Abstract
Purpose
The aim of this study was to investigate the factors influencing the volume of the filtration bleb after Ex-PRESS® surgery.
Methods
This was a retrospective non-randomized study. After excluding patients who had undergone an additional glaucoma surgery, 99 glaucoma patients who underwent Ex-PRESS® surgeries and were followed up for >2 years were analyzed. We used anterior segment optical coherence tomography to measure the bleb volume, and compared the volume at 1 year with that at 2 years after surgery. We also examined potential influencing factors, including age, number of medications, central corneal thickness, type of glaucoma, preoperative intraocular pressure (IOP), postoperative IOP, reduction ratio of IOP, history of trabeculotomy, and operation method (Ex-PRESS® surgery only or simultaneous cataract surgery).
Results
Ex-PRESS® surgeries significantly decreased the IOP from 26.3±9.7 mmHg to 11.6±3.7 mmHg after 24 months (P<0.001). The success rate at 2 years was 81.2% (IOP ≥21 mmHg or ≥20% reduction from the baseline IOP). The results showed that age, postoperative IOP, glaucoma type, and simultaneous cataract surgeries influenced the filtration-bleb volume.
Conclusion
Advanced age, higher postoperative IOP, pseudo-exfoliation glaucoma, and simultaneous cataract surgeries were all found to decrease the volume of the filtration bleb.
Keywords: Ex-PRESS, glaucoma, bleb, size, factors, volume
Introduction
Trabeculectomy is the most common glaucoma surgery. In trabeculectomy, a bleb is formed in the sub-Tenon space to receive the outflow of aqueous humor, thereby lowering the intraocular pressure (IOP). The morphology of the bleb after trabeculectomy is an important clinical parameter and has been analyzed in numerous reports, these studies have revealed, for example, that the wall thickness, height, and width of the bleb correlate with IOP.1–5 However, reports on the influence of the bleb volume are rare.6,7 The main cause of unsuccessful trabeculectomy is that the bleb becomes too small and is crushed. Nonetheless, it is important to maintain the bleb for a long period of time in order to provide continuous low IOP. Thus, it is useful to measure the volume of the bleb periodically. The relatively difficult clinical method of slit-lamp examination was previously used for this purpose. The more recently developed anterior segment optical coherence tomography (AS-OCT) approach has made it easier to measure the volume of blebs.
There are 2 main surgical methods used to make blebs, the conventional trabeculectomy and the trabeculectomy with a mini shunt called Ex-PRESS® (Alcon Laboratories, Fort Worth, TX, USA) (Ex-PRESS). Many studies have compared conventional trabeculectomy and trabeculectomy with Ex-PRESS.8–11 Ex-PRESS exhibits similar outcomes with fewer early postoperative complications because of its low rate of over-filtration. Other advantages of Ex-PRESS are that the lumen of the device is standardized constantly, and the outflow is predictable.
In this study, we measured the volume of blebs of patients who underwent trabeculectomy with Ex-PRESS. Under the assumption that large blebs would contribute to low IOP, we investigated the factors influencing the bleb volume.
Subjects and methods
Subjects
This was a retrospective, non-randomized observational study. We analyzed the cases of 99 consecutive patients (110 eyes) who underwent a trabeculectomy with Ex-PRESS for the first time at Toyama University Hospital and were followed for >2 years. There were 8 patients who dropped out during the 2 years follow-up. We used data of their final visit. Since Ex-PRESS is contraindicated for use in patients with uveitis or primary angle-closure glaucoma, we performed trabeculectomy with Ex-PRESS only for patients with primary open-angle glaucoma (POAG) or pseudo-exfoliation glaucoma (PEXG). There were 11 patients who underwent trabeculectomy with Ex-PRESS in both eyes, and in these cases, we used unilateral data of the eye that was operated earlier. All subjects were recruited during the period from January 2013 to March 2016. All patients underwent a comprehensive ophthalmic examination, including refraction, Goldmann gonioscopy, Goldmann applanation tonometry (GAT), fundus examination, automated perimetry (Humphrey Field Analyzer; Carl Zeiss Meditec, Dublin, CA, USA), and measurement of central corneal thickness (CCT) and anterior chamber depth with AS-OCT (CASIA SS-1000; Tomey, Nagoya, Japan). Two glaucoma specialists (NT and AH) diagnosed all the cases of glaucoma.
The patients had already used tolerated glaucoma medications, but required further treatment to lower their IOP due to the progression of their visual field disorder. The research protocol was approved by the Institutional Review Board of the University of Toyama, and the procedures used conformed to the tenets of the Declaration of Helsinki. After the nature and possible consequences of the study were explained to the patients, written informed consent was obtained from each patient.
