study
| NGVB Study Number | Principle Investigator | All Cell Samples | Negative Control Samples | Transduced Samples | Total Number of Control Cells Tested | Total Number of Transduced Cells Tested | Mean Number of Transduced Cells per Assay | Ratio of Test Cell to HEK293 Cell | Supernatant Samples Assayed | Negative Control Samples | Transduced Supernatant Samples | Total Volume of Negative Control Samples (mL) | Total Volume of Transduced Test Article (mL) | Mean Volume of Transduced Test Article (mL) | Ratio of Test Volume (mL) to 105 HEK293 Cell |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10-8 | Junghans | 12 | 0 | 12 | 0 | 1.28E+07 | 1.07E+06 | 6.2 | |||||||
| 11-13 | Mackall | 53 | 0 | 53 | 0 | 4.86E+08 | 9.16E+06 | 6.0 | |||||||
| 12-8 | Rosenberg | 91 | 0 | 91 | 0 | 1.12E+09 | 1.23E+07 | 5.6 | |||||||
| 13-4 | Curran | 24 | 0 | 24 | 0 | 9.20E+08 | 3.80E+07 | 5.3 | 2 | 0 | 2 | 0 | 173 | 86.5 | 2.3 |
| 13-7 | Davila | 39 | 0 | 39 | 0 | 1.51E+09 | 3.88E+07 | 5.3 | |||||||
| 13-8 | Junghans | 1 | 0 | 1 | 0 | 1.00E+06 | 1.00E+06 | 5.0 | |||||||
| 13-32 | Tang | 7 | 0 | 7 | 0 | 1.40E+07 | 2.00E+06 | 8.6 | 7 | 0 | 7 | 0 | 34 | 4.9 | 8.2 |
| 14-4 | Mackall | 13 | 0 | 13 | 0 | 1.22E+08 | 9.38E+06 | 6.1 | |||||||
| 14-5 | Kiem | 4 | 0 | 4 | 0 | 45 | 11.5 | 4.4 | |||||||
| 15-9 | Junghans | 7 | 0 | 7 | 0 | 7.00E+06 | 1.00E+06 | 5.0 | |||||||
| 15-10 | Kochenderfer | 10 | 0 | 10 | 0 | 1.40E+08 | 1.40E+07 | 5.4 | |||||||
| 15-14 | Archer | 6 | 3 | 3 | 13 | 14 | 4.7 | 8.5 | |||||||
| 15-15 | O’Cearbhaill | 10 | 1 | 9 | 1.05E+07 | 2.01E+08 | 2.23E+07 | 5.4 | |||||||
| Total | 267 | 1 | 266 | 1.05E+07 | 4.53E+09 | 19 | 3 | 16 | 13 | 266 | |||||
| Mean | 1.35E+07 | 5.8 | 26.9 | 5.9 |
Subject Follow-Up
Of the 282 transduced products analyzed, 271 were infused into 270 subjects (Table 3). The difference between analyzed products and infused subjects varied. One product was not infused due to a change in clinical status, and four subjects are awaiting infusion. Seven subjects had both cell and supernatant samples submitted and analyzed for the same product. One subject had two different products infused.
Table 3.
