Abstract
Purpose of Review:
We aim to give an overview of the epidemiology and treatment trends of testicular germ cell tumors (TGCT), with an emphasis on recent trends.
Recent Findings:
The incidence of TGCT appears to be increasing, particularly in developed countries, although the reasons are not well understood. There is evidence of racial differences in predisposition to TGCT, with white men having highest risk and men of African or Asian descent having lower risk. In the United States, the incidence of TGCT among Hispanics appears to be rising most quickly. A recent genomic analysis indicates there is no highly penetrant major TGCT susceptibility gene. Incorporation of multi-disciplinary care has led to excellent long-term cure rates, however access to care and insurance remain barriers in young men. Recent treatment trends have centered on maximizing oncologic outcomes while minimizing long-term morbidity.
Summary:
Emerging population-level data provides critical insight into the evolving demographics of TGCT, which may allow for elucidation of biologic and environmental determinants of TGCT. Further, identification of socioeconomic barriers to excellent clinical outcomes will allow for targeted interventions to patients with unique demographic and socioeconomic considerations. Treatment trend analyses suggest that the field is moving towards minimizing treatment-related morbidity.
Keywords: testicular cancer, trends, epidemiology, demographics
Introduction
Testicular germ cell tumors (TGCT) are the most common malignancies affecting young men, and the incidence appears to be increasing worldwide. The advent of cisplatin-based chemotherapy, and incorporation of multidisciplinary teams of surgeons, radiologists, oncologists, radiation oncologists, and pathologists has led to dramatic improvements in cure in most cases, yet there are concerns for long-term treatment-related morbidity. Our aim is to review epidemiologic trends in TGCT, explore risk factors, and give a broad overview of treatment patterns.
Epidemiology
TGCT is the most common malignancy affecting young men between 15–44 years of age, and the mean age of diagnosis is 33.(1) In 2018, there will be an estimated 9,310 new cases of TGCT diagnosed in the United States, and approximately 400 men will die of disease.(2) There is substantial variability in the reported incidence of TGCT worldwide. An analysis from the International Agency for Research on Cancer (IARC) indicates age-standardized rates of TGCT varied from less than 1/100,000 person-years in Africa and Asia to over 9/100,000 person-years in Northern and Western Europe.(1)
Rising Incidence
Numerous reports dating back over 50 years have noted an increasing incidence of TGCT worldwide.(3–5) In the United States, the reported incidence has steadily increased to approximately 6.8 per 100,000.(6) Additionally, there appears to be substantial difference in the rising rates between countries, and even within a country (Figure 1A). For instance, the incidence of TGCT in Switzerland between 1974–1987 has remained relatively stable, but there are substantial differences in the incidence in men living in rural (6.8/100,000 person-years) vs. urban (10.7/100,000 person-years) areas. Some of the geographic variability and increasing incidence may be attributable to detection bias, increased patient and physician awareness, increased availability of ultrasounds.(7) Nonetheless, it is unlikely that such biases, which are essentially lead time biases, will substantially impact the overall trends noted. TGCT is a disease that typically affects young men, with few competing causes of mortality. Although more access to care or vigilance might lead to an earlier diagnosis, the overall incidence should remain stable.
Figure 1A. Estimated age-standardized rates of incident cases of testicular cancer, worldwide in 2012.
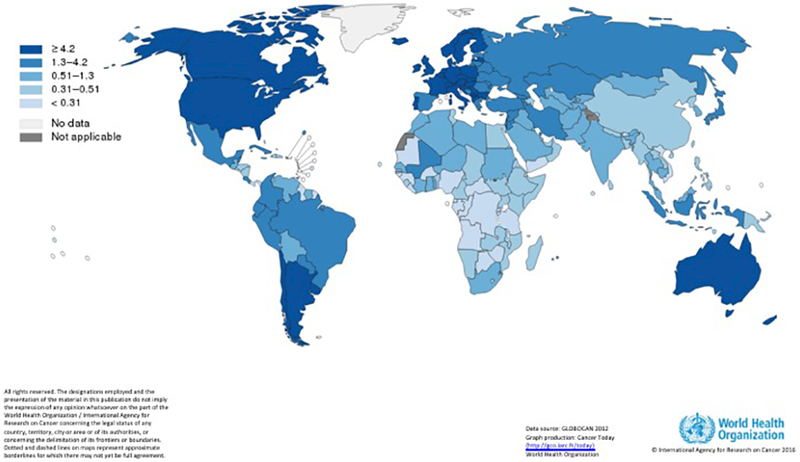
Source: GLOBOCAN 2012, Graph production: Cancer Today, (http://gco.iarc.fr/today), World Health Organization
Racial Differences
TGCT is a disease that mainly affects White Caucasian men, both in the US and globally. The reported incidence of TGCT in Scandinavia is ten times higher than the rates in Africa and Asia.(8) While such differences can be attributed to environmental causes, there are also substantial difference in the incidence of TGCT among racial groups within a country. In the United States, the highest incidence of TGCT is among Caucasian whites (6.57/100,000 person-years), followed by Hispanics (3.88/100,000 person-years), Asians (1.6/100,000 person-years), and Blacks (1.2/100,000 person-years). Additionally, rates of TGCT appear to be rising most quickly among Hispanics.(9) Woldu and colleagues found that the average age at diagnosis of TGCT was 5 years younger for Hispanic men compared to white men, and they were significantly more like to harbor non-seminomatous vs. seminomatous histology.(10) Reasons for such differences in incidence and trends remain speculative. An allele distribution of TGCT risk loci across races demonstrates that Hispanic men have a distribution of alleges similar to that of higher risk European men compared to lower risk African men.(11) Studies of immigrants from lower to higher rate countries find that the first-generation of migrants do not appear to have an increased rate of TGCT, but subsequent generations do.(12, 13) Ghazarian and colleagues speculate that the rising incidence in Hispanic men may be due to an increase in 2nd and higher generations of immigrants, whereby a high risk but lower incidence group is being exposed to environmental factor(s) that lead to a more rapid rise in the rate of TGCT.(9)
Mortality
Despite the reported rise of TGCT incidence, mortality rates from TGCT have continued to decline in more highly developed countries for several decades.(1, 14) In the United States, testicular cancer mortality comprises approximately 4% of annual incidence.(2) The advent of cisplatin-based chemotherapy, improvements in surgical techniques, and incorporation of multi-disciplinary care teams has led to this success story. In developed countries the cure rate of stage I disease approaches 100%. (15) Even in metastatic disease, cure rates for good, intermediate, and poor-risk disease are approximately 90%(16), 70%(17), 48%, respectively.(18) There is substantial global variability of mortality rates, which are largely inverse of that seen in the incidence rates. TGCT mortality is highest in low-income countries compared to higher income countries (Figure 1B).(1) Within the United States, a recent analysis of NCDB indicates worse outcomes in racial minorities, and those with lower socioeconomic status and ‘underinsurance.’(19) Such findings highlight the notion that reliable access to care is required to prevent needless mortality in an otherwise healthy population.
