1. Introduction to clock biology and disease relevance
Chronobiology is a study of biological rhythms, and the predominant rhythms are those of daily, or circadian, dimension. The circadian rhythms of tissue and organismal functions are driven by a circadian clock system consisting of cell-autonomous, self-sustainable molecular oscillators. In both the core loop and the secondary, stabilization loop of the oscillator, positive (CLOCK/NPAS2, BMAL1, RORs) and negative (PERIODs, CRYPTOCHROMEs, REV-ERBs) factors form transcription-translation negative feedback (Fig. 1) [1]. Several other feedback loops have been shown to superimpose on this basic framework, playing important roles to regulate the core oscillator. The cellular oscillators throughout the body are synchronized by a master pacemaker, the suprachiasmatic nuclei (SCN) in the hypothalamus, in response to photic signals throughout the day. The circadian clock, through both systemic signals originated from the SCN and local control by tissue oscillators, drives downstream gene expression. While the circadian transcriptome is highly tissue specific, overall half of the genes in mammalian genomes display circadian expression in at least one tissue [2], underscoring a prevalent clock control of physiology.
Figure 1.
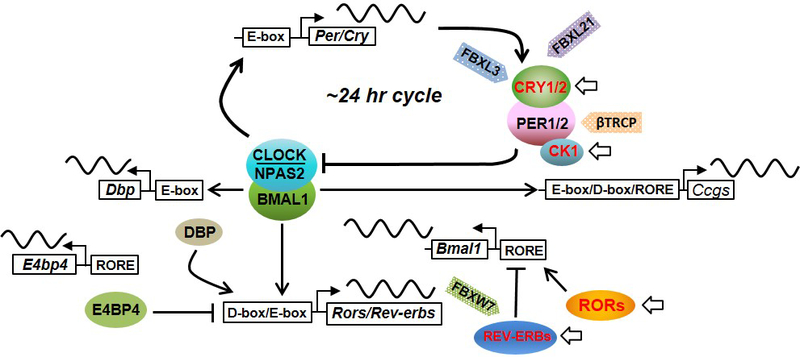
The circadian oscillator and representative drug candidates. The cell-autonomous core oscillator consists of interlocked feedback loops. In core and secondary loops, positive and negative factors form transcription-translation negative feedback. Several other feedback loops (e.g., DBP loop) have been shown to superimpose on this basic framework to fine-tune circadian oscillation. The circadian clock, including both systemic control originated from the SCN and local oscillators, drives downstream gene expression, either directly via circadian response elements including E-box, RORE, and D-box, recognized by CLOCK/BMAL1, REV-ERBs and RORs, and DBP respectively, or indirectly by clock-controlled transcription factors, epigenetic regulators and post-transcriptional and post-translational machineries. A number of small molecules have been identified or developed to target core clock components or proximal regulatory factors (highlighted in red with arrows). Adapted from [5] permission of Frontiers in Neurology via the Creative Commons CC-BY license.
Consistently, circadian dysregulation has been broadly implicated in various diseases and aging. Studies from shift workers and mouse circadian disruption models have revealed pervasive pathophysiological consequences of clock dysregulation [3]. In mice, both genetic and environmental means have been employed to disrupt the clock and, in some cases, downstream effectors that connect dysfunctional clocks and disease phenotypes have been identified. A complementary line of evidence comes from circadian characterization in diseases and aging conditions where dysregulated, often damped, circadian gene expression and physiological rhythms are routinely observed [4, 5]. Finally, human genetic studies, particularly of familial sleep disorders [6], have established clinical relevance of clock genes in human disease. Collectively, studies thus far revealed an important role of robust rhythms for health and fitness.