Follow-Up Testing for RCR by Study Infused with Transduced Cell Products
| IU VPF Study Number | Principle Investigator | Number of Products Infused | Number of Subjects Infused | Subjects with RCR Follow-up | Method of RCR Detection | Level of Sensitivity per DNA |
|---|---|---|---|---|---|---|
| 10-8 | Junghans | 12 | 12 | 5 | qPCR | <10 copies/0.2 mcg DNA |
| 11-13 | Mackall | 53 | 53 | 53 | qPCR | <10 copies/0.2 mcg DNA |
| 12-8 | Rosenberg | 91 | 90 | 80 | S+/L− | 1 FFU/mL |
| 13-4 | Curran | 26 | 26 | 26 | qPCR | <10 copies/0.2 mcg DNA |
| 13-7 | Davila | 39 | 39 | 39 | qPCR | <10 copies/0.2 mcg DNA |
| 13-8 | Junghans | 1 | 1 | 1 | qPCR | <10 copies/0.2 mcg DNA |
| 13-32 | Tang | 7 | 7 | 7 | qPCR | <10 copies/0.2 mcg DNA |
| 14-4 | Mackall | 12 | 12 | 10 | qPCR | <10 copies/0.2 mcg DNA |
| 14-5 | Kiem | 4 | 4 | 4 | qPCR | <10 copies/0.2 mcg DNA |
| 15-9 | Junghans | 7 | 7 | 2 | qPCR and S+/L− | <10 copies/0.2 mcg DNA, 1 FFU/mL |
| 15-10 | Kochenderfer | 10 | 10 | 9 | qPCR | <10 copies/0.2 mcg DNA |
| 15-14 | Archer | 0 | 0 | 0 | N/A | N/A |
| 15-15 | O’Cearbhaill | 9 | 9 | 5 | qPCR | <10 copies/0.2 mcg DNA |
| Total | 271 | 270 | 241 |
FFU, focus forming unit; mcg, microgram.
Of the 270 subjects treated, 241 (89%) had blood tested at least 30 days after infusion for evidence of RCR. The majority of subjects were evaluated by qPCR analysis using primers and probe for the GALV envelope (160 of 241, 66%). The remaining subjects were evaluated by the S+/L− assay (without amplification) representing 81 of 241subjects tested (34%). Regardless of testing method, all subjects tested were negative for RCR.
Assay Performance
The performance of the RCR assay was evaluated. As the assay is performed under Good Manufacturing Practice, any variation from the defined procedure is documented. We found out-of-specification or deviations in 17 of the 286 samples tested (5.9%). There were five contaminated samples in the amplification phase that required the investigator to send additional samples for repeated testing (1.7%). The 12 remaining tests were completed by repeating the S+/L− portion of the assay using reserve samples from the amplification phase. The need for repeating the S+/L− assays were as follows: six samples on the same assay required retesting due to a defective control flask. In five samples, the S+/L− assay control did not reach confluence within the time period required by the standard operating procedure. There was contamination of one sample in the S+/L− assay.
The assay sensitivity is 1 focus forming unit of RCR per 1 mL test article media incubated with ≥2 × 105 HEK293 cells or one test article cell co-cultured with ≥5 HEK293 cells. The ratio of test article to HEK293 cells was established to minimize receptor interference between vector and RCR; all assays reported were reviewed and met the appropriate ratio.
Discussion
An important safety concern for patients seeking retroviral gene therapy is exposure to RCR. In this manuscript, we report a multi-institutional study including samples from 10 research groups involved with 14 clinical trials. No evidence of RCR was found in 282 transduced cell products tested in a rigorous RCR assay. Also, there was no evidence of RCR in the 241 research subjects screened for RCR post-treatment.
The NGVB recently reported on the lack of replication-competent lentivirus (RCL) in ex vivo transduced T cells used in cancer immunotherapy trials.32 In that study, 460 cell products were tested with no evidence of RCL. Although the potential risk of RCR is likely higher than that of current third-generation lentiviral vectors, our current study provides additional support for the safety of retroviral vectors. RCL has not been reported with current HIV-1-based systems, and this finding likely relates to deletion of HIV-1 accessory genes, use of self-inactivating long terminal repeats (LTRs), the separation of packaging sequences on to different plasmids, and the use of transient transfection production methods that limit the time for vector generation to less than 1 week.33 While methods for transient transduction of retroviral vectors are well described,34 many retroviral vectors are generated in packaging cell lines, which require clone selection, expansion for master cell bank generation, and expansion for vector production, a process that typically takes weeks to months during which time recombinations could occur. Furthermore, retroviral vector systems generally retain most of the components needed to generate a gammaretrovirus including the wild-type LTR.