Figure 1B. Estimated age-standardized rates of deaths due to testicular cancer, worldwide in 2012 (Reprinted with Permission from the International Agency for Research on Cancer).

Source: GLOBOCAN 2012, Graph production: Cancer Today, (http://gco.iarc.fr/today), World Health Organization
Heritability
Familial clustering of TGCT has been noted for decades, in particular for male siblings who have a 4–8 fold higher risk of TGCT.(20–22) Recent genomic studies suggest heritability of TGCT at 49%, and known single nucleotide polymorphisms (SNP) represent only a minority of heritability.(23, 24) Targeted analysis of multiplex TGCT families has identified rare gene mutations, such as DNAAF1 and related ciliary-microtubule genes, as likely culprits of TGCT in certain cases, however these high-impact mutations are rare.(25) Numerous genome-wide association studies have identified multiple loci associated with TGCT, many of which contain genes which are involved in germ cell development and pluripotency, DNA damage response, mitochondrial function, and kinetochore function.(26–30) These reports combined with epidemiologic evidence has led to speculation of a TGCT susceptibility gene which might be suitable for clinical testing. Recently, Litchfield and colleagues performed germ-line whole-exome sequencing of 919 TGCT cases and compared those to cancer-free controls and found no individual variant or genes that support the notion of a major, high-penetrance TGCT susceptibility gene.(31) Taken together, the development of TGCT is likely a polygenic process with multiple potential loci of susceptibility, combined with environmental exposures and epigenetic modification that lead to disease phenotype.(32)
Treatment Trends
Clinically Localized Disease
Patients with localized disease following radical orchiectomy can be managed in a number of ways. Guidelines-based recommendations for localized seminoma include active surveillance, single-dose carboplatin, or radiation to the retroperitoneum, while localized NSGCT can be managed with surveillance, cisplatin-based chemotherapy (1 cycle of bleomycin, etoposide, and cisplatin [BEP]), or retroperitoneal lymph node dissection (RPLND). Regardless of the strategy employed, long-term survival approaches 100% as most contemporary data suggests the ability to salvage the estimated 20–30% of patients with occult metastatic disease who recur during surveillance.(15, 33–37) Given high cure rates with all management strategies, significant attention is paid to minimizing long-term effect of adjuvant chemotherapy and radiotherapy in testis cancer survivors, which include cardiovascular disease, secondary malignancies, and various toxicities.(38, 39)
A risk-adapted approach has been advocated to decide management strategies, with adjuvant therapy used in cases of perceived higher risk seminoma (i.e. stromal invasion of the rete testis and/or tumor diameter >4cm).(40, 41) However, these risk factors lack validation.(15) As seminoma has a lower risk of occult metastatic disease than NSGCT, active surveillance is the preferred strategy by most centers. Recent analyses of the National Cancer Database (NCDB) confirms an increasing utilization of surveillance for clinically localized seminoma from 25% in 1998 to over 60% in more recently, and even higher rates of surveillance at TGCT referral centers (Figure 2).(42–44) While use of adjuvant therapies has declined in recent years, the proportion of chemotherapy use has increased significantly compared to radiotherapy, which was the mainstay treatment for decades.(24, 44) This shift followed the publication of trials demonstrating equivalent recurrence-free survival of single-dose carboplatin and radiotherapy, but an improved adverse effect profile with chemotherapy.(45, 46) Further, if patients have relapse after radiotherapy and require chemotherapy, long-term risks including cardiac disease and secondary malignancies, are compounded.
Figure 2. Testicular cancer treatment patterns stratified by hospital volume. (A) Stage I seminoma, (B) stage I nonseminomatous germ cell tumor, (C) management of stage II to III nonseminomatous germ cell tumor following chemotherapy.
