MHCII+/CD206− (M1) and MHCII−/CD206+ (M2) constitute two distinct populations of macrophages that are in close apposition to the cholinergic neurons in the rat gastric myenteric plexus (MP). Abdominal surgery (6 h) activates M1 macrophage leading to inflammation in the gastric MP correlated with the delayed gastric emptying, which was abolished by central vagal stimulation via intracisternal injection of RX-77368. Vagal stimulation linked with the cephalic phase may have potential beneficial effects to curtail postoperative gastric ileus.
Keywords: abdominal surgery, central vagal activation, gastric emptying, M1 and M2 macrophages, TRH agonist
Abstract
Inflammation plays a role in abdominal surgery (AS)-induced intestinal ileus that is alleviated by electrical vagal stimulation. Intracisternal injection of RX-77368, the stable thyrotropin-releasing hormone agonist, activates dorsal motor nucleus neurons and gastric vagal efferent discharges. We investigated the gastric inflammation induced by AS and the modulation by intracisternal RX-77368 in rats. RX-77368 (50 ng/rat) or saline was injected followed, 1 h later, by laparotomy and small intestinal/cecal manipulation. The sham group had anesthesia alone. After 6 h, gastric emptying (GE) and the inflammation in gastric corpus were determined. AS inhibited GE by 72% vs. control and doubled the number of M1-like macrophage immunoreactive for major histocompatibility complex class II (MHCII; M1 marker) but not for cluster of differentiation 206 (CD206; M2 marker) (MHCII+/CD206−) while there was no change in M2-like macrophages (MHCII−/CD206+). AS increased mRNA levels of interleukin-1β (IL-1β) and tumor necrosis factor α (TNF-α) by 1.7- and 1.5-fold, respectively, in the gastric submucosa plus muscle layers and the infiltration of neutrophils labeled by myeloperoxidase by 9.5-fold in the muscularis externa. RX-77368 inhibited AS-related gastric changes while not altering these parameters in the sham group. There was a significant negative correlation between GE and IL-1β (r = −0.46), TNF-α (r = −0.44), M1 macrophage (r = −0.82), and neutrophils (r = −0.91). The M2-like macrophages and IL-10 expression were unchanged by AS with intracisternal saline or RX-77368. These data indicate that AS activates gastric M1 macrophages and increases proinflammatory cytokines expression, which are prevented by central vagal activation and may contribute to the correlated dampening of postoperative gastric ileus.
NEW & NOTEWORTHY MHCII+/CD206− (M1) and MHCII−/CD206+ (M2) constitute two distinct populations of macrophages that are in close apposition to the cholinergic neurons in the rat gastric myenteric plexus (MP). Abdominal surgery (6 h) activates M1 macrophage leading to inflammation in the gastric MP correlated with the delayed gastric emptying, which was abolished by central vagal stimulation via intracisternal injection of RX-77368. Vagal stimulation linked with the cephalic phase may have potential beneficial effects to curtail postoperative gastric ileus.
convincing evidence indicates that intestinal manipulation during abdominal surgery (AS) initiates a local inflammation within the intestinal muscularis externa that plays a role in intestinal ileus (10, 21, 22, 57). In particular, this was supported by the demonstration that chemical inactivation or genetic depletion of the intestinal muscularis macrophage network prevents intestinal inflammation and postoperative ileus (POI) in rodents (57). However so far, macrophages and inflammatory response induced by AS and their role in the ileus have been studied primarily in the intestine while rarely investigated in the stomach (7, 43). Importantly, little is known about the phenotypes and distribution of macrophages in the gastric myenteric plexus. Macrophages encompass the classical (M1) and alternative (M2) phenotypes that are thought to be at the extremes and generated in function of cues in the microenvironment (2, 32, 44). M1 macrophages are typically characterized by the expression of proinflammatory cytokines such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), inducible nitric oxide (NO) synthase, and major histocompatibility complex class II (MHCII) molecules. M2 macrophages are identified by their signature expression of a variety of markers, including arginase-1, mannose receptor [also known as cluster of differentiation 206 (CD206)], and anti-inflammatory cytokines such as IL-10 and transforming growth factor-β (27).
The identification of intestinal inflammation as a relevant mechanism contributing to POI, opened new venues for therapeutic strategies (3, 45, 54). Of importance was the identification of a vagus nerve-based inflammatory reflex in which the efferent arm encompasses the cholinergic anti-inflammatory properties of the vagus (4, 52). It is well established that brain medullary thyrotropin-releasing hormone (TRH)-receptor 1 (TRH-R1) signaling located in neurons of the dorsal motor nucleus of the vagus plays a physiological role in the brain stimulation of gastric vagal efferent activity and thereby contributes to the vagal regulation of gastric secretory-motor function including during the cephalic phase (31, 50, 59). However, whether central vagal activation modulates a gastric inflammatory response is still unknown. Previously, we showed that the activation of TRH-R1 signaling in the brainstem by acute cold exposure or intracisternal injection of TRH or the stable TRH agonist RX-77368 prevented postoperative gastric ileus (POGI) in rats as monitored 90 min post-AS (29, 47). This was indicative of an interaction between central vagal stimulation and the prevention of POGI.
In the present study we first assessed the spatial configuration and the distribution of M1 and M2 macrophages and their relationship with cholinergic neurons in the gastric myenteric plexus that are so far largely unexplored. We then examined whether AS induces gastric inflammation and the activation states of macrophages (M1/M2) in the gastric myenteric plexus at 6 h postsurgery. Lastly, we investigated whether central vagal activation by intracisternal RX-77368, as a pretreatment influences AS-induced gastric M1 activation, inflammatory markers, and delayed gastric emptying.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (Harlan, San Diego, CA) weighing 280–310 g were housed under controlled conditions of temperature (22–24°C) and light (from 6:00 am to 6:00 pm) for at least 1 wk before the experiments. Animals had free access to Purina chow (Prolab RMH 2500; PMI Nutrition International, Brentwood, MO) and tap water. Rats were deprived of food, but not water, for 16 h before the start of treatment. At the end of experiments, rats were euthanized by CO2 inhalation. All experimental protocols (No. 05058-02) were approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Greater Los Angeles Healthcare System that is under the auspices of the Office of Laboratory Animal Welfare Assurance of Compliance (A3002-01) in accordance with National Institutes of Health guidelines.
Intracisternal injection.
Fasted rats (n = 22) were anesthetized with isoflurane (2 to 3% vapor concentration in oxygen; Ethrane; Ohmeda Pharmaceutical, Liberty Corner, NJ) and mounted on ear bars of a Kopf stereotaxic frame (Model 900) as previously described (59). The atlanto-occipital membrane was punctured using a Hamilton microliter syringe, and confirmation of needle placement into the cisterna magna was assessed by aspirating cerebrospinal fluid into the syringe. The volume of intracisternal injection was 10 µl. Animals recovered from anesthesia within 2 to 3 min postinjection.
Abdominal/sham surgery.