The selection of the retroviral packaging cell line is an important factor in the risk of RCR development. A variety of packaging cell lines were developed in the 1980s and 1990s, and RCR was reported.4, 5, 6, 7, 8, 11, 12 Subsequent modification in packaging cell lines appear to decrease the chance of recombination and PG13 cells appears to be the preferred cell line for many investigators.10 One advantage of the PG13 cell line is that it was generated in NIH 3T3 cells, a murine-derived cell line. The GALV envelope used in PG13 cell lines is a feline-derived virus, and the receptor for the envelope is not present on murine cells. If an RCR did develop in a PG13 cell, the virus would not be expected to propagate within the culture.
Whether the finding of RCR free vector with the PG13 cell line applies to other methods and cell lines will depend on characteristics of the vector and cell line. Additional testing will require study before the safety data noted here can be extrapolated to other systems. One of the products included in this study utilized the Phoenix-GALV packaging cell line, which is based on the human HEK293 cell line. While the experience here is limited, it generated vector that was RCR free in the vector product, in transduced CD34+ cells, and in research subjects.
The US FDA requires subjects treated with retroviral products to be monitored for RCR post-infusion. Currently, there is no guidance requiring a specific assay, and the majority of investigators chose a qPCR-based assay for the viral envelope, predicting that any RCR would contain the GALV envelope. One investigator chose the S+/L− assay (without HEK293 amplification). All samples tested were negative providing further support for the safety of current retroviral packaging systems.
While all NGVB investigators within the RCR study time frame were invited to participate in this study, four did not provide data on patient follow-up and the 93 RCR assays performed for their trials were not included in this analysis. While we excluded these samples due to lack of follow-up, the cell products tested in the NGVB were all negative for RCR.
In summary, RCR has not been detected in cell products manufactured for clinical use. Participants evaluated post-infusion were also without evidence of RCR exposure. It should be noted all but one study utilized the PG13 cell line. Given the known development of RCR in other cell lines and envelopes, additional studies would be required before extending these findings to other packaging cell lines. Given this caveat, screening cell products for RCR does not add additional assurance of safety and should no longer be required when a well-characterized cell line and the resulting retroviral vector product have been successfully screened for RCR.
Materials and Methods
Collection of Study Data
The NGVB is an NIH-sponsored resource that assists gene-therapy investigators in meeting FDA-required testing (https://www.NGVBCC.org/). Only samples intended for in vivo administration were included in the analysis. Investigators with study agreements agreeing to participate were sent a list of their test articles with the dates of assay initiation and completion. Participants were asked to supply the following information: (1) production method (stable packaging cell line versus transient transduction); (2) envelope pseudotype; (3) clinical indication; (4) clinicaltrial.gov identifier; (5) if the product was administered to the subject; (6) if the clinical vector product was shown to be RCR free prior to use in the clinical trial; (7) the results of post-infusion RCR screening (if performed ≥30 days infusion); and (8) the method of post-infusion testing and the level of sensitivity of the assay. For research subjects screened for RCR after product administration, the site and method of screening was at the investigators’ discretion.
Cell Line and Positive Control Preparation
HEK293 (American Type Culture Collection [ATCC], Manassas, VA; catalog CRL-1573) and Mink GALV SEATO cells (a kind gift of M. Eiden and C. Wilson, NIH, Bethesda, MD) were maintained in D10 medium (DMEM; Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2 mM L-glutamine (Invitrogen), 1 mM sodium pyruvate, and 100 units/mL penicillin and 100 μg/mL streptomycin (Invitrogen). PG4 cells (ATCC, CRL-2032) were maintained in MyCoys 5A media with 10% FBS and 100 units/mL penicillin and 100 μg/mL streptomycin. All cell lines were maintained at 37°C and 5% CO2.