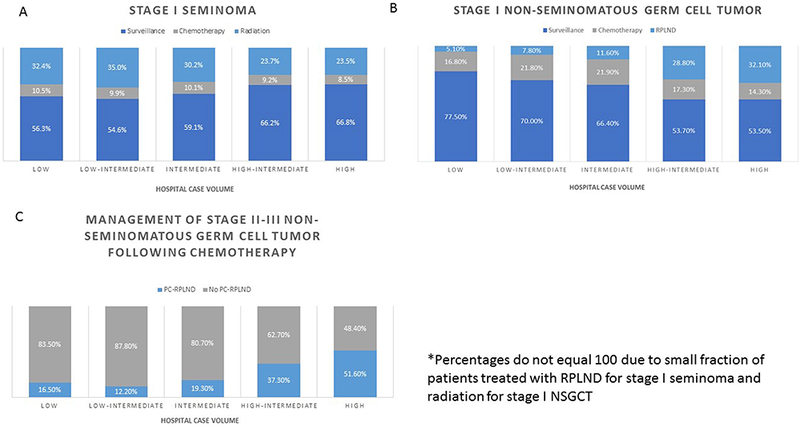
RPLND – retroperitoneal lymph node dissection, PC-RPLND – post-chemotherapy retroperitoneal lymph node dissection
Source: Woldu SL, Matulay JT, Clinton TN, Singla N, Krabbe L-M, Hutchinson RC, et al. Impact of hospital case volume on testicular cancer outcomes and practice patterns. Urologic Oncology: Seminars and Original Investigations. 2018;36(1):14.e7-.e5.
Similar to seminoma, long-term survival approaches 100% with any upfront strategy due to efficacy of salvage therapies. Unlike seminoma, risk of occult disease is higher with NSGCT and a risk-adapted approach is more established. The presence of lymphovascular invasion (LVI) or invasion of the tunica vaginalis are incorporated into the AJCC staging, signifying at least pT2 disease and mark the distinction between clinical stage IA and IB disease. Patients with clinical stage IB disease have a ~50% recurrence rate, compared to an approximately 15% recurrence rate in clinical stage IA disease.(47–49) Embryonal carcinoma predominance has also been reported as marker of occult disease, although the effect is less definitive than LVI.(48, 50) Clinical stage IA NSGCT can be managed with a choice of surveillance, adjuvant chemotherapy, or primary nerve-sparing RPLND though surveillance is the preferred strategy given significant overtreatment rates. There is more controversy for higher risk clinical stage IB NSGCT with respect to adjuvant treatment versus surveillance.(33, 51) Both chemotherapy (BEP) and nerve-sparing RPLND represent viable adjuvant treatment strategies. Although a randomized trial comparing one cycle of BEP vs. RPLND for clinical stage I NSGCT showed superiority of BEP over RPLND,(52) this study has been criticized due to the notion that the RPLND was performed by less skilled surgeons as evidenced by high “in-field” recurrence rates. A single cycle of BEP is the standard for adjuvant chemotherapy based on results from SWENOTECA, demonstrating safety of dose reduction and an attempt to minimize dose related toxicity.(53, 54) Recent analysis of the NCDB indicate that ~75% of men with clinical stage IA NSGCT receive surveillance, compared to ~50% of men with clinical stage IB.(55) Compared to guideline recommendations, this suggests an underutilization of surveillance in clinical stage IA. In the United States, there appears to be a higher utilization of RPLND and decreased utilization of surveillance at referral centers (Figure 2).(43)
Marker Only Elevation Disease
TGCT is unique with the incorporation of STMs into clinical staging. Although not common, a proportion of patients may have elevated AFP and hCG despite no evidence of distant disease on imaging studies or in the contralateral testicle (clinical stage IS). This situation is very rare for seminoma, and a conservative approach is warranted with repeat of STM measurement and imaging studies.(56) For clinical stage IS NSGCT, treatment recommendations are controversial with suggestions of primary chemotherapy for good-risk disseminated cancer or RPLND.(56, 57) Given the rarity of clinical stage IS disease, analysis of treatment trends is limited to large nationwide databases where there are significant questions about the validity of STM interpretation.(58)
Retroperitoneal Lymph Node Positive Disease
For stage IIA/IIB seminoma, the traditional treatment has been radiotherapy (59, 60) although 3 cycles of BEP or 4 cycles of etoposide and cisplatin (EP) is an alternative option. There are no randomized studies comparing radiotherapy with chemotherapy, a recent meta-analysis by Giannatempo and colleagues indicate that radiotherapy and chemotherapy appear to be equally effective.(61) As expected, chemotherapy was associated with acute toxicity, while long-term toxicity was more frequent in radiotherapy.(61) NCDB analysis indicates decreasing utilization of radiotherapy and increased utilization of chemotherapy, although a clear trend is not indicated. In the last year of analysis (2012), radiotherapy (vs. chemotherapy) was still used in over 70% of clinical stage IIA cases and approximately 50% of stage IIB cases.(62) In an effort to minimize long-term morbidity in TGCT survivors, there are two ongoing prospective clinical trials (SEMS and PRIMETEST) of RPLND for seminoma involving the retroperitoneal lymph nodes.(63)
There is similar controversy regarding treatment of node-positive, marker-negative NSGCT where options include RPLND or primary cisplatin-based chemotherapy (BEPx3 or EPx4).(56, 64) Chemotherapy provides early treatment of possible micrometastatic disease, however RPLND is the only treatment for possible teratoma. There are no randomized trials comparing chemotherapy with RPLND in this setting, though both approaches are associated with high cancer specific survival rates.(65) No nationwide analyses are available to address recent trends in the management of stage II NSGCT, although Memorial Sloan Kettering Cancer Center reports an increasing utilization of primary chemotherapy, in particular for patients with retroperitoneal lymphadenopathy >2cm, outside the primary landing zones, or multifocal disease.(65)
Metastatic Disease
Once TGCT has metastasized to organs beyond the retroperitoneum (cM1 disease) or serum tumor markers are significantly elevated (S2 or S3), cisplatin-based chemotherapy is the mainstay of treatment. Chemotherapy regimens are generally driven by International Germ Cell Cancer Collaborative Group (IGCCCG) categorization of disease into either good, intermediate, or poor-risk.(18, 66) Good-risk disease is typically managed with 3 cycles of BEP or 4 cycles of EP.(67–71) The standard regimen for Intermediate and poor risk disease is 4 cycles of BEP or alternatively 4 cycles of etoposide, ifosfamide, cisplatin (VIP).(17, 72) In poor-risk cancers, alternative regimens have been proposed and show promise in the first-line setting including paclitaxel, ifosfamide, cisplatin (TIP)(73) though randomized trials are required to demonstrate equivalency or superiority to standard regimens. As an example, addition of high-dose carboplatin chemotherapy and hematopoietic stem-cell rescue did not show improved outcomes for poor-risk TGCT over BEP alone in the first-line setting.(74) Treatment trends for utilization of various chemotherapy regimens based on IGCCCG risk criteria are not reported.