AS was performed as previously described (21) with some modifications. Rats were anesthetized with isoflurane, the abdomen was shaved, and the area was treated with 70% alcohol (Fisher Scientific) followed by the topical povidone-iodine Dynarex (Mfg. Part No. 36532301) antiseptic. After a median laparotomy (3 to 4 cm), the cecum was exteriorized, placed in saline-soaked gauze, and gently manipulated between two fingers for 3 min. The small intestine was then exteriorized and ran throughout its entire length for 5 min with compression applied by a sterile cotton applicator moistened with saline using a strength similar to writing pressure. Thereafter, the small intestine and cecum covered with gauze soaked in saline remained exteriorized for an additional 5 min before being placed back into the abdominal cavity. The incision was stitched in layers with 4-0 Coated VICRYL Plus Antibacterial (polyglactin 910) suture (Medline Industries, Broadview, IL). Anesthesia and surgery lasted for ~20 min and were conducted between 9:00 to 11:00 am. Sham surgery consisted of exposing rats to the same duration of anesthesia as the AS groups but without surgery, thereby avoiding any possible confounding influence of anesthesia. Afterward, animals were housed singly without access to food or water for the subsequent 6-h experimental period.
Immunostaining of resident macrophages and cholinergic neurons in the gastric myenteric plexus of naïve rats.
The gastric corpus was harvested from two naïve rats and processed to obtain longitudinal muscle/myenteric plexus (LMMP) preparation. Double immunostaining was then performed to label macrophages with 1) MHCII and CD206 (24); 2) ED2, a marker for resident macrophages (12, 40) and cuprolinic blue, a neuronal marker (17); and 3) ED2 and the cholinergic neuronal marker peripheral choline acetyltransferase (pChAT) (35).
Effects of central vagal stimulation and AS on gastric emptying, macrophages, and cytokines.
Rats received intracisternal injection with either saline or RX-77368 [pGlu-His-(3,3′-dimethyl)-Pro-NH2] (50 ng/rat; Ferring Pharmaceuticals, Feltham, Middlesex, UK; dissolved in saline) and 1 h later, each group underwent either AS (6 to 7 rats /group) or sham operation (4 to 5 rats/group). The dose of RX-77368 was based on our previous dose-response studies showing maximal vagal activation of gastric efferent discharges and vagal cholinergic-dependent stimulation of gastric motor function lasting 1 to 2 h postinjection (26, 37). At 6 h postsurgery, all groups were euthanized to determine: gastric emptying of nonnutrient viscous solution, the number of macrophages immunolabeled by MHCII+/CD206− (M1 like) and MHCII−/CD206+ (M2 like) in gastric corpus LMMP, myeloperoxidase (MPO)-positive cells in whole mount gastric corpus muscularis externa, and cytokine mRNA levels (IL-1β, TNF-α, and IL-10) in gastric corpus submucosal plus muscle layers.
Determination of gastric emptying.
The gastric emptying of a nonnutrient viscous solution was determined by the phenol red/methyl cellulose method as described in our previous studies (47). Briefly, 20 min before the end of the 6-h postsurgery, all groups received a 1.5-ml orogastric gavage of a nonnutrient viscous solution consisting of 0.05% phenol red mixed with 1.5% methylcellulose (Sigma-Aldrich, St. Louis, MO) and were euthanized 20 min later. The abdominal cavity was opened, and gastric pylorus and cardia were clamped. The stomach was removed, opened along the great curvature, and flushed three times with 100 ml of 0.1 N NaOH. The suspension settled for 1 h at room temperature, and 5 ml of the supernatant were then added to 0.5 ml of 20% trichloroacetic acid (wt/vol; Sigma-Aldrich). The solution was centrifuged at 4°C for 20 min at 3,000 rpm, and 3 ml of supernatant were mixed with 4 ml of 0.5 N NaOH. Phenol red recovered from the stomach of the rat euthanized immediately after gavage of the solution served as a standard. The absorbance of the samples was determined at a wavelength of 560 nm (Shimadzu 260 spectrophotometer, Kyoto, Japan). Gastric emptying was calculated as percent emptying = (1 absorbance of test sample/absorbance of standard) × 100.
Cells labeling in whole mount gastric corpus preparations.
The gastric corpus was dissected and rinsed with PBS prepared with 0.1% diethylpyrocarbonate-treated water. The anterior wall was placed in PBS containing nifedipine (10−6 M) to avoid muscle contraction, pinned flat in a Sylgard-coated petri dish, and fixed overnight in 0.1 M sodium phosphate buffer (pH 7.4) containing 4% paraformaldehyde and 14% picric acid. After being rinsed in PBS, each tissue sample was divided into two parts and processed, respectively, for whole mount preparations of the muscularis externa and LMMP as described previously (31, 59). Briefly, the mucosa was scraped off, the submucosa was peeled, and the muscularis externa was collected for MPO staining. The circular muscle was further removed using fine forceps, and LMMP whole mount preparation including the myenteric plexus adhering to the longitudinal muscle was used for immunohistochemistry.
Double labeling with MHCII and CD206 was performed on LMMP whole mount preparations of gastric corpus. After the free-floating preparations were washed three times at 10-min intervals with PBS, they were incubated in 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA) in 0.3% Triton-X 100/PBS (PBS-T) for 30 min at room temperature. This was followed by an overnight incubation with the mixture of a mouse antibody against rat MHCII (1:200; AbD Serotec, Raleigh, NC), a marker for M1 macrophages (24), and a rabbit antibody against CD206 (1:1000; Abcam, Cambridge, MA), a marker for M2 macrophages (24, 28). Whole mount LMMP preparations were then washed with PBS three times at 10-min intervals and incubated for 2 h at room temperature with the mixture of Rhodamine Red-X-conjugated donkey anti-mouse IgG and FITC-conjugated donkey anti-rabbit IgG (1:100. Jackson ImmunoResearch). After being washed three times in PBS, preparations were mounted on slides (Superfrost/Plus; Fisher Scientific, Pittsburgh, PA) with anti-fade mounting media (Vector, Burlingame, CA). To minimize variability in staining, immunohistochemical detection in the whole mount preparations from all animals was processed simultaneously as a single batch. Negative controls were performed without primary antibodies. The macrophages labeled with MHCII but not CD206 (MHCII+/CD206−) or CD206 but not MHCII (MHCII−/CD206+) were counted in 20 optical fields/rat (475 × 475 μm with one myenteric ganglion/field) under ZEISS Axioskop 2 plus upright microscope with epifluorescence (Carl Zeiss Microscopy; Thornwood, NY).
In the gastric LMMP preparations from the gastric corpus, we then characterize myenteric neurons using a slightly modified cuprolinic blue staining (17) as we previously detailed (59) combined with immunohistochemistry for ED2. Briefly, free-floating gastric LMMP whole mount preparations were incubated in 0.3% H2O2 in PBS to remove the activity of endogenous peroxidase followed by a wash with PBS and 0.05 M sodium acetate buffer (pH 5.6). The preparations were then stained for 2 h at 42°C in cuprolinic blue (0.3% quinolinic phthalocyanine in 0.05 M sodium acetate-1.0 M magnesium chloride buffer, pH 4.9; Electron Microscopy Sciences, Fort Washington, PA). After being washed in PBS, preparations were incubated in 10% normal donkey serum (Jackson ImmunoResearch) in PBS-Tween-20 (Sigma-Aldrich) for 30 min at room temperature. This was followed by an overnight incubation at 4°C with a mouse monoclonal antibody ED2 (1:200; AbD Serotec), and then a 1-h incubation at room temperature with biotinylated donkey anti-mouse secondary antibody (1:1,000; Jackson ImmunoResearch). After incubation with the standard biotin-avidin-horseradish peroxidase complex, preparations were processed for coloration in presence of 3,3′-diaminobezidine tetrahydrochloride and hydrogen peroxide. The myenteric neurons were stained as light blue color while the macrophages were labeled as brown color. Immunohistochemical controls were routinely performed following the same procedure except that the primary antibody was replaced by PBS-T.