2. The clock as target for drug discovery
There is a growing interest to exploit circadian rhythms as a therapeutic target. Independent of the traditional chronotherapy with the primary aim to optimize dosing time, active manipulation of circadian rhythms is gaining attention in recent years [7]. Melatonin is an endogenous hormone whose nighttime production in the pineal gland is directly controlled by the SCN [8]. In turn, melatonin synchronizes peripheral tissues as a robust circadian output and impinges on the SCN where melatonin receptors are abundantly expressed. Melatonin and its synthetic derivatives have shown broad biological activities, including sleep-promoting, anti-oxidant and neurological effects [8, 9]. Dietary manipulation of circadian timing, exemplified by the time-restricted feeding paradigm [10], illustrates further the therapeutic potential of circadian manipulation against metabolic and cardiovascular diseases as well as aging in mice and Drosophila. Coupled with the realization that the protein targets of 56% of top-selling drugs are expressed from genes with circadian expression pattern [2], these animal studies underscore the potential of clock-targeting drugs.
To search for clock-targeting drug candidates, one approach involves chemical screens that employ phenotypic assays measuring circadian reporter expression; various compounds, including clinical drugs, natural products, and synthetic molecules, have been identified to alter the cardinal features of circadian rhythms, namely period, phase and amplitude [11–13]. Several studies succeeded in demonstrating core oscillator components (CRYs, RORs) or proximal regulators (Casein kinase I or CKI) as direct targets of these clock-modulating compounds [14–16]. A complementary approach to circadian modulator discovery focuses on specific clock components. Four predominant classes of drug targets include GPCRs, ion channels, kinases and nuclear receptors. In accordance, much effort has been dedicated to REV-ERBs and RORs [17, 18], the two families of nuclear receptors playing antagonistic roles in the circadian oscillator. CKI and several GPCRs, including melanopsin receptor, vasopressin receptors, and a newly characterized Gz-coupled receptor, are also excellent targets for compound development to evoke period change and phase shift with potential applications in photic entrainment, jet-lag and shiftwork disorder [12, 19, 20]. Overall, an increasing number of compounds have been reported to modulate activities of clock components and/or the clock itself, show sufficient bioavailability, and mitigate circadian and physiological deficiencies in preclinical disease models [12, 14, 15, 17]. These pioneering studies on the one hand affirm the therapeutic strategy of targeting the clock, on the other also reveal several key issues as discussed below.
3. Key issues to advance chronomedicine
Chronomedicine is a general term for therapeutics targeting biological rhythms. Below four key issues are discussed, corresponding to different stages of drug discovery pipeline (Fig. 2).
Figure 2.
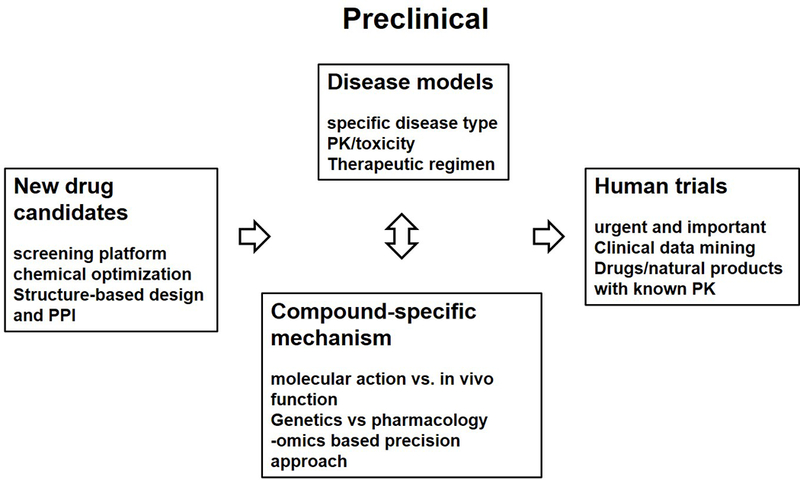
Four key areas of interests in drug discovery for chronomedicine. Please see text for details. PPI: protein-protein interaction. PK: pharmacokinetics. Human trials are of critical urgency and importance to validate clock-targeting therapeutics. Established drugs or natural products with known PK and safety profiles are ideally positioned to advance to trials.