Post-Chemotherapy RPLND
Following chemotherapy for seminoma, an FDG positron-emission tomography (PET) scan is typically recommended to assess >3cm residual disease for viable germ cell tumor.(75) In the event of PET avid disease in the retroperitoneum and normal serum tumor markers following chemotherapy, typical management is post-chemotherapy RPLND (PC-RPLND), salvage chemotherapy, or tumor excision following confirmatory biopsy.(18) Following chemotherapy for NSGCT, PET scan is not typically utilized. Some investigators advocate for the selective use of PC-RPLND based on residual mass size and absence of teratoma in the orchiectomy specimen.(76) This strategy avoids “double therapy” in the majority of patients who have a complete clinical response to chemotherapy alone. Others advocate for PC-RPLND in all patients due the risk of chemotherapy-resistant disease in the retroperitoneum (viable germ cell tumor or teratoma). (65, 77, 78) In the United States, utilization of PC-RPLND appears to be highest at centers of excellence, a finding that may be related to experience with this more complicated surgery (Figure 2).(43)
Sperm Cryopreservation
Testicular cancer is associated with subfertility at baseline(79) and all subsequent treatments are risk factors for further declines in fertility potential. As such, the NCCN recommends discussion of sperm banking (cryopreservation) at the initial work-up of suspected TGCT and recommends sperm banking prior to RPLND, chemotherapy, or radiation treatment.(56) Despite these risks, the reported use of sperm banking is low.(80, 81) Even at high-volume institutions, only 30% of patients received chemotherapy chose to bank sperm.(82) Reasons for low utilization may include logistical concerns, cost, and knowledge of fertility risks associated with testicular cancer treatment. In a recent cost-effectiveness analysis, sperm banking was more cost-effective strategy than other assisted reproductive techniques in those patients interested in paternity. The estimated cost of sperm banking is $745 in the first year and $343 for each additional year of cryopreservation.(83)
Conclusion
Epidemiologic studies suggest a rising incidence of TGCT, in particular for developed countries. Additionally, racial variability in incidence and trends suggest a combination of biologic predisposition with environmental exposure is playing a role in the development of TGCT. Although TGCT has largely been shown to have a significant degree of heritability, there does not appear to be a major gene or variant linked with the development of testicular cancer. Treatment trends indicate an increased understanding of the long-term morbidity associated with adjuvant therapy for localized disease. Despite high cure rates overall, patients with lack of adequate access to care suffer high rates of mortality from TGCT.
Key Points.
Although there is substantial heterogeneity in reporting and bias in detection across countries, a wide body of literature suggests a rising incidence of testicular germ cell tumor worldwide.
There does not appear to be a major, high-penetrance gene mutation that confers a substantial risk of testicular germ cell tumor, but TGCT is a highly heritable disease and genome-wide association studies suggest multiple loci of susceptibility.
Clinically localized testicular cancer can be treated with active surveillance, adjuvant single-dose carboplatin or radiotherapy (seminoma) or adjuvant cisplatin-based chemotherapy or retroperitoneal lymph node dissection (NSGCT). All strategies have long-term survival rates approaching 100%, with high rates of salvage in patients who experience recurrence during surveillance.
Advanced testicular cancer often requires multi-disciplinary approaches involving chemotherapy and consolidative surgery. There is ongoing debate about the utility of PC-RPLND following complete response to chemotherapy for NSGCT.
While cure rates for TGCT are high, this requires complex coordination of multi-disciplinary care. Patients with lower socioeconomic status, minorities, and underinsured patients suffer from high rates of mortality.
Acknowledgements
None
Financial Support and Sponsorship
This work was supported by the National Institutes of Health (T32 CA136515 Ruth L. Kirschstein Institutional National Research Award to S.L.W.) and the Dedman Family Scholarship in Clinical Care (to A.B.).
Footnotes
Conflicts of Interest
None
References and Recommended Reading:
Papers of particular interest, published within the annual period of review, (the last 2 years) have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Znaor A, Lortet-Tieulent J, Jemal A, Bray F. International Variations and Trends in Testicular Cancer Incidence and Mortality. European Urology. 2014;65(6):1095–106. [DOI] [PubMed] [Google Scholar]
- 2.Society AC. Key Statistics for Testicular Cancer: The American Cancer Society; 2018. [updated January 4th, 2018. Available from: https://www.cancer.org/cancer/testicular-cancer/about/key-statistics.html.
- 3.Clemmesen J A DOUBLING OF MORBIDITY FROM TESTIS CARCINOMA IN COPENHAGEN, 1943–1962. Acta Pathologica Microbiologica Scandinavica. 1968;72(2):348–9. [DOI] [PubMed] [Google Scholar]
- 4.Chia VM, Quraishi SM, Devesa SS, Purdue MP, Cook MB, McGlynn KA. International trends in the incidence of testicular cancer, 1973–2002. Cancer Epidemiology Biomarkers and Prevention. 2010;19(5):1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman MP, Esteve J, Damiecki P, Arslan A, Renard H. Trends in cancer incidence and mortality. IARC scientific publications. 1993(121):1–806. [DOI] [PubMed] [Google Scholar]
- 6.Nigam M, Aschebrook-Kilfoy B, Shikanov S, Eggener S. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World Journal of Urology. 2015;33(5):623–31. [DOI] [PubMed] [Google Scholar]
- 7.Huyghe E, Matsuda T, Thonneau P. Increasing incidence of testicular cancer worldwide: a review. The Journal of urology. 2003;170(1):5–11. [DOI] [PubMed] [Google Scholar]
- 8.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer; 2013. [Available from: http://globocan.iarc.fr.