Additionally, we performed double labeling with ED2 and pChAT in gastric corpus LMMP whole mount preparations as previously detailed (59). Free-floating preparations were incubated overnight for 5 nights at 4°C with the mixture of mouse monoclonal antibody ED2 (1:200; AbD Serotec) and pChAT rabbit antibody (1:4,000; gift from Dr. H. Kimura and J. P. Bellier, Shiga University of Medical Science, Otsu, Japan). The mixture of secondary antibodies and double immunostaining procedure were used as described above.
MPO histochemistry.
Free-floating gastric muscularis externa whole mount preparations from the corpus were cut into 0.5 × 1 cm pieces and used for MPO histochemistry procedures as described (22). Briefly, freshly prepared whole mounts were stained with a mixture of 15 mg Hanker-Yates reagent (Polysciences, Warrington, PA), 10 ml 0.1 M Tris·HCl (pH 7.6), and 100 μl 1% hydrogen peroxide (Sigma-Aldrich) for 10 min. MPO-positive cells were counted under the Zeiss Axioskop 2 plus upright microscope (Carl Zeiss Microscopy) in five randomly chosen fields (340 × 260 µm/field) in each specimen at a magnification of ×200 .
Cytokine determinations by RT-qPCR in gastric submucosa plus muscle layers.
The posterior wall of gastric corpus was separated into mucosa and the submucosa plus muscle layers, and tissues were snapped frozen in dry ice and stored at −80°C until processed for RT-qPCR as in our previous studies (58). Total RNA was extracted using RNA-Bee (TEL-TEST) according to manufacturer’s recommended protocol and then followed by DNAse-I treatment. cDNA was synthesized in a total volume of 20 μl reaction by using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Briefly, total RNA (2 µg) was denatured at 65°C for 5 min and then followed by the procedures of the reverse transcription that was stopped by incubation at 85°C for 5 min. The reaction mixture was then incubated with RNase H at 37°C for 30 min to remove total RNA template. Real-time quantitative PCR for rat IL1β, TNF-α, and IL10 was performed in duplicates using StepOnePlus Real-Time PCR System (Applied Biosystems) in a 25-μl reaction volume. The optimized reaction contained 12.5 μl of SABiosciences RT2 SYBR Green ROX qPCR MasterMix (Qiagen, Valencia, CA), 0.5 μl each of oligonucleotide primers (10 μM), 1 μl of the cDNA synthesis reaction, and 10.5 μl of H2O. Selected forward and reverse primers are listed in Table 1. The housekeeping gene rat GAPDH was also analyzed as an internal control. Thermal conditions were as follows: 95°C for 4 min followed by 40 cycles of 95°C for 10 s, 57°C for 10 s, and 72°C for 20 s with a final extension cycle of 10 min at 72°C. The specificity of the amplification reaction was determined by performing a melting curve analysis of the PCR fragments. Each target gene was normalized with GAPDH and calculated using the comparative ΔΔCt method. Results were calculated by the equation 2–ΔΔCt and expressed as fold change in reference to the control group as in our previous studies (58).
Table 1.
Primers used for real-time PCR
| Gene | Forward | Reverse | Size, bp |
|---|---|---|---|
| IL-1β | CAGGAAGGCAGTGTCACTCA | AGACAGCACGAGGCATTTTT | 232 |
| TNF-α | CCTCACACTCAGATCATCTTCTCA | GTGGGTGAGGAGCACATAG | 237 |
| IL-10 | CCTGCTCTTACTGGCTGGAG | TGTCCAGCTGGTCCTTCTTT | 161 |
| GAPDH | AGACAGCCGCATCTTCTTGT | TGATGGCAACAATGTCCACT | 142 |
Statistics analysis.
Data are expressed as means ± SE. Changes in the number of macrophages; mRNA levels of IL-1β, TNF-α, and IL-10; infiltration of neutrophils; and gastric emptying induced by intracisternal saline or RX-77368 plus AS compared with intracisternal saline/sham group were analyzed by one-way ANOVA followed by Dunnett’s post hoc comparisons or two-way ANOVA with intracisternal pretreatment and AS fixed factors followed by post hoc comparisons using Bonferroni’s method. Correlations between gastric emptying and parameters for M1 and M2 macrophages were performed using the Pearson correlation coefficient and simple linear regression. Differences between groups were considered significant when P < 0.05.
RESULTS
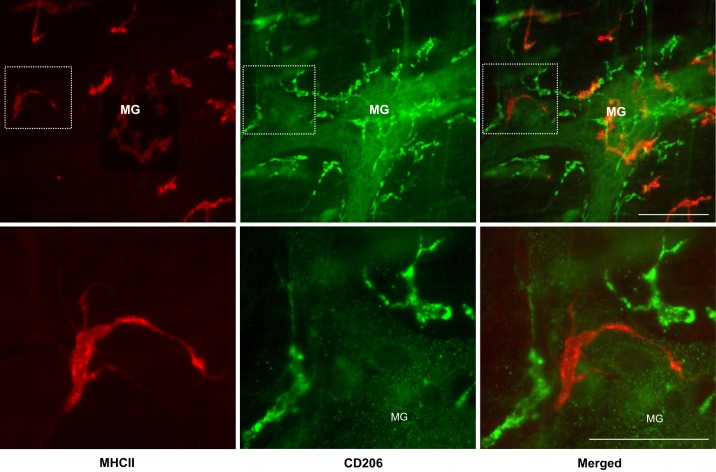
First, we assessed macrophage phenotype distribution in gastric corpus myenteric plexus of naïve rats. Double immunostaining showed that marker antibodies MHCII for M1 (24) and CD206 for M2 (24, 28) distinctively labeled two populations of resident macrophages at the level of gastric corpus myenteric plexus: macrophages immunoreactive for MHCII but not for CD206 (MHCII+/CD206−) and those immunoreactive for CD206 but not for MHCII (MHCII−/CD206+) with rarely double-labeled macrophages (MHCII+/CD206+) (Fig. 1, top). The MHCII+/CD206− macrophages displayed often a single thread-like filament projecting away from the cell body. They were distributed on or around ganglia in a highly variable or patchy pattern. By contrast, MHCII−/CD206+ showed relatively ramified processes with spines at opposing poles of a macrophage's soma (Fig. 1, bottom), and they also often outlined the perimeter of ganglia (Fig. 1, top).
Fig. 1.
Double immunostaining of major histocompatibility complex class II (MHCII; red) and cluster of differentiation 206 (CD206; green) in gastric myenteric plexus of naïve rats. Bottom: high magnification of insets at top. Two populations of macrophages MHCII+/CD206− (M1 like) and MHCII−/CD206+ (M2 like) were distinctively labeled by marker antibodies without overlap on the merged image. The former labeled as red color distributed on or around ganglia in a highly variable or patchy pattern while the latter labeled as green color often outlined the perimeter of ganglia. MG, myenteric ganglia. Bars = 100 µm.