The preoperative (baseline) IOP was the mean of the IOPs recorded at 3 of the patient’s visits while on preoperative treatment. The IOP was measured using GAT.
Surgical techniques
All patients were operated on by 1 surgeon (NT). Retrobulbar anesthesia was administered. A standard fornix-based conjunctival incision was made to gain exposure to the scleral bed adjacent to the limbus. A single 3.5- mm2 square scleral flap was created. Mitomycin C (MMC) solution (0.04 mg/mL) was applied for 4 minutes. At this point, the eye was a completely enclosed space, and thus, the MMC solution could not flow into the anterior chamber. The treated area was then irrigated with ~100 mL of balanced salt solution. If the patient needed simultaneous cataract surgery, it was performed at this time. Phacoemulsification was performed with a WhiteStar Signature system (Abbott Medical Optics, Santa Ana, CA, USA).
Regarding the surgical indications for cataract surgery, since this was a retrospective study, no clear criteria were established, and cataract surgery was performed according to the judgment of the operator. The scleral flap was lifted, and a 25-gauge needle was horizontally inserted into the anterior chamber at the surgical limbus to create a path for the Ex-PRESS (model P50); the 25-gauge needle was inserted into the anterior chamber from the sclera–cornea transition zone parallel with the iris. The Ex-PRESS was then inserted into the anterior chamber. The scleral flap was sutured using 10–0 nylon, while adjusting the tension on the sutures to maintain the anterior chamber depth with a slow flow of aqueous humor around the margins of the scleral flap. The conjunctiva was meticulously closed with 10–0 nylon sutures. We confirmed that there was no leakage from the blebs.
Postoperative medication
The postoperative treatments consisted of topical steroids, antibiotics, and non-steroidal anti-inflammatory drugs (NSAIDs). The steroids and antibiotics were reduced over the 4 weeks following the interventions. NSAIDs were reduced over a 12-week period after the interventions. Anti-glaucoma medications were added at the discretion of the physicians. We counted a compounding agent as 2 medications.
Evaluation of the volume of blebs
We excluded data from patients who underwent additional glaucoma surgeries within 2 years post operation, and from patients who underwent a needling procedure or had bleb leaks within 2 years post operation. In total, we evaluated the blebs of 77 eyes. At 1 and 2 years after trabeculectomy with Ex-PRESS, filtering bleb images (12×12 mm square) were acquired via AS-OCT. We used the CASIA Bleb Assessment Software package (version 6P.1; Tomey) to analyze the size of the bleb automatically. In principle, AS-OCT measured the size of the hypo-reflective area compared with the surroundings with a C-scan (XY cross section) (Figure 1). In addition, while changing the position of the Z axis, we measured the hypo-reflective area of each section and calculated the volume of the bleb by integrating each hypo-reflective area. The thickness of 1 slice was about 10 µm. We did not perform any additional manual measurements. The volume of each bleb was measured twice by 2 researchers, and the results of these measurements were used to check the accuracy of the automatic measurement. We defined the mean value of the 2 measurements as the volume of the bleb. Based on the bleb volumes at 1 and 2 years after surgery, we obtained the rate of change in the volume of the bleb over the first 2 years postoperation. We calculated the bleb retention rate by dividing the volume of the bleb after 2 years by that after 1 year.
Figure 1.

Example of the result of measuring the volume of bleb with CASIA. SS-1000; Tomey, Nagoya, Japan.
Abbreviation: OS, oculus sinister.
We conducted a statistical analysis to determine whether various factors influenced the size or retention rate of the blebs. These factors were age, sex, CCT, glaucoma type (POAG or PEXG), number of preoperative anti-glaucoma medications, history of previous glaucoma surgery, preoperative IOP, postoperative IOP, reduction ratio of IOP, history of trabeculotomy, and operation method (Ex-PRESS surgery alone [Single surgery] or phacoemulsification + intraocular lens implantation and Ex-PRESS surgery simultaneously [Triple surgery]).
Definition of success
We used 2 main criteria of successful treatment. Success according to Criterion A was defined as postoperative IOP ≤21 mmHg or a ≥20% reduction from the baseline on 2 consecutive visits with or without glaucoma medications after the first postoperative month. Success according to Criterion B was defined as postoperative IOP ≤15 mmHg or a ≥20% reduction from the baseline on 2 consecutive visits with or without glaucoma medications after the first postoperative month. Cases were considered treatment failures if neither of the success criterions was met at 2 consecutive visits after the first postoperative month. Eyes requiring additional glaucoma surgery and those that developed phthisis or showed loss of light perception were classified as failures.