- 9.Ghazarian AA, Trabert B, Graubard BI, Schwartz SM, Altekruse SF, McGlynn KA. Incidence of testicular germ cell tumors among US men by census region. Cancer. 2015;121(23):4181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. •.Woldu SL, Aydin AM, Rao AV, Hutchinson RC, Singla N, Clinton TN, et al. Differences at Presentation and Treatment of Testicular Cancer in Hispanic Men: Institutional and National Hospital-based Analyses. Urology. 2018;112:103–11. NCDB analysis reporting that Hispanic patients are being diagnosed with TGCT approximately 5 years younger than non-Hispanic white patients and more likely to have non-seminomatous histology. [DOI] [PubMed] [Google Scholar]
- 11.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parkin DM, Iscovich J. Risk of cancer in migrants and their descendants in Israel: II. Carcinomas and germ-cell tumours. International journal of cancer. 1997;70(6):654–60. [DOI] [PubMed] [Google Scholar]
- 13.Hemminki K, Li X. Cancer risks in Nordic immigrants and their offspring in Sweden. European journal of cancer (Oxford, England : 1990). 2002;38(18):2428–34. [DOI] [PubMed] [Google Scholar]
- 14.Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Møller H. Trends in testicular cancer incidence and mortality in 22 European countries: Continuing increases in incidence and declines in mortality. International journal of cancer. 2006;118(12):3099–111. [DOI] [PubMed] [Google Scholar]
- 15. •.Pierorazio PM, Albers P, Black PC, Tandstad T, Heidenreich A, Nicolai N, et al. Non–risk-adapted Surveillance for Stage I Testicular Cancer: Critical Review and Summary. European Urology. 2018. Systematic review of studies evaluating the clinical efficacy of non-risk adapted active surveillance for clinically localized (stage I) testicular cancer [DOI] [PubMed] [Google Scholar]
- 16.Jones RH, Vasey PA. Part II: testicular cancer--management of advanced disease. The Lancet Oncology. 2003;4(12):738–47. [DOI] [PubMed] [Google Scholar]
- 17.de Wit R, Stoter G, Sleijfer DT, Neijt JP, ten Bokkel Huinink WW, de Prijck L, et al. Four cycles of BEP vs four cycles of VIP in patients with intermediate-prognosis metastatic testicular non-seminoma: a randomized study of the EORTC Genitourinary Tract Cancer Cooperative Group. European Organization for Research and Treatment of Cancer. Br J Cancer. 1998;78(6):828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group . Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(2):594–603. [DOI] [PubMed] [Google Scholar]
- 19. ••.Macleod LC, Cannon SS, Ko O, Schade GR, Wright JL, Lin DW, et al. Disparities in Access and Regionalization of Care in Testicular Cancer. Clinical Genitourinary Cancer. 2018. NCDB analysis reporting worse outcomes for patients who are non-white minorities, have lower socioeconomic status, and/or have unfavorable insurance [DOI] [PubMed] [Google Scholar]
- 20.Forman D, Oliver RTD, Brett AR, Marsh SGE, Moses JH, Bodmer JG, et al. Familial testicular cancer: A report of the UK family register, estimation of risk and an HLA class 1 sib-pair analysis. British Journal of Cancer. 1992;65(2):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemminki K, Li X. Familial risk in testicular cancer as a clue to a heritable and environmental aetiology. Br J Cancer. 2004;90(9):1765–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kharazmi E, Hemminki K, Pukkala E, Sundquist K, Tryggvadottir L, Tretli S, et al. Cancer Risk in Relatives of Testicular Cancer Patients by Histology Type and Age at Diagnosis: A Joint Study from Five Nordic Countries. Eur Urol. 2015;68(2):283–9. [DOI] [PubMed] [Google Scholar]
- 23.Litchfield K, Thomsen H, Mitchell JS, Sundquist J, Houlston RS, Hemminki K, et al. Quantifying the heritability of testicular germ cell tumour using both population-based and genomic approaches. Scientific reports. 2015;5:13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mucci LA, Hjelmborg JB, Harris JR, Czene K, Havelick DJ, Scheike T, et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. Jama. 2016;315(1):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litchfield K, Levy M, Dudakia D, Proszek P, Shipley C, Basten S, et al. Rare disruptive mutations in ciliary function genes contribute to testicular cancer susceptibility. Nature communications. 2016;7:13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung CC, Kanetsky PA, Wang Z, Hildebrandt MA, Koster R, Skotheim RI, et al. Meta-analysis identifies four new loci associated with testicular germ cell tumor. Nature genetics. 2013;45(6):680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristiansen W, Karlsson R, Rounge TB, Whitington T, Andreassen BK, Magnusson PK, et al. Two new loci and gene sets related to sex determination and cancer progression are associated with susceptibility to testicular germ cell tumor. Human molecular genetics. 2015;24(14):4138–46. [DOI] [PubMed] [Google Scholar]