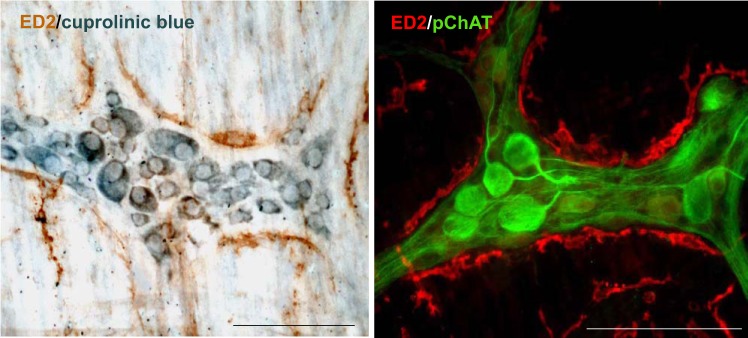
Double staining of cuprolinic blue and ED2 showed that the individual cytoplasm of myenteric neurons stained with cuprolinic blue was clearly defined with the blue-stained Nissl substance forming a ring around the unstained nucleus while the dendrites and processes of the neurons lacked staining. The myenteric ganglia were outlined by macrophages labeled by ED2 as brown color (Fig. 2, left). No immunoreactivity was observed with the omission of ED2 antibody (data not shown). The gastric cholinergic myenteric neurons and their processes labeled immunohistochemically by antibody against pChAT are in line with macrophages labeled by ED2. Both appeared to contact morphologically under ×200 magnification in the whole mount preparations of myenteric plexus (Fig. 2, right).
Fig. 2.
Left: double staining of ED2 (macrophage marker) with cuprolinic blue (neuronal marker); right: ED2 with peripheral choline acetyltransferase (pChAT; a marker for peripheral cholinergic neurons) in the gastric myenteric plexus of naïve rats. Macrophages labeled by ED2 as brown color often outlined the perimeter of ganglia, and myenteric neuron cell bodies were labeled as blue color (left). The soma and processes of the cholinergic neurons labeled by pChAT as green color conform to macrophages labeled as red color, and both appeared to be in close apposition or contact morphologically (right) in the whole mount preparations of myentric plexus. Bar = 100 µm.
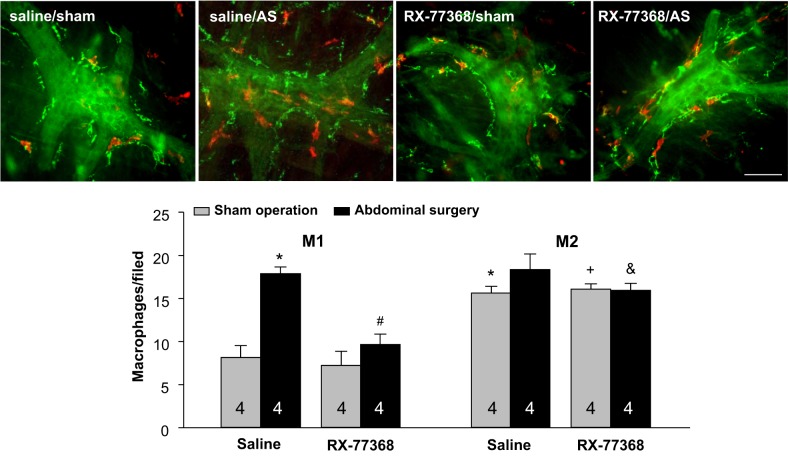
We then assessed whether AS and central vagal activation influence the number of MHCII+/CD206− and MHCII−/CD206+ macrophages in the gastric myenteric plexus (Fig. 3, top). In the intracisternal saline/sham group, the number of MHCII−/CD206+ macrophages (green color) is significantly higher than MHCII+/CD206− macrophages (red color) with a ratio 1.9:1 (15.6 ± 0.8 vs. 8.2 ± 0.3/field, P < 0.001). In intracisternal saline-treated rats, AS induced a significant 2.2.-fold increase in the number of MHCII+/CD206− macrophages compared with saline intracisternal/sham group at 6 h post-AS (18.9 ± 0.8 vs. 8.2 ± 0.3/field, P < 0.001) while there was no change in the number of MHCII−/CD206+ macrophages by AS. Treatment with the stable TRH analogue RX-77368 (50 ng ic) 1 h before AS completely blocked the AS-induced increased number of MHCII+/CD206− macrophages (RX-77368/AS: 9.7 ± 1.2 vs. saline/AS: 18.9 ± 0.8 /field, P < 0.001) while not influencing the number of these macrophages in the sham group (RX-77368/sham: 7.2 ± 0.5 vs. saline/sham: 8.2 ± 0.3/field in saline/sham group) (Fig. 3, bottom). A two-way ANOVA indicated a significant effect of both intracisternal RX-77368 over saline in AS groups [F(1,15) = 34.9, P < 0.001] and the AS over sham operation in intracisternal saline groups [F(1,15) = 61.6, P < 0.001] on the number of MHCII+/CD206− macrophages. The interaction between intracisternal injection and surgery was also significant [F(1,15) = 22.0, P < 0.001]. No significant changes were observed for MHCII−/CD206+ cell number by either AS or RX-77368 (Fig. 3).
Fig. 3.
Intracisternal injection of RX-77368 abolished abdominal surgery (AS)-induced increase in the number of MHCII+/CD206− (M1 like) macrophages in the gastric myenteric plexus assessed 6-h postsurgery. Top: double staining of MHCII (red) and CD206 (green) in rats treated with intracisternal saline (10 µl, −1 h)/sham operation; intracisternal saline/AS; intracisternal RX-77368 (50 ng/rat, −1 h)/sham operation; and intracisternal RX-77368 (50 ng/rat, −1 h)/AS. Bar = 100 µm. Bottom: quantitative analysis of macrophages counted in 20 optical fields/rat (475 × 475 μm/field) with each field containing a ganglium. M1, MHCII+/CD206−; M2, MHCII−/CD206+. Each column represents means ± SE of no. of rats indicated at the bottom. *P < 0.05 vs. M1 in saline/sham; #P < 0.05 vs. M1 in saline/AS group; +P < 0.05 vs. M1 in RX-77368/sham group; &P < 0.05 vs. M1 in RX-77368/AS group.
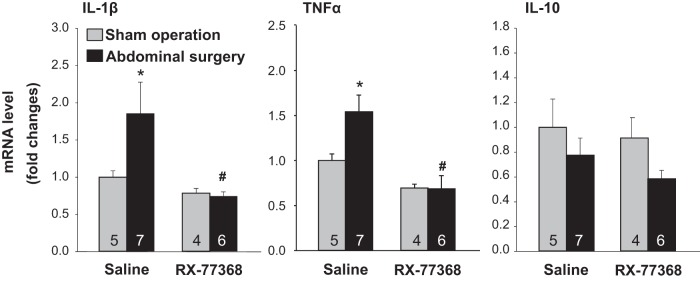
We next assessed whether cytokine markers for M1 and M2 activation were altered in the gastric submucosa plus muscle layers by AS and modulated by intracisternal RX-77368. Real-time quantitative PCR showed that AS significantly increased mRNA levels of the proinflammatory cytokines IL-1β and TNF-α (1.7- and 1.5-fold, respectively) compared with intracisternal saline/sham group at 6 h post-AS (Fig. 4). RX-77368 injected intracisternally 1 h before AS completely blocked AS-induced upregulation of IL-1β and TNF-α expression while not altering IL-1β and TNF-α mRNA levels in the sham group (Fig. 4). Two-way ANOVA indicated a significant influence of intracisternal RX-77368 compared with saline on IL-1β or TNF-α mRNA levels in AS groups [F(1,21) = 8.2, P < 0.01 and F(1,21) = 16.2, respectively, P < 0.001] and surgery over sham operation in intracisternal saline groups [F(1,21) = 3.6, P < 0.05 and F(1,12) = 3.4, P = 0.05, respectively]. The interaction between intracisternal injection and surgery was also significant [F(1,15) = 4.3, P < 0.05 and F(1,12) = 3.6, P < 0.05]. The IL-10 mRNA level was not changed by either AS or intracisternal RX-77368 (Fig. 4).