The positive control for the assay utilizes the GALV SEATO virus. The NGVB maintains this cell bank, and a vial of cells are thawed, placed in a 75-cm2 flask, and expanded over three passages into three 175-cm2 flasks. After three passages, media is changed on confluent flasks and media is harvested after approximately 24 hr and filtered through a 0.45-μm filter. The media is aliquoted into cryovials, and the virus stock is stored at ≤ –70°C for no more than 5 years. The tissue culture infective dose 50 (TCID50) of the virus stock is determined on PG4 cells.
RCR Assays
Amplification Phase
HEK293 cells are expanded to the number required based on the test articles to be assayed. On day −1, assay control flasks are prepared by plating eight 25-cm2 flasks with 2 × 106 HEK293 cells per flask; three flasks will be used for negative control and five flasks will be used for the positive control. For liquid test articles, HEK293 cells are plated in flasks of different sizes to attain a test-volume-to-HEK293-cell ratio within a specified range (Table 4). Similarly, cell test articles are plated at specific test-cell-to-HEK293-cell ratio, also shown in Table 4.
Table 4.
Passage Methodology and Ratio of Test Article to HEK293 Cells
| Test Supernatant Volume | HEK293 Cells | Flask Size | Split 1 | Split 2 | Split ≥ 3 |
|---|---|---|---|---|---|
| 1–4 mL | 2 × 106 | 25 cm2 | 1:5 25 cm2 | 1:5 25 cm2 | 1:5 25 cm2 |
| 10–15 mL | 5 × 106 | 75 cm2 | 1:5 75 cm2 | 1:5 75 cm2 | 1:5 75 cm2 |
| 30–50 mL | 1.5 × 107 | 175 cm2 | 1:10 75 cm2 | 1:5 75 cm2 | 1:5 75 cm2 |
| 60–90 mL | 2 × 107 | 300 cm2 | 1:10 175 cm2 | 1:10 175 cm2 | 1:5 75 cm2 |
| 90–120 mL | 3 × 107 | 450 cm2 | 1:10 175 cm2 | 1:10 175 cm2 | 1:5 75 cm2 |
| Test Cell Number | HEK293 Cells | Flask Size | Split 1 | Split 2 | Split ≥ 3 |
| 2 to 4 × 105 | 2 × 106 | 25 cm2 | 1:5 25 cm2 | 1:5 25 cm2 | 1:5 25 cm2 |
| 5 to 10 × 105 | 5 × 106 | 75 cm2 | 1:5 75 cm2 | 1:5 75 cm2 | 1:5 75 cm2 |
| 1.5 to 3 × 106 | 1.5 × 107 | 175 cm2 | 1:10 75 cm2 | 1:5 75 cm2 | 1:5 75 cm2 |
| 2 to 4 × 106 | 2 × 107 | 300 cm2 | 1:10 175 cm2 | 1:10 175 cm2 | 1:5 75 cm2 |
| 3 to 6 × 106 | 3 × 107 | 450 cm2 | 1:10 175 cm2 | 1:10 175 cm2 | 1:5 75 cm2 |
On day 0, medium is removed from the supernatant test article and control flasks and control and test articles are added. For the negative control, 1 mL of D10 medium is added. For the positive control, GALV SEATO virus at the TCID50 (in 1 mL of media) is added to five 25-cm2 flasks. Test articles are added to the appropriate flasks at specified ratios according to Table 4. Polybrene at a final concentration of 8 μg/mL is added to all controls and supernatant test articles. Cultures are then incubated for 2–4 hr, after which time medium is removed from control and supernatant flask, fresh medium is added, and cultures are returned to the incubator. Cells are split at a 1:5 ratio, decreasing the size of flasks at split 1 as shown in Table 4.
Cultures are maintained in log-phase growth, passaging a minimum of five times over a 3-week period. At the end of this period, a media change is performed when cells are confluent and the media is collected 24 hr after the exchange. The media is filtered through a 0.45-μm filter and either used immediately or frozen at −70°C to 80°C for later testing. Reserve samples are also stored at −70°C to 80°C in case repeat testing is required.