- 28.Ruark E, Seal S, McDonald H, Zhang F, Elliot A, Lau K, et al. Identification of nine new susceptibility loci for testicular cancer, including variants near DAZL and PRDM14. Nature genetics. 2013;45(6):686–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalgaard MD, Weinhold N, Edsgard D, Silver JD, Pers TH, Nielsen JE, et al. A genome-wide association study of men with symptoms of testicular dysgenesis syndrome and its network biology interpretation. Journal of medical genetics. 2012;49(1):58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, McGlynn KA, Rajpert-De Meyts E, Bishop DT, Chung C, Dalgaard MD, et al. Meta-analysis of five genome-wide association studies identifies multiple new loci associated with testicular germ cell tumor. Nature genetics. 2017;49(7):1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litchfield K, Loveday C, Levy M, Dudakia D, Rapley E, Nsengimana J, et al. Large-scale Sequencing of Testicular Germ Cell Tumour (TGCT) Cases Excludes Major TGCT Predisposition Gene. European Urology. 2018. [DOI] [PubMed] [Google Scholar]
- 32.Woldu SL, Amatruda JF, Bagrodia A. Testicular germ cell tumor genomics. Curr Opin Urol. 2017;27(1):41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tandstad T, Dahl O, Cohn-Cedermark G, Cavallin-Stahl E, Stierner U, Solberg A, et al. Risk-Adapted Treatment in Clinical Stage I Nonseminomatous Germ Cell Testicular Cancer: The SWENOTECA Management Program. Journal of Clinical Oncology. 2009;27(13):2122–8. [DOI] [PubMed] [Google Scholar]
- 34.Gumus M, Bilici A, Odabas H, Ustaalioglu BBO, Kandemir N, Demirci U, et al. Outcomes of surveillance versus adjuvant chemotherapy for patients with stage IA and IB nonseminomatous testicular germ cell tumors. World Journal of Urology. 2017;35(7):1103–10. [DOI] [PubMed] [Google Scholar]
- 35.Tandstad T, Smaaland R, Solberg A, Bremnes RM, Langberg CW, Laurell A, et al. Management of Seminomatous Testicular Cancer: A Binational Prospective Population-Based Study From the Swedish Norwegian Testicular Cancer Study Group. Journal of Clinical Oncology. 2011;29(6):719–25. [DOI] [PubMed] [Google Scholar]
- 36.Sturgeon JF, Moore MJ, Kakiashvili DM, Duran I, Anson-Cartwright LC, Berthold DR, et al. Non–Risk-Adapted Surveillance in Clinical Stage I Nonseminomatous Germ Cell Tumors: The Princess Margaret Hospital’s Experience. European Urology. 2011;59(4):556–62. [DOI] [PubMed] [Google Scholar]
- 37.Kollmannsberger C, Tandstad T, Bedard PL, Cohn-Cedermark G, Chung PW, Jewett MA, et al. Patterns of Relapse in Patients With Clinical Stage I Testicular Cancer Managed With Active Surveillance. Journal of Clinical Oncology. 2015;33(1):51–7. [DOI] [PubMed] [Google Scholar]
- 38.Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB. Cardiovascular Disease Mortality After Chemotherapy or Surgery for Testicular Nonseminoma: A Population-Based Study. Journal of Clinical Oncology. 2015;33(28):3105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Travis LB, Curtis RE, Fraumeni JJF, Boice JJD, Storm H, Andersson M, et al. Risk of Second Malignant Neoplasms Among Long-term Survivors of Testicular Cancer. JNCI: Journal of the National Cancer Institute. 1997;89(19):1429–39. [DOI] [PubMed] [Google Scholar]
- 40.Tandstad T, Ståhl O, Dahl O, Haugnes HS, Håkansson U, Karlsdottir Å, et al. Treatment of stage I seminoma, with one course of adjuvant carboplatin or surveillance, risk-adapted recommendations implementing patient autonomy: a report from the Swedish and Norwegian Testicular Cancer Group (SWENOTECA). Annals of Oncology. 2016;27(7):1299–304. [DOI] [PubMed] [Google Scholar]
- 41.Warde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M, et al. Prognostic Factors for Relapse in Stage I Seminoma Managed by Surveillance: A Pooled Analysis. Journal of Clinical Oncology. 2002;20(22):4448–52. [DOI] [PubMed] [Google Scholar]
- 42.Matulewicz RS, Oberlin DT, Sheinfeld J, Meeks JJ. The Evolving Management of Patients With Clinical Stage I Seminoma. Urology. 2016;98:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. ••.Woldu SL, Matulay JT, Clinton TN, Singla N, Krabbe L- M, Hutchinson RC, et al. Impact of hospital case volume on testicular cancer outcomes and practice patterns. Urologic Oncology: Seminars and Original Investigations. 2018;36(1):14.e7–e5. NCDB analysis reporting higher volume institutions have improved outcomes for patients with advanced NSGCT. Additionally, there are significant differences in treatment patterns; higher volume institutions are more likely to treat stage I seminoma with surveillance, stage I NSGCT with primary RPLND, and advanced NSGCT with post-chemotherapy RPLND. [DOI] [PubMed] [Google Scholar]
- 44.Gray Phillip J, Lin Chun C, Sineshaw H, Paly Jonathan J, Jemal A, Efstathiou Jason A. Management trends in stage I testicular seminoma: Impact of race, insurance status, and treatment facility. Cancer. 2015;121(5):681–7. [DOI] [PubMed] [Google Scholar]
- 45.Oliver RTD, Mead GM, Rustin GJS, Joffe JK, Aass N, Coleman R, et al. Randomized Trial of Carboplatin Versus Radiotherapy for Stage I Seminoma: Mature Results on Relapse and Contralateral Testis Cancer Rates in MRC TE19/EORTC 30982 Study (ISRCTN27163214). Journal of Clinical Oncology. 2011;29(8):957–62. [DOI] [PubMed] [Google Scholar]