3.1. Expand the repertoire of targets and drug candidates
For the screening approach, additional screening platforms (e.g., single-cell, high-content imaging) or molecular events (e.g., protein modification, degradation or subcellular localization) can be exploited to design novel assays. If focusing on particular clock components, structural biology is a powerful tool to open new venues for drug discovery. Recent studies have reported structures of apo and ligand-bound core clock proteins, as well as protein complexes [1]. For example, the PAS domain, an ancient structural motif present in CLOCK, BMAL1 and PER proteins, are evolutionarily conserved and known for ligand binding in microbial proteins. Structure characterization of mammalian PAS domains, including those of clock proteins, have unveiled internal pockets potentially capable of ligand binding. Studies of CRYs have also illustrated highly dynamic intermolecular interactions at the FAD-binding surface in the photolyase homology domain. Protein-protein interactions (PPIs) are a new class of targetable molecular events, especially for traditionally intractable proteins such as non-receptor type transcription factors [21]. Structural studies have revealed key amino acids, or so-called hotspots, that mediate energetically important interactions within the circadian protein complexes. PPIs are more commonly targeted by peptides or biologic drugs; however, detailed structural and biochemical characterization should be conducive to the design of small molecule modulators of clock proteins.
3. 2. Define and refine disease target and therapeutic regimen
The list of clock-related diseases continues to expand. Many clock-targeting compounds have been shown to display beneficial effects toward certain diseases or phenotypes in mice (e.g., metabolic or mood dysregulation), serving as the initial evidence for the validity of this therapeutic strategy. Circadian preclinical studies, however, are still in a nascent stage. First, many high-priority disease targets have not been directly tested for clock-targeting drug candidates, including, but certainly not limited to, most cancer types, neurodegenerative diseases, and other age-related decline. Second, apart from jetlag, most of the clock-related disease symptoms or age-related decline are chronic in nature. Both preventive and therapeutic paradigms should be tested. Prevention is especially important for age-related chronic diseases where delay of symptom onset will have strong impact on disease rate and duration. For example, if effective prevention leads to five-year delay in the onset of the Alzheimer’s disease, disease incidence can be reduced by half [22]. Furthermore, pharmacokinetic and toxicity tests should be considered in a chronic context, whenever feasible. Finally, clock-targeting compounds may be utilized in combination therapies to achieve optimal therapeutic index. Although mechanistically unclear, studies have been reported showing beneficial effects of melatonin co-administration [9]. It is conceivable that, as a variation of traditional chronotherapy, potent and specific clock-target compounds can manipulate circadian timing for optimal effects of other therapeutics.
3.3. Improve Mechanistic knowledge for precision chronomedicine
The preclinical stage is particularly important to understand mechanism of action because, other than blood and limited biopsy types, it is virtually impossible to obtain time-series clinical information on how drugs work. Recent mouse studies of clock-targeting compounds provide further evidence for mechanistic complexity. Both positive (agonists) or negative (antagonists or inverse agonists) ligands of the same target can lead to similar beneficial effects. This is exemplified by studies of compounds targeting RORs and REV-ERBs in metabolic and mood disorder [14, 17, 23]. Conversely, compounds with similar activities may elicit highly divergent cellular responses. Likewise, genetic and pharmacological manipulations of the same nature (e.g., genetic knockout and pharmacological inhibition) could lead to highly distinct outcomes, due to confounding factors such as developmental or pleiotrophic effects.
For mechanistic investigation, the effects on the oscillator and putative clock-regulated targets should be characterized, ideally at both transcript and protein levels, over a circadian time course. With the advent of systems biology era [24], integrative -omics analysis represents a precision chronomedicine approach to elucidate dose-specific cellular and systemic mechanisms, and identify therapeutic biomarkers potentially extrapolatable to human.
3.4. Human relevance and applications
This is perhaps the most important next step for drug-based chronomedicine (as opposed to behavioral/environmental intervention). Search of the public database at ClinicalTrials.Gov using “circadian” as the key word revealed >100 observational and interventional clinical trials. Within the interventional category, in addition to the traditional chronotherapy involving disease-specific drugs, the vast majority of strategies targeting circadian rhythms are bright light exposure, meal timing, and melatonin. None involves a novel chronotherapeutic drug. Sleep or activity monitoring is the most convenient and least invasive approach for human circadian studies/trials. Physiological monitoring, predominantly levels of circulating hormones, neurotransmitters, nutrients and metabolites, are often used for secondary outcome. It is encouraging that a number of studies also investigate expression of clock genes or clock-controlled genes, to evaluate molecular rhythms or derive molecular signatures. Such molecular data will prove invaluable information on the core oscillator, and can be readily corroborated with preclinical studies described above.