Statistical analysis
A Wilcoxon signed-rank test was used to compare IOP levels and numbers of glaucoma medications. An analysis of variance test was used for factors influencing the volume of blebs. In order to see the correlation between the volume of blebs and postoperative IOP, the Spearman coefficient was examined. A Kaplan–Meier survival analysis and log-rank tests were used for comparison of the success rates. All of the statistical analyses were performed with JMP Pro 11 software (SAS, Cary, NC, USA). Significance was defined as P-values <0.05.
Results
Ophthalmic data
The characteristics of the patients are shown in Table 1. We analyzed the cases of 99 patients: 53 males and 46 females. The mean (±SD) values for all 99 patients were as follows: age at the time of surgery, 70.8±10.2 years; CCT, 528±36 µm; follow-up period, 35.6±9.7 months; number of preoperative glaucoma medications, 4.2±1.0; and preoperative IOP, 26.3±9.7 mmHg. Seventy-four eyes already had undergone cataract surgery and underwent a trabeculectomy with Ex-PRESS alone, and the other 25 eyes were phakic and underwent cataract surgery and a trabeculectomy with Ex-PRESS® simultaneously. There were 2 patients who did not undergo simultaneous cataract surgery with phakic eyes. Twenty patients had previously undergone trabeculotomy (including Trabectome® or Canaloplasty). We classified the subtypes of glaucoma as POAG (n=39), PEXG (n=56), and secondary glaucoma (n=4).
Table 1.
Characteristics of 99 glaucoma patients who underwent trabeculectomy with an Ex-PRESS mini tube shunt (mean ± SD)
| Items | Mean ± SD |
|---|---|
| Age (years) | 70.8±10.2 |
| Gender (male/female), n | 53/46 |
| CCT (µm) | 528±36 |
| Pre-IOP (mmHg) | 26.3±9.7 |
| Pre-medications | 4.2±1.0 |
| History of trabeculotomy, n (%) | 20/99 (20.2%) |
| Type of glaucoma (POAG/PEXG), n | 39/56 |
| Methods of surgery (single/triple), n | 60/17 |
| Follow-up periods (months) | 35.6±9.7 |
| Post-IOP at 1 year (mmHg) | 11.2±3.7 |
| Post-medications at 1 year | 1.3±1.6 |
| Post-IOP at 2 years (mmHg) | 11.6±3.7 |
| Post-medications at 2 years | 2.0±1.7 |
| Volume of bleb at 1 year (mm3) | 5.28±6.41 |
| Volume of bleb at 2 years (mm3) | 4.02±5.26 |
| Bleb reduction rate (%) | 74.9±29.9 |
Notes: Post-medications, postoperative glaucoma medications; Pre-medications, preoperative glaucoma medications; Single, trabeculectomy with Ex-PRESS® alone; Triple, trabeculectomy with Ex-PRESS® and phacoemulsification and intraocular lens implantation simultaneously.
Abbreviations: CCT, central corneal thickness; IOP, preoperative intraocular pressure; PEXG, pseudo-exfoliation glaucoma; POAG, primary open-angle glaucoma; Post-IOP, postoperative intraocular pressure.
Postoperative IOP and success rate
We performed a Kaplan–Meier analysis to determine the success rates of the 99 eyes for both Criterion A and B (Figure 2). When using Criterion A, the success rates at 1 year and 2 years were 83.8% and 81.7%, and they were 79.8% and 76.7% when using Criterion B, respectively. Table 1 summarizes the postoperative data of the Ex-PRESS® surgeries. The mean postoperative IOP was 11.2±3.7 mmHg after 1 year, and 11.6±3.7 mmHg after 2 years; both values were significantly lower than the preoperative IOP (P<0.0001). The mean number of glaucoma medications was significantly decreased compared with the preoperative value (P<0.0001). The number of patients who required no glaucoma medication was 41 (45.0%) after 1 year and 27 (29.7%) after 2 years. The reasons for treatment failure were as follows: additional glaucoma surgery was required in 13 eyes, patients with postoperative IOP ≥20% reduction from the baseline on 2 consecutive visits was in 4 eyes and loss of light sensation developed in 1 eye. In all cases with postoperative IOP ≥20% reduction from the baseline on 2 consecutive visits, the preoperative IOP was <12 mmHg.
Figure 2.

Kaplan–Meier survival curves for Criterion A (postoperative IOP ≤21 mmHg or ≥20% reduction from the baseline) and Criterion B (postoperative IOP ≤15 mmHg or ≥20% reduction from the baseline).
Abbreviation: IOP, intraocular pressure.
The volume of blebs and influencing factors