- 46.Oliver RTD, Mason MD, Mead GM, von der Maase H, Rustin GJS, Joffe JK, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. The Lancet. 2005;366(9482):293–300. [DOI] [PubMed] [Google Scholar]
- 47.Klepp O, Olsson AM, Henrikson H, Aass N, Dahl O, Stenwig AE, et al. Prognostic factors in clinical stage I nonseminomatous germ cell tumors of the testis: multivariate analysis of a prospective multicenter study. Swedish-Norwegian Testicular Cancer Group. Journal of Clinical Oncology. 1990;8(3):509–18. [DOI] [PubMed] [Google Scholar]
- 48.Vergouwe Y, Steyerberg EW, Eijkemans MJC, Albers P, Habbema JDF. Predictors of Occult Metastasis in Clinical Stage I Nonseminoma: A Systematic Review. Journal of Clinical Oncology. 2003;21(22):4092–9. [DOI] [PubMed] [Google Scholar]
- 49.Albers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C, et al. Risk Factors for Relapse in Clinical Stage I Nonseminomatous Testicular Germ Cell Tumors: Results of the German Testicular Cancer Study Group Trial. Journal of Clinical Oncology. 2003;21(8):1505–12. [DOI] [PubMed] [Google Scholar]
- 50.Albers P, Miller Greg A, Orazi A, Ulbright Thomas M, Albers J, Donohue John P, et al. Immunohistochemical assessment of tumor proliferation and volume of embryonal carcinoma identify patients with clinical stage a nonseminomatous testicular germ cell tumor at low risk for occult metastasis. Cancer. 2006;75(3):844–50. [DOI] [PubMed] [Google Scholar]
- 51.Hamilton RJ. Active Surveillance for Stage I Testicular Cancer: A Four-decade-old Experiment Proven Correct. Eur Urol. 2018. [DOI] [PubMed] [Google Scholar]
- 52.Albers P, Siener R, Krege S, Schmelz H- U, Dieckmann K- P, Heidenreich A, et al. Randomized Phase III Trial Comparing Retroperitoneal Lymph Node Dissection With One Course of Bleomycin and Etoposide Plus Cisplatin Chemotherapy in the Adjuvant Treatment of Clinical Stage I Nonseminomatous Testicular Germ Cell Tumors: AUO Trial AH 01/94 by the German Testicular Cancer Study Group. Journal of Clinical Oncology. 2008;26(18):2966–72. [DOI] [PubMed] [Google Scholar]
- 53.Tandstad T, Stahl O, Hakansson U, Dahl O, Haugnes HS, Klepp OH, et al. One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Annals of oncology : official journal of the European Society for Medical Oncology. 2014;25(11):2167–72. [DOI] [PubMed] [Google Scholar]
- 54.Kerns SL, Fung C, Monahan PO, Ardeshir-Rouhani-Fard S, Abu Zaid MI, Williams AM, et al. Cumulative Burden of Morbidity Among Testicular Cancer Survivors After Standard Cisplatin-Based Chemotherapy: A Multi-Institutional Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018:JCO2017770735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. ••.Weiner Adam B, Pearce Shane M, Eggener Scott E. Management trends for men with early‐stage nonseminomatous germ cell tumors of the testicle: An analysis of the National Cancer Database. Cancer. 2017;123(2):245–52. NCDB analysis reporting ~75% of stage IA NSGCT and ~50% of stage IB NSGCT is currently being managed by surveillance and surveillance is increasingly being utilized for stage IA disease over the past decade. [DOI] [PubMed] [Google Scholar]
- 56.Giligan T BC, Carneiro B, Chism D, Cost N, Derweesh IH. Testicular Cancer Version 2.2018. National Comprehensive Cancer Network, Inc.; 2018. [Google Scholar]
- 57.Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, et al. Guidelines on Testicular Cancer: 2015 Update. European Urology. 2015;68(6):1054–68. [DOI] [PubMed] [Google Scholar]
- 58.Kamran SC, Seisen T, Markt SC, Preston MA, Trinh Q- D, Frazier LA, et al. Contemporary Treatment Patterns and Outcomes for Clinical Stage IS Testicular Cancer. European Urology. 2018;73(2):262–70. [DOI] [PubMed] [Google Scholar]
- 59.Classen J, Schmidberger H, Meisner C, Souchon R, Sautter-Bihl ML, Sauer R, et al. Radiotherapy for stages IIA/B testicular seminoma: final report of a prospective multicenter clinical trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(6):1101–6. [DOI] [PubMed] [Google Scholar]
- 60.Chung PW, Gospodarowicz MK, Panzarella T, Jewett MA, Sturgeon JF, Tew-George B, et al. Stage II testicular seminoma: patterns of recurrence and outcome of treatment. Eur Urol. 2004;45(6):754–59; discussion 9–60. [DOI] [PubMed] [Google Scholar]
- 61.Giannatempo P, Greco T, Mariani L, Nicolai N, Tana S, Fare E, et al. Radiotherapy or chemotherapy for clinical stage IIA and IIB seminoma: a systematic review and meta-analysis of patient outcomes. Annals of oncology : official journal of the European Society for Medical Oncology. 2015;26(4):657–68. [DOI] [PubMed] [Google Scholar]
- 62.Glaser SM, Vargo JA, Balasubramani GK, Beriwal S. Stage II Testicular Seminoma: Patterns of Care and Survival by Treatment Strategy. Clinical Oncology.28(8):513–21. [DOI] [PubMed] [Google Scholar]
- 63. •.Hu B, Daneshmand S. Retroperitoneal Lymph Node Dissection as Primary Treatment for Metastatic Seminoma. Advances in urology. 2018;2018:7978958 Review article reporting on the rationale and trials evaluating RPLND for the treatment of seminoma [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weissbach L, Bussar-Maatz R, Flechtner H, Pichlmeier U, Hartmann M, Keller L. RPLND or primary chemotherapy in clinical stage IIA/B nonseminomatous germ cell tumors? Results of a prospective multicenter trial including quality of life assessment. Eur Urol. 2000;37(5):582–94. [DOI] [PubMed] [Google Scholar]