Indicator Phase
On days 14–15 of the amplification phase, a vial of PG4 cells are thawed and expanded. On day −1 of the assay, cells are plated in 6-well dishes with 4 mL of media and 1 × 105 cells per well. On day 0, the media is removed from the wells. There are two sets of controls. The “amplified” negative and positive controls are set up using the respective media from the corresponding amplification-phase negative and positive controls. 1 mL from the respective negative control cultures are filtered (0.45 μm) and added to two wells of PG4 undiluted. These control for adequate amplification of virus during the 3 weeks of culture.
In addition, a new set of “direct” negative controls and “direct” positive controls are evaluated using diluted GALV SEATO virus. 1 mL of media is added to three “direct” negative control wells. The “direct” positive control consists of GALV SEATO virus that is of a known potency and is plated at five serial log dilutions, with at least two dilutions below the TCID50.
For each test article culture, three wells are evaluated, two wells containing 1 mL of undiluted amplification phase media and one well containing 1 mL of amplification media diluted 1:100. Polybrene at a final concentration of 8 μg/mL is added to all control and test articles.
Cultures are incubated from 2 to 4 hr, and the media is removed and replaced with 4 mL of media. On day 2, the medium is removed from each well and replaced. On day 4, wells are inspected for foci using an inverted microscope (if wells are not confluent they are re-fed and read on days 5–6). The foci are enumerated independently by two technicians. The PG4 assay is acceptable if the following three criteria are met: (1) no foci are observed in the negative control wells; (2) foci are noted at a dilution which is 2-log more concentrated than the TCID50 dilution in the direct positive control; and (3) one or more wells of the five amplification-positive controls originally inoculated at the TCID50 contain foci. A test article is considered positive for RCR if one or more foci are detected.
Author Contributions
Conceptualization, Methodology, and Investigation, K.C., L.D.; Resources, all authors; Writing – First Draft, K.C.; Writing – Review and Editing, all authors; Funding Acquisition, K.C. (for the NGVB), C.L.M., M.L.D., K.J.C., R.P.J., J.Y.T., J.N.K., R.O., G.A., H.-P.K., S.A.R. (for clinical trial samples).
Conflicts of Interest
J.N.K. has research funding from Kite, a Gilead company, and Celgene and also holds patents on CAR T-cell technology. M.L.D. is a consultant for Celyad, Novartis, Precision Biosciences, and Servier and receives research funds from Celgene.
Acknowledgments
The authors would like to thank Sue Koop, Stephanie Baker, Lilith Reeves, Scott Witting, and the NGVB technicians involved with RCR testing. The National Gene Vector Biorepository is supported by a grant from the NHLBI (P40HL116242, PI K.C.). Additional investigator funding is as follows: J.N.K., NCI Intramural Funding; R.O., NIH/NCI support grant P30 CA008748 and NIH P01CA190174-01A1A; H.-P.K., R01CA114218; G.A., P50-CA190991 and R01-CA177476; K.J.C., the St. Baldrick's Foundation and the William Lawrence and Blanche Hughes Foundation; R.J.B., the Carson Family Charitable Trust and the Annual Terry Fox Run for Cancer Research organized by the Canada Club of New York, Kate’s Team; I.R., NCI PO1 CA008748 and NCI PO1 CA008748-T Cell Therapies.
References
- 1.Glaxo Smith Kline (2016). StrimvelisTM receives European marketing authorisation to treat very rare disease, ADA-SCID. https://us.gsk.com/en-us/media/press-releases/2016/strimvelistm-receives-european-marketing-authorisation-to-treat-very-rare-disease-ada-scid/.
- 2.U.S. Food and Drug Administration (2017). FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm581216.htm.