Fig. 4.
Intracisternal injection of RX-77368 inhibited AS-induced upregulation of IL-1β and TNF-α mRNA in the gastric submucosal plus muscle layers at 6 h postsurgery detected by real-time RT-qPCR. Each target gene was normalized with GAPDH and calculated using the comparative ΔΔCt method. Results calculated by the equation 2–ΔΔCt were expressed as fold change in reference to the control group. Each column represents means ± SE of no. of rats indicated at the bottom. *P < 0.05 vs. saline/sham; #P < 0.05 vs. saline/AS group.
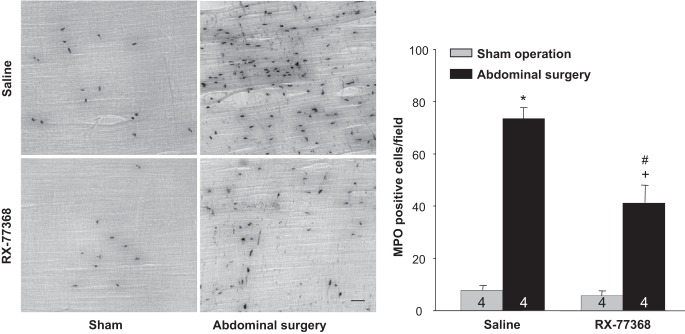
To assess further the gastric inflammatory response induced by AS with intracisternal saline or RX-77368 pretreatment, we determined MPO by histochemistry in the gastric muscularis externa (Fig. 5, left). Only a small number of positive MPO-stained cells were found distributed in the gastric muscularis externa of sham groups treated with either intracisternal saline or RX-77368. However, AS led to a significant increase in neutrophils extravasation in the gastric muscularis externa at 6 h postsurgery that was diminished by the intracisternal injection of RX-77368 1 h before AS (Fig. 5, left). The neutrophil counts indicated a 9.4-fold increase of recruited neutrophils in intracisternal saline/AS group over the intracisternal saline/sham group (73.6 ± 4.1 vs. 7.8 ± 1.9 MPO-positive cells/field, P < 0.001). The TRH agonist significantly reduced the increase in neutrophil counts in response to AS by 51% (41.3 ± 2.4 vs. 73.6 ± 4.1 MPO-positive cells/field in RX-77368/AS vs. saline/AS, P < 0.001) even though values are still significant higher than the RX-77368/sham group (41.3 ± 2.4 vs. 5.7 ± 1.9 MPO-positive cells/field, P < 0.001). No significant difference was found in the sham groups with intracisternal saline or RX-77368 (Fig. 5, right). A two-way ANOVA indicated a significant effect of both intracisternal RX-77368 over saline in AS groups [F(1,15) = 39.1, P < 0.001] and AS over sham operation in intracisternal saline groups [F(1,15) = 339.3, P < 0.001] on the number of MPO positively stained cells. The interaction between intracisternal injection and surgery was also significant [F(1,15) = 30.3, P < 0.001].
Fig. 5.
Intracisternal injection of RX-77368 decreased AS-induced neutrophils infiltration in the gastric muscularis externa (ME) at 6 h postsurgery. Left: myeloperoxidase (MPO) histochemistry was performed on whole mount preparations of the gastric muscularis externa from rats treated with intracisternal saline (10 µl, −1 h)/sham operation; intracisternal saline/AS; intracisternal RX-77368 (50 ng/rat, −1 h)/sham operation; and intracisternal RX-77368 (50 ng/rat, −1 h)/AS. Bar = 100 µm. Right: MPO-positive cells were counted under the microscope in 5 randomly chosen fields (340 × 260 µm/field) in each specimen at a magnification of ×200. Each column represents means ± SE of no. of rats indicated at the bottom. *P < 0.05 vs. saline/sham; #P < 0.05 vs. saline/AS group. +P < 0.05 vs. RX77368/sham group.
We examined functional consequence of intracisternal RX-77368 anti-inflammatory effect on POGI. In rats treated with intracisternal saline, AS significantly delayed gastric emptying of a viscous nonnutrient solution compared with the sham group (20.8 ± 3.2 vs. 74.9 ± 3.2%, P < 0.001), which represents a 72% inhibition of gastric transit still maintained at 6 h after AS. Treatment with intracisternal RX-77368 (50 ng/rat) 1 h before AS reduced significantly the AS-induced delayed gastric emptying (RX-77368/AS: 46.3 ± 4.9% vs. saline/AS: 20.8 ± 3.2%, P < 0.01; vs. saline/sham: 74.9 ± 3.2%, P < 0.01) although there was still a remaining significant 38% inhibition of gastric emptying. In the sham group, intracisternal injection of RX-77368 did not significantly influence gastric emptying compared with saline (78.5 ± 5.4 vs. 74.9 ± 3.2%, P < 0.01) (Fig. 6). Two-way ANOVA indicated a significant influence of intracisternal injection [F(1,21) = 4.5, P < 0.05] and surgery [F(1,21) = 68.5, P < 0.001].
Fig. 6.
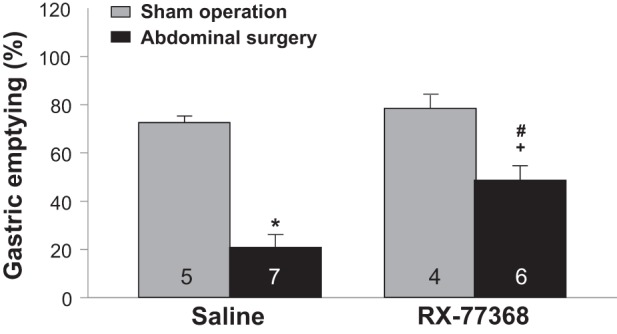
Intracisternal injection of RX-77368 dampened postoperative gastric ileus. Gastric emptying of a viscous nonnutrient solution was monitored during the 5:40-6:00-h period after AS or sham operation in rats injected intracisternally with saline (10 µl, −1 h) or intracisternal RX-77368 (50 ng/rat, −1 h). Each column represents means ± SE of no. of rats indicated. *P < 0.05 vs. saline/sham group; #P < 0.05 vs. saline/AS group; +P < 0.05 vs. RX-7736/sham group.