- 65.Stephenson AJ, Bosl GJ, Motzer RJ, Bajorin DF, Stasi JP, Sheinfeld J. Nonrandomized Comparison of Primary Chemotherapy and Retroperitoneal Lymph Node Dissection for Clinical Stage IIA and IIB Nonseminomatous Germ Cell Testicular Cancer. Journal of Clinical Oncology. 2007;25(35):5597–602. [DOI] [PubMed] [Google Scholar]
- 66.International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. Journal of Clinical Oncology. 1997;15(2):594–603. [DOI] [PubMed] [Google Scholar]
- 67.de Wit R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM, Fossa SD, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(6):1629–40. [DOI] [PubMed] [Google Scholar]
- 68.Saxman SB, Finch D, Gonin R, Einhorn LH. Long-term follow-up of a phase III study of three versus four cycles of bleomycin, etoposide, and cisplatin in favorable-prognosis germ-cell tumors: the Indian University experience. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(2):702–6. [DOI] [PubMed] [Google Scholar]
- 69.de Wit R, Stoter G, Kaye SB, Sleijfer DT, Jones WG, ten Bokkel Huinink WW, et al. Importance of bleomycin in combination chemotherapy for good-prognosis testicular nonseminoma: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(5):1837–43. [DOI] [PubMed] [Google Scholar]
- 70.Bajorin DF, Sarosdy MF, Pfister DG, Mazumdar M, Motzer RJ, Scher HI, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11(4):598–606. [DOI] [PubMed] [Google Scholar]
- 71.Kondagunta GV, Bacik J, Bajorin D, Dobrzynski D, Sheinfeld J, Motzer RJ, et al. Etoposide and cisplatin chemotherapy for metastatic good-risk germ cell tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(36):9290–4. [DOI] [PubMed] [Google Scholar]
- 72.Nichols CR, Catalano PJ, Crawford ED, Vogelzang NJ, Einhorn LH, Loehrer PJ. Randomized comparison of cisplatin and etoposide and either bleomycin or ifosfamide in treatment of advanced disseminated germ cell tumors: an Eastern Cooperative Oncology Group, Southwest Oncology Group, and Cancer and Leukemia Group B Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16(4):1287–93. [DOI] [PubMed] [Google Scholar]
- 73.Feldman DR, Hu J, Dorff TB, Lim K, Patil S, Woo KM, et al. Paclitaxel, Ifosfamide, and Cisplatin Efficacy for First-Line Treatment of Patients With Intermediate- or Poor-Risk Germ Cell Tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016;34(21):2478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motzer RJ, Nichols CJ, Margolin KA, Bacik J, Richardson PG, Vogelzang NJ, et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(3):247–56. [DOI] [PubMed] [Google Scholar]
- 75.De Santis M, Becherer A, Bokemeyer C, Stoiber F, Oechsle K, Sellner F, et al. 2–18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(6):1034–9. [DOI] [PubMed] [Google Scholar]
- 76.Beck SD, Foster RS, Bihrle R, Ulbright T, Koch MO, Wahle GR, et al. Teratoma in the orchiectomy specimen and volume of metastasis are predictors of retroperitoneal teratoma in post-chemotherapy nonseminomatous testis cancer. The Journal of urology. 2002;168(4 Pt 1):1402–4. [DOI] [PubMed] [Google Scholar]
- 77.Oldenburg J, Alfsen GC, Lien HH, Aass N, Wæhre H, Fosså SD. Postchemotherapy Retroperitoneal Surgery Remains Necessary in Patients With Nonseminomatous Testicular Cancer and Minimal Residual Tumor Masses. Journal of Clinical Oncology. 2003;21(17):3310–7. [DOI] [PubMed] [Google Scholar]
- 78.Steyerberg EW, Gerl A, Fossá SD, Sleijfer DT, de Wit R, Kirkels WJ, et al. Validity of predictions of residual retroperitoneal mass histology in nonseminomatous testicular cancer. Journal of Clinical Oncology. 1998;16(1):269–74. [DOI] [PubMed] [Google Scholar]
- 79.Peng X, Zeng X, Peng S, Deng D, Zhang J. The Association Risk of Male Subfertility and Testicular Cancer: A Systematic Review. PLoS ONE. 2009;4(5):e5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Girasole CR, Cookson MS, Smith JA Jr., Ivey BS, Roth BJ, Chang SS. Sperm banking: use and outcomes in patients treated for testicular cancer. BJU international. 2007;99(1):33–6. [DOI] [PubMed] [Google Scholar]
- 81.Post CM, Jain A, Degnin C, Chen Y, Craycraft M, Hung AY, et al. Current Practice Patterns Surrounding Fertility Concerns in Stage I Seminoma Patients: Survey of United States Radiation Oncologists. Journal of adolescent and young adult oncology. 2018. [DOI] [PubMed] [Google Scholar]
- 82.Sonnenburg DW, Brames MJ, Case-Eads S, Einhorn LH. Utilization of sperm banking and barriers to its use in testicular cancer patients. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(9):2763–8. [DOI] [PubMed] [Google Scholar]
- 83.Gilbert K, Nangia AK, Dupree JM, Smith JF, Mehta A. Fertility preservation for men with testicular cancer: Is sperm cryopreservation cost effective in the era of assisted reproductive technology? Urologic Oncology: Seminars and Original Investigations. 2018;36(3):92.e1–e9. [DOI] [PubMed] [Google Scholar]