Mirroring these on-going clinical trials, sleep, mobility and blood tests are routinely conducted in clinical visits, while molecular or genomic data are usually deficient. Most clinical samples are collected at time of visit or post-mortem in the extreme case, and the specific time of collection is not always on record. In a recent study [25], a computational tool was reported to assign time series to unordered clinical samples, which revealed dysregulated, but not disrupted, rhythms in hepatocellular carcinoma. This approach can be applied to illuminate circadian rhythms of other clock-related diseases, advancing our understanding of circadian etiology and design of targeted choronomedine.
4. Conclusion
Pharmacological manipulation of circadian rhythms is emerging as a therapeutic strategy against myriad diseases. Clock-modulating compounds, identified via screening and/or chemical modification, are able to manipulate oscillatory properties such as period, phase and amplitude or alter activities of clock proteins. Accumulating evidence from cellular and in vivo animal studies supports further exploitation of these compounds as drug candidates against jetlag, chronic diseases (e.g., sleep, metabolic and mood disorders) and aging. Moving forward, several key areas will prove fruitful, including broadening the search for drug candidates, refining disease targets and animal models, deepening molecular and omics-based mechanistic understanding, and advancing novel drug candidates to human trials.
5. Expert opinion
Although still a relatively new topic, direct manipulation of circadian rhythms is an innovative therapeutic strategy with great potential. Many debilitating diseases are chronic and systemic in nature. As opposed to targeting a specific cellular pathway, which may incur unwanted effects on a linked pathway over disease progression, holistic modulation of the circadian clock network represents a new drug discovery route. Initially set out to search for compounds that can modulate clock components or overall clock parameters, investigators have applied clock-modulating small molecules to disease models and observed a broad array of potent efficacies. It is an elegant idea to match overall circadian phenotypes in diseases with modulatory compounds; for example, period- and phase-resetting compounds can be applied to circadian sleep disorders, jetlag and shiftwork disorder, and amplitude enhancers for metabolic and mood disorders and age-related decline. However, one should exercise caution to avoid oversimplification. Both stimulatory and inhibitory activities for the same target can be beneficial; conversely, compounds of the same functional group (agonists or antagonists) can evoke distinct cellular mechanisms. These observations together highlight a compound-specific mechanism of action that was previously underappreciated.
In the near future, an urgent task for the field is to conduct human trials and establish therapeutic utility clinically (Fig. 2). Especially for circadian sleep and shiftwork disorders where period and phase changes in behavior and physiology can be monitored easily, drug development efforts focusing on established targets (e.g., CKI, CRY and GPCRs) may prove fruitful. For other clock-related diseases with more complex and variable circadian phenotypes such as metabolic, mood and neurodegenerative diseases, it is important to characterize the circadian rhythms associated with the particular disease, combining molecular studies with sophisticated biostatistics and bioinformatics tools. Such information could define the predominant circadian signature of the disease for rational design of possible therapeutic regimens. On the preclinical side, we expect to identify new circadian therapeutic targets and drug candidates, refine disease models and strengthen mechanistic studies in both depth and breadth to understand molecular signatures characteristic of drug effects. As mentioned above, existing animal studies have already revealed important lessons regarding the choice of circadian therapeutics for different disease types and the unexpected complexity in compound mechanism of action. With these general principles in hand, future preclinical studies should strive to achieve specific alignment between compound action and disease characteristics (e.g., circadian signature) that resemble human settings. Finally, the typical pipeline of drug discovery applies here, including pharmacokinetic studies and medicinal chemistry efforts. Given that most clock-related diseases are chronic, preventive route and chronic toxicity should be evaluated routinely for chronomedicine. Overall, we expect exciting growth in chronobiological understanding and drug discovery.
Acknowledgements
The author thanks Dr. Seung-Hee Yoo for critical reading and artwork.
Funding:
This work is in part supported by the Robert A. Welch Foundation (AU-1731) and NIH/NIA (R01AG045828).