- 3.Cornetta K., Morgan R.A., Anderson W.F. Safety issues related to retroviral-mediated gene transfer in humans. Hum. Gene Ther. 1991;2:5–14. doi: 10.1089/hum.1991.2.1-5. [DOI] [PubMed] [Google Scholar]
- 4.Muenchau D.D., Freeman S.M., Cornetta K., Zwiebel J.A., Anderson W.F. Analysis of retroviral packaging lines for generation of replication-competent virus. Virology. 1990;176:262–265. doi: 10.1016/0042-6822(90)90251-l. [DOI] [PubMed] [Google Scholar]
- 5.Bodine D.M., McDonagh K.T., Brandt S.J., Ney P.A., Agricola B., Byrne E., Nienhuis A.W. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 1990;87:3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarpa M., Cournoyer D., Muzny D.M., Moore K.A., Belmont J.W., Caskey C.T. Characterization of recombinant helper retroviruses from Moloney-based vectors in ecotropic and amphotropic packaging cell lines. Virology. 1991;180:849–852. doi: 10.1016/0042-6822(91)90105-k. [DOI] [PubMed] [Google Scholar]
- 7.Otto E., Jones-Trower A., Vanin E.F., Stambaugh K., Mueller S.N., Anderson W.F., McGarrity G.J. Characterization of a replication-competent retrovirus resulting from recombination of packaging and vector sequences. Hum. Gene Ther. 1994;5:567–575. doi: 10.1089/hum.1994.5.5-567. [DOI] [PubMed] [Google Scholar]
- 8.Bosselman R.A., Hsu R.-Y., Bruszewski J., Hu S., Martin F., Nicolson M. Replication-defective chimeric helper proviruses and factors affecting generation of competent virus: expression of Moloney murine leukemia virus structural genes via the metallothionein promoter. Mol. Cell. Biol. 1987;7:1797–1806. doi: 10.1128/mcb.7.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller A.D., Rosman G.J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–982. 984–986, 989–990. [PMC free article] [PubMed] [Google Scholar]
- 10.Miller A.D., Garcia J.V., von Suhr N., Lynch C.M., Wilson C., Eiden M.V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J. Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chong H., Starkey W., Vile R.G. A replication-competent retrovirus arising from a split-function packaging cell line was generated by recombination events between the vector, one of the packaging constructs, and endogenous retroviral sequences. J. Virol. 1998;72:2663–2670. doi: 10.1128/jvi.72.4.2663-2670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrett E., Miller A.R.-M., Goldman J.M., Apperley J.F., Melo J.V. Characterization of recombination events leading to the production of an ecotropic replication-competent retrovirus in a GP+envAM12-derived producer cell line. Virology. 2000;266:170–179. doi: 10.1006/viro.1999.0052. [DOI] [PubMed] [Google Scholar]
- 13.Donahue R.E., Kessler S.W., Bodine D., McDonagh K., Dunbar C., Goodman S., Agricola B., Byrne E., Raffeld M., Moen R. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornetta K., Nguyen N., Morgan R.A., Muenchau D.D., Hartley J.W., Blaese R.M., Anderson W.F. Infection of human cells with murine amphotropic replication-competent retroviruses. Hum. Gene Ther. 1993;4:579–588. doi: 10.1089/hum.1993.4.5-579. [DOI] [PubMed] [Google Scholar]
- 15.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.L., Fraser C.C., Cavazzana-Calvo M., Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 16.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 17.Stein S., Ott M.G., Schultze-Strasser S., Jauch A., Burwinkel B., Kinner A., Schmidt M., Krämer A., Schwäble J., Glimm H. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 18.Ott M.G., Schmidt M., Schwarzwaelder K., Stein S., Siler U., Koehl U., Glimm H., Kühlcke K., Schilz A., Kunkel H. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 19.Boztug K., Schmidt M., Schwarzer A., Banerjee P.P., Díez I.A., Dewey R.A., Böhm M., Nowrouzi A., Ball C.R., Glimm H. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun C.J., Boztug K., Paruzynski A., Witzel M., Schwarzer A., Rothe M., Modlich U., Beier R., Göhring G., Steinemann D. Gene therapy for Wiskott-Aldrich syndrome—long-term efficacy and genotoxicity. Sci. Transl. Med. 2014;6:227ra33. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 21.Cornetta K., Wilson C.A. Safety of retroviral vectors: regulatory and technical considerations. In: Dropulic B., Carter B.J., editors. Concepts in genetic medicine. John Wiley & Sons, Inc.; 2008. pp. 277–288. [Google Scholar]
- 22.U.S. Food and Drug Administration (2006). Guidance for Industry: Supplemental Guidance on Testing for Replication Competent Retrovirus in Retroviral Vector Based Gene Therapy Products and During Follow-up of Patients in Clinical Trials Using Retroviral Vectors. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM078723.pdf. [DOI] [PubMed]
- 23.U.S. Food and Drug Administration (1998). Guidance for Human Somatic Cell Therapy and Gene Therapy. https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm081670.pdf.