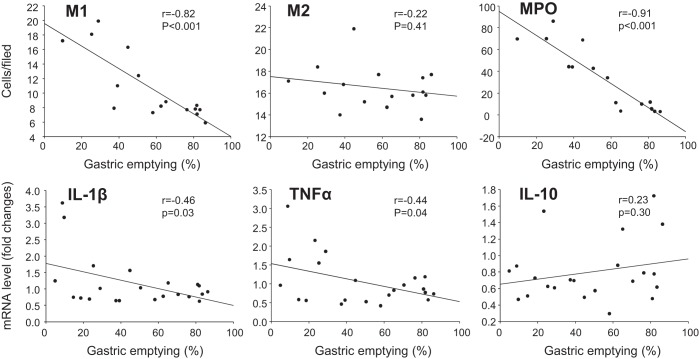
We then assessed the correlations between gastric emptying with parameters for M1 macrophages and inflammation. The Pearson correlation test revealed a significant negative correlation between gastric emptying and MHCII+/CD206− macrophages (r = −0.82, P < 0.001) in the gastric myenteric plexus, MPO-positive cells (r = −0.91, P < 0.001) in the muscularis externa, and mRNA levels of IL-1β (r = −0.46, P < 0.03) and TNF-α (r = −0.44, P < 0.05) in the gastric submucosa plus muscle layer. No significant correlations were found between gastric emptying and MHCII−/CD206+ macrophages (r = −0.22, P = 0.41) or IL-10 (r = 0.23, P = 0.30) (Fig. 7).
Fig. 7.
Correlations between gastric emptying with parameters for M1 and M2 macrophages. The Pearson correlation coefficient and simple linear regression were performed between gastric emptying with M1 (MHCII+/CD206−) or M2 (MHCII−/ CD206+) macrophages in the gastric myenteric plexus, MPO-positive cells in the muscularis externa, and mRNA levels of IL-1β, TNF-α, and IL-10 in the gastric submucosa plus muscle layer. P < 0.05 was considered significant.
DISCUSSION
In the present study, we identified M1 vs. M2 macrophage phenotypes with a distinct spatial configuration and morphological relationships with cholinergic neurons at the level of the gastric myenteric plexus in naïve rats that so far were largely unknown. Although morphologically indistinguishable using routine methods of examination, M1 and M2 can be identified and differentiated according to their cell surface-specific markers in murine and human central nerve system (15) and intestine (8). Flow cytometry combined with cell surface markers have been used to isolate M1 and M2 macrophages from the mouse bone marrow, spleen, peritoneum and lung (55), and human brain (8, 38) for phenotypic analysis. However, most of these markers are not available for such an approach in rats (33, 41, 49). In this study, we used double immunostaining and selected two marker antibodies, MHCII and CD206, to distinguish M1 and M2 in the rat gastric LMMP preparation. The MHCII occurs mainly on antigen-presenting cells including macrophages (19). M1 macrophages in the healthy brain parenchyma display elevated expression levels of MHCII in response to interferon-γ and lipopolysaccharide, the M1 prototypical activating stimuli (15, 51). In particular, in the rat ileum muscularis, under basal conditions only few resident macrophages expressed MHCII as detected by immunohistochemistry while lipopolysaccharide increased this population in a time-dependent manner (51). MHCII immunostaining was also successfully used to label macrophages in the rat gastrointestinal muscularis externa (39). Even though MHCII is also expressed in the gut dendritic cells that can be labeled by MHCII immunostaining (20), these cells are mainly localized in the lamina propria, Peyers patches, isolated lymphoid follicles, and mesenteric lymph nodes but rarely in the muscularis externa (9). Thus MHCII has the characteristic of a relatively selective marker to label and detect M1 macrophages in the muscularis externa. Given that macrophages are very plastic cells, an approach using combinations of monoclonal antibody anti-MHCII with a polyclonal antibody anti-CD206, a marker well validated and extensively used to detect M2 by immunohistochemistry (23, 27, 46) but not expressed in M1 (14), would provide insight to their activation outcomes. Here, we showed that the MHCII+/CD206− or MHCII−/CD206+ cells constituted two distinct populations of resident macrophages distributed at the level of rat gastric myenteric plexus while MHCII+/CD206+ macrophages were rarely found. MHCII+/CD206− (M1 like) macrophages were distributed on or around gastric corpus ganglia in a highly variable pattern, which is similar to that demonstrated by MHCII immunohistochemistry in the myenteric plexus of the rat small intestine (39). By contrast, MHCII−/CD206+ (M2 like) macrophages often outlined the perimeter of ganglia in gastric LMMP preparations and distinctly located from M1-like macrophages, These data are indicative that double immunostaining of MHCII and CD206 is a suitable approach to distinguish specifically M1 from M2 in the rat gastric myenteric plexus.
The combination of the pan neuronal marker cuprolinic blue (17) with a monoclonal antibody ED2, an established marker of macrophage maturity rather than M2 activation in vivo in the rat (12, 40), showed that individual myenteric neuron cytoplasm was stained as blue color and myenteric ganglia were outlined by macrophages labeled as brown color. These data provide anatomical support for possible interaction between neurons and macrophages in the rat gastric myenteric plexus. Recent findings have established a bidirectional functional interaction between macrophages and neurons in the mouse intestinal muscularis where macrophages directly regulate the activity of enteric neurons and peristalsis via secretion of bone morphogenetic protein-2 (34). Conversely, macrophages surrounding ganglia and neuronal cell bodies are able to sense neuronal signals (16). Moreover, in the present study, double labeling of ED2 with polyclonal antibody against pChAT, the marker for peripheral cholinergic neurons (35), also showed that the processes of the cholinergic myenteric neurons conform to macrophages labeled by ED2 and both appeared to contact morphologically. Therefore, such a morphological relationship of resident macrophages in close apposition to the cholinergic myenteric neurons would be consistent with being an end target of this pathway in the rat gastric myenteric plexus as described in the murine small intestine (6, 30).
We then quantitatively analyzed the changes in the number of two phenotypically distinct subpopulations, MHCII+/CD206− (M1 like) and MHCII−/CD206+ (M2 like) macrophages, at the level of rat gastric myenteric plexus under the inflammatory phase of POI starting ~3 to 4 h after AS in the intestinal muscularis (3, 21). Our data showed that AS increased by 2.2-fold the number of MHCII+/CD206− macrophages compared with the sham group in gastric whole mount LMMP at 6 h post-AS. By contrast, there were no significant changes observed in the number of MHCII−/CD206+ macrophages, suggesting that AS induced macrophage activation toward to M1 but not M2 state in the gastric myenteric plexus. M1 activation is thought to contribute to inflammation by initiating the secretion of proinflammatory cytokines such as IL-1β and TNF-α (13, 25). This was confirmed by real-time qPCR and MPO histochemistry. Along with a significant increase in the number of MHCII+/CD206− macrophages in the gastric myenteric plexus, there was a 1.5- and 1.7-fold increase of the gene expression of TNF-α and IL-1β, respectively, in the gastric submucosa plus muscle layers and a 9.5-fold infiltration of neutrophils labeled by MPO in the gastric corpus muscularis externa. Consistent with the lack of changes in the number of M2 macrophages, IL-10, a signature of M2 anti-inflammatory cytokine (27) was not altered by AS. The increased M1 population and recruitment of leucocytes could produce NO and prostaglandins that inhibit the contractile activity of the smooth muscle cells leading to POI (42, 53). In the present study, gastric emptying was inhibited by 72% compared with sham group at 6 h post-AS and there was a high negative correlation between values of gastric emptying with parameters of inflammation most prominently M1 (r = −0.82) and MPO (r = −0.91) but also IL-1β and TNF-α (r = −0.46 and r = −44, respectively). Collectively, the previous and present studies indicate that AS and intestinal manipulation activate resident muscularis macrophages not only in the intestine (1, 18, 23) but also in the gastric corpus LMMP preparation that is associated with the inflammatory markers. In particular, we showed that AS promotes M1 activation in the gastric LMMP and inflammation in the gastric muscularis externa associated with POGI.