Footnotes
Declaration of Interest:
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
6. Annotated bibliography
- 1.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 2017. March;18(3):164–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.**.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proceedings of the National Academy of Sciences of the United States of America 2014. November 11;111(45):16219–24. A comprehensive characterization of circadian gene expression throughout the body and important insights on circadian expression of drug targets. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science 2016. November 25;354(6315):994–99. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder AM, Colwell CS. How to fix a broken clock. Trends Pharmacol Sci 2013. November;34(11):605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gloston GF, Yoo SH, Chen ZJ. Clock-Enhancing Small Molecules and Potential Applications in Chronic Diseases and Aging. Front Neurol 2017;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones CR, Huang AL, Ptacek LJ, Fu YH. Genetic basis of human circadian rhythm disorders. Exp Neurol 2013. May;243:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrow SN, Solari R, Willson TM. The importance of chronobiology to drug discovery. Expert Opin Drug Discov 2012. July;7(7):535–41. [DOI] [PubMed] [Google Scholar]
- 8.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. Journal of physiology, Paris 2011. December;105(4–6):170–82. [DOI] [PubMed] [Google Scholar]
- 9.Rivara S, Pala D, Bedini A, Spadoni G. Therapeutic uses of melatonin and melatonin derivatives: a patent review (2012 – 2014). Expert opinion on therapeutic patents 2015. April;25(4):425–41. [DOI] [PubMed] [Google Scholar]
- 10.*.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell metabolism 2012. June 6;15(6):848–60. A pioneering study on a robust circadian dietary paradigm with profound interventional efficacies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoch MP, Kondratov RV. Pharmacological modulators of the circadian clock as potential therapeutic drugs: focus on genotoxic/anticancer therapy. Handb Exp Pharmacol 2013(217):289–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallach T, Kramer A. Chemical chronobiology: Toward drugs manipulating time. FEBS Lett 2015. June 22;589(14):1530–38. [DOI] [PubMed] [Google Scholar]
- 13.He B, Chen Z. Molecular Targets for Small-Molecule Modulators of Circadian Clocks. Curr Drug Metab 2016;17(5):503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.*.He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell metabolism 2016. April 12;23(4):610–21. Describes screening, physiological benefits, and target identification of a natural clock amplitude-enhancing small molecule. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.*.Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, et al. Identification of Small Molecule Activators of Cryptochrome. Science 2012. July 12 Describes a novel CRY-activating compound. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chun SK, Jang J, Chung S, Yun H, Kim NJ, Jung JW, et al. Identification and validation of cryptochrome inhibitors that modulate the molecular circadian clock. ACS Chem Biol 2014. March 21;9(3):703–10. [DOI] [PubMed] [Google Scholar]
- 17.*.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov 2014. Mar;13(3):197–216. A comprehensive summary of compounds targeting circadian nuclear receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A 2012. January 10;109(2):582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones KA, Hatori M, Mure LS, Bramley JR, Artymyshyn R, Hong SP, et al. Small-molecule antagonists of melanopsin-mediated phototransduction. Nat Chem Biol 2013. October;9(10):630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doi M, Murai I, Kunisue S, Setsu G, Uchio N, Tanaka R, et al. Gpr176 is a Gz-linked orphan G-protein-coupled receptor that sets the pace of circadian behaviour. Nature communications 2016. February 17;7:10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laraia L, McKenzie G, Spring DR, Venkitaraman AR, Huggins DJ. Overcoming Chemical, Biological, and Computational Challenges in the Development of Inhibitors Targeting Protein-Protein Interactions. Chem Biol 2015. June 18;22(6):689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American journal of public health 1998. September;88(9):1337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Son GH, Chung S, Ramirez, VD, Kim K Pharmacological Modulators of Molecular Clock and their Therapeutic Potentials in Circadian Rhythm-Related Diseases. Medicinal Chemistry (Los Angeles) 2016;6:12. [Google Scholar]
- 24.Millius A, Ueda HR. Systems Biology-Derived Discoveries of Intrinsic Clocks. Front Neurol 2017;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anafi RC, Francey LJ, Hogenesch JB, Kim J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc Natl Acad Sci U S A 2017. May 16;114(20):5312–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