- 24.Printz M., Reynolds J., Mento S.J., Jolly D., Kowal K., Sajjadi N. Recombinant retroviral vector interferes with the detection of amphotropic replication competent retrovirus in standard culture assays. Gene Ther. 1995;2:143–150. [PubMed] [Google Scholar]
- 25.Miller A.D., Bonham L., Alfano J., Kiem H.P., Reynolds T., Wolgamot G. A novel murine retrovirus identified during testing for helper virus in human gene transfer trials. J. Virol. 1996;70:1804–1809. doi: 10.1128/jvi.70.3.1804-1809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forestell S.P., Dando J.S., Böhnlein E., Rigg R.J. Improved detection of replication-competent retrovirus. J. Virol. Methods. 1996;60:171–178. doi: 10.1016/0166-0934(96)02052-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen J., Reeves L., Cornetta K. Safety testing for replication-competent retrovirus associated with gibbon ape leukemia virus-pseudotyped retroviral vectors. Hum. Gene Ther. 2001;12:61–70. doi: 10.1089/104303401450979. [DOI] [PubMed] [Google Scholar]
- 28.Duffy L., Koop S., Fyffe J., Cornetta K. Extended S+/L− assay for detecting replication competent retroviruses (RCR) pseudotyped with the RD114 viral envelope. Preclinica. 2003 May–June, 53–59. [Google Scholar]
- 29.Bear A.S., Morgan R.A., Cornetta K., June C.H., Binder-Scholl G., Dudley M.E., Feldman S.A., Rosenberg S.A., Shurtleff S.A., Rooney C.M. Replication-competent retroviruses in gene-modified T cells used in clinical trials: is it time to revise the testing requirements? Mol. Ther. 2012;20:246–249. doi: 10.1038/mt.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z., Blair M., Thorner L. PG-4 cell plaque assay for xenotropic murine leukemia virus. J. Virol. Methods. 1999;81:47–53. doi: 10.1016/s0166-0934(99)00064-6. [DOI] [PubMed] [Google Scholar]
- 31.Horn P.A., Topp M.S., Morris J.C., Riddell S.R., Kiem H.P. Highly efficient gene transfer into baboon marrow repopulating cells using GALV-pseudotype oncoretroviral vectors produced by human packaging cells. Blood. 2002;100:3960–3967. doi: 10.1182/blood-2002-05-1359. [DOI] [PubMed] [Google Scholar]
- 32.Cornetta K., Duffy L., Turtle C.J., Jensen M., Forman S., Binder-Scholl G., Fry T., Chew A., Maloney D.G., June C.H. Absence of Replication-Competent Lentivirus in the Clinic: Analysis of Infused T Cell Products. Mol. Ther. 2018;26:280–288. doi: 10.1016/j.ymthe.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schambach A., Zychlinski D., Ehrnstroem B., Baum C. Biosafety features of lentiviral vectors. Hum. Gene Ther. 2013;24:132–142. doi: 10.1089/hum.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pear W.S., Nolan G.P., Scott M.L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]