We next obtained evidence that central vagal activation can modulate gastric inflammatory response to AS and alleviate POGI. In previous studies, the influence of vagus on POI was exclusively tested using electrical stimulation at the cervical level during the intestinal manipulation. This prevented AS-induced intestinal inflammation and improved the course of POI in mice (10, 45, 54). In the present study, pretreatment with intracisternal RX-77368 at a low dose 1 h before AS in rats completely suppressed AS-induced 2.2-fold increase in the number of M1 gastric myenteric preparations and the upregulation of proinflammatory cytokines IL-1β and TNF-α gene expression in the gastric submucosa plus muscle layers monitored at 6 h after AS. It reduced also AS-induced 9.5-fold increase of neutrophil infiltration by 51% in the gastric muscle externa and curtailed gastric emptying inhibition from 72 to 38%. Whether the partial reduction of neutrophils infiltration and POGI by intracisternal RX-77368 reflects a suboptimal regimen of administration (50 ng/rat, −7 h before determination) or mechanisms not sensitive to vagal stimulation will need to be further investigated. It should be noted that RX-77368 did not modify gastric emptying in the sham group, which indicates that the prevention of POGI is unlikely to be related to a direct prokinetic action (26). IL-10 derived from M2-like macrophages is well known to suppress the expression of inflammatory cytokines released by M1-like macrophages (11) and was reported to play a role in the intestinal recovery from POI in mice (48). In our study, intracisternal RX-77368 completely abolished the increases in the number of M1 at the level of gastric myenteric plexus induced by AS while not altering M2-like cells. We also found that in sham group, intracisternal RX-77368 did not alter the number of M2 macrophages or level of IL-10 mRNA. These data lend support to our hypothesis that central vagal stimulation can deactivate M1 resident muscularis macrophages in the stomach that are involved in AS-induced gastric inflammation and delayed gastric emptying. This is in agreement with reports that vagal signaling to cholinergic myenteric neurons and subsequent release of acetylcholine acting through nicotinic acetylcholine receptors lead to the attenuation of macrophages activation in the rat liver and blood and in the mouse stomach (5, 30, 36) while not suppressing the release of the anti-inflammatory cytokine IL-10 by macrophages (5). There is also in line with nicotinic acetylcholine receptors shown to be localized on resident macrophages in the rodent gastric and intestinal myenteric plexus (36, 56).
In summary, we specifically distinguished M1 from M2 in the rat gastric myenteric plexus by using the double immunostaining of MHCII (M1 marker) and CD206 (M2 marker) showing that MHCII+/CD206− (M1 like) and MHCII−/CD206+ (M2 like) macrophages constituted two distinct populations of resident macrophages. The resident macrophages labeled by ED2 are in close apposition to the gastric cholinergic myenteric neurons, and both appeared to contact morphologically. AS activates M1 macrophage and increases the expression of proinflammatory cytokines leading to inflammation in the gastric myenteric plexus correlated with the delayed gastric emptying. Central vagal stimulation abolished the increase in the number of M1 and proinflammatory cytokine productions, reduced neutrophil infiltration, and dampened POGI monitored 6 h after AS. It can also be speculated that vagal stimulation linked with the cephalic phase (50) may have a potential beneficial effect to curtail POGI.
GRANTS
This research was supported by the Veteran Administration Research Career Scientist Award, Middleton Award, Veterans Affairs Merit Award (to Y. Taché), and National Institute of Diabetes and Digestive and Kidney Diseases Grant P30-DK-41301 (Animal Core).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.-Q.Y. and Y.T. conceived and designed research; P.-Q.Y. performed experiments; P.-Q.Y. analyzed data; P.-Q.Y. and Y.T. interpreted results of experiments; P.-Q.Y. prepared figures; P.-Q.Y. drafted manuscript; P.-Q.Y. and Y.T. edited and revised manuscript; Y.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Honghui Liang for excellent technical assistance.
REFERENCES
- 1.Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil 16, Suppl 2: 54–60, 2004. doi: 10.1111/j.1743-3150.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 2.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol 181: 3733–3739, 2008. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 3.Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut 58: 1300–1311, 2009. doi: 10.1136/gut.2008.169250. [DOI] [PubMed] [Google Scholar]
- 4.Bonaz B, Sinniger V, Pellissier S. Vagal tone: effects on sensitivity, motility, and inflammation. Neurogastroenterol Motil 28: 455–462, 2016. doi: 10.1111/nmo.12817. [DOI] [PubMed] [Google Scholar]
- 5.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 6.Cailotto C, Gomez-Pinilla PJ, Costes LM, van der Vliet J, Di Giovangiulio M, Némethova A, Matteoli G, Boeckxstaens GE. Neuro-anatomical evidence indicating indirect modulation of macrophages by vagal efferents in the intestine but not in the spleen. PLoS One 9: e87785, 2014. doi: 10.1371/journal.pone.0087785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HK, Lee YJ, Lee YH, Park JP, Min K, Park H. Inflammatory responses in the muscle coat of stomach and small bowel in the postoperative ileus model of guinea pig. Yonsei Med J 54: 1336–1341, 2013. doi: 10.3349/ymj.2013.54.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cipriani G, Gibbons SJ, Kashyap PC, Farrugia G. Intrinsic gastrointestinal macrophages: their phenotype and role in gastrointestinal motility. Cell Mol Gastroenterol Hepatol 2: 120–130.e1, 2016. doi: 10.1016/j.jcmgh.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol 8: 435–446, 2008. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol 6: 844–851, 2005. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 11.Deng B, Wehling-Henricks M, Villalta SA, Wang Y, Tidball JG. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol 189: 3669–3680, 2012. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dijkstra CD, Döpp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. Adv Exp Med Biol 186: 409–419, 1985. [DOI] [PubMed] [Google Scholar]
- 13.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80: 1298–1307, 2006. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezekowitz RA, Gordon S. Alterations of surface properties by macrophage activation: expression of receptors for Fc and mannose-terminal glycoproteins and differentiation antigens. Contemp Top Immunobiol 13: 33–56, 1984. [DOI] [PubMed] [Google Scholar]
- 15.Franco R, Fernández-Suárez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131: 65–86, 2015. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 164: 378–391, 2016. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holst MC, Powley TL. Cuprolinic blue (quinolinic phthalocyanine) counterstaining of enteric neurons for peroxidase immunocytochemistry. J Neurosci Methods 62: 121–127, 1995. doi: 10.1016/0165-0270(95)00064-X. [DOI] [PubMed] [Google Scholar]
- 18.Hori M, Kita M, Torihashi S, Miyamoto S, Won KJ, Sato K, Ozaki H, Karaki H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am J Physiol Gastrointest Liver Physiol 280: G930–G938, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Hori M, Nobe H, Horiguchi K, Ozaki H. MCP-1 targeting inhibits muscularis macrophage recruitment and intestinal smooth muscle dysfunction in colonic inflammation. Am J Physiol Cell Physiol 294: C391–C401, 2008. doi: 10.1152/ajpcell.00056.2007. [DOI] [PubMed] [Google Scholar]
- 20.Janeway CA, Travers P, Walport M, Shlomchik MJ. The major histocompatibility complex and its functions. In: Immunobiology: The Immune System in Health and Disease (5th ed.). New York: Garland Science, 2001, chapt 5. https://www.ncbi.nlm.nih.gov/books/NBK27156/. [Google Scholar]
- 21.Kalff JC, Carlos TM, Schraut WH, Billiar TR, Simmons RL, Bauer AJ. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology 117: 378–387, 1999. doi: 10.1053/gast.1999.0029900378. [DOI] [PubMed] [Google Scholar]
- 22.Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg 228: 652–663, 1998. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalff JC, Türler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, Billiar TR, Simmons RL, Bauer AJ. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg 237: 301–315, 2003. doi: 10.1097/01.SLA.0000055742.79045.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11: 750–761, 2011. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Stewart KN, Bishop E, Marek CJ, Kluth DC, Rees AJ, Wilson HM. Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo. J Immunol 180: 6270–6278, 2008. doi: 10.4049/jimmunol.180.9.6270. [DOI] [PubMed] [Google Scholar]
- 26.Maeda-Hagiwara M, Taché Y. Central nervous system action of TRH to stimulate gastric emptying in rats. Regul Pept 17: 199–207, 1987. doi: 10.1016/0167-0115(87)90063-2. [DOI] [PubMed] [Google Scholar]
- 27.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27: 451–483, 2009. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 28.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 13: 453–461, 2008. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 29.Martinez V, Wu SV, Taché Y. Intracisternal antisense oligodeoxynucleotides to the thyrotropin-releasing hormone receptor blocked vagal-dependent stimulation of gastric emptying induced by acute cold in rats. Endocrinology 139: 3730–3735, 1998. doi: 10.1210/endo.139.9.6195. [DOI] [PubMed] [Google Scholar]
- 30.Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, Vanden Berghe P, Boeckxstaens GE. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63: 938–948, 2014. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 31.Miampamba M, Yang H, Sharkey KA, Taché Y. Intracisternal TRH analog induces Fos expression in gastric myenteric neurons and glia in conscious rats. Am J Physiol Gastrointest Liver Physiol 280: G979–G991, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosser DM. The many faces of macrophage activation. J Leukoc Biol 73: 209–212, 2003. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 34.Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158: 300–313, 2014. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. J Chem Neuroanat 18: 31–40, 2000. doi: 10.1016/S0891-0618(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 36.Nemethova A, Michel K, Gomez-Pinilla PJ, Boeckxstaens GE, Schemann M. Nicotine attenuates activation of tissue resident macrophages in the mouse stomach through the β2 nicotinic acetylcholine receptor. PLoS One 8: e79264, 2013. doi: 10.1371/journal.pone.0079264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O-Lee TJ, Wei JY, Taché Y. Intracisternal TRH and RX 77368 potently activate gastric vagal efferent discharge in rats. Peptides 18: 213–219, 1997. doi: 10.1016/S0196-9781(96)00281-1. [DOI] [PubMed] [Google Scholar]
- 38.Perego C, Fumagalli S, Zanier ER, Carlino E, Panini N, Erba E, De Simoni MG. Macrophages are essential for maintaining a M2 protective response early after ischemic brain injury. Neurobiol Dis 96: 284–293, 2016. doi: 10.1016/j.nbd.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Phillips RJ, Powley TL. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci 169: 12–27, 2012. doi: 10.1016/j.autneu.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polfliet MM, Fabriek BO, Daniëls WP, Dijkstra CD, van den Berg TK. The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology 211: 419–425, 2006. doi: 10.1016/j.imbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 41.Rubio-Navarro A, Guerrero-Hue M, Martín-Fernandez B, Cortegano I, Olivares-Alvaro E, de Las Heras N, Alía M, de Andrés B, Gaspar ML, Egido J, Moreno JA. Phenotypic characterization of macrophages from rat kidney by flow cytometry. J Vis Exp 18: 1–5, 2016. doi: 10.3791/54599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarz NT, Kalff JC, Türler A, Engel BM, Watkins SC, Billiar TR, Bauer AJ. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology 121: 1354–1371, 2001. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- 43.Schwarz NT, Kalff JC, Türler A, Speidel N, Grandis JR, Billiar TR, Bauer AJ. Selective jejunal manipulation causes postoperative pan-enteric inflammation and dysmotility. Gastroenterology 126: 159–169, 2004. doi: 10.1053/j.gastro.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 44.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stakenborg N, Wolthuis AM, Gomez-Pinilla PJ, Farro G, Di Giovangiulio M, Bosmans G, Labeeuw E, Verhaegen M, Depoortere I, D’Hoore A, Matteoli G, Boeckxstaens GE. Abdominal vagus nerve stimulation as a new therapeutic approach to prevent postoperative ileus. Neurogastroenterol Motil 29: e13075, 2017. doi: 10.1111/nmo.13075. [DOI] [PubMed] [Google Scholar]
- 46.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 176: 287–292, 1992. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stengel A, Goebel M, Luckey A, Yuan PQ, Wang L, Taché Y. Cold ambient temperature reverses abdominal surgery-induced delayed gastric emptying and decreased plasma ghrelin levels in rats. Peptides 31: 2229–2235, 2010. doi: 10.1016/j.peptides.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoffels B, Schmidt J, Nakao A, Nazir A, Chanthaphavong RS, Bauer AJ. Role of interleukin 10 in murine postoperative ileus. Gut 58: 648–660, 2009. doi: 10.1136/gut.2008.153288. [DOI] [PubMed] [Google Scholar]
- 49.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol 175: 342–349, 2005. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 50.Taché Y, Yang H, Miampamba M, Martinez V, Yuan PQ. Role of brainstem TRH/TRH-R1 receptors in the vagal gastric cholinergic response to various stimuli including sham-feeding. Auton Neurosci 125: 42–52, 2006. doi: 10.1016/j.autneu.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torihashi S, Ozaki H, Hori M, Kita M, Ohota S, Karaki H. Resident macrophages activated by lipopolysaccharide suppress muscle tension and initiate inflammatory response in the gastrointestinal muscle layer. Histochem Cell Biol 113: 73–80, 2000. doi: 10.1007/s004180050009. [DOI] [PubMed] [Google Scholar]
- 52.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296, 2007. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Türler A, Kalff JC, Moore BA, Hoffman RA, Billiar TR, Simmons RL, Bauer AJ. Leukocyte-derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann Surg 244: 220–229, 2006. doi: 10.1097/01.sla.0000229963.37544.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Bree SH, Nemethova A, Cailotto C, Gomez-Pinilla PJ, Matteoli G, Boeckxstaens GE. New therapeutic strategies for postoperative ileus. Nat Rev Gastroenterol Hepatol 9: 675–683, 2012. doi: 10.1038/nrgastro.2012.134. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Yu X, Cao Q, Wang Y, Zheng G, Tan TK, Zhao H, Zhao Y, Wang Y, Harris DC. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunol 14: 6, 2013. doi: 10.1186/1471-2172-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 57.Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, Kalff JC. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56: 176–185, 2007. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu SV, Yuan PQ, Wang L, Peng YL, Chen CY, Taché Y. Identification and characterization of multiple corticotropin-releasing factor type 2 receptor isoforms in the rat esophagus. Endocrinology 148: 1675–1687, 2007. doi: 10.1210/en.2006-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan PQ, Kimura H, Million M, Bellier JP, Wang L, Ohning GV, Taché Y. Central vagal stimulation activates enteric cholinergic neurons in the stomach and VIP neurons in the duodenum in conscious rats. Peptides 26: 653–664, 2005. doi: 10.1016/j.peptides.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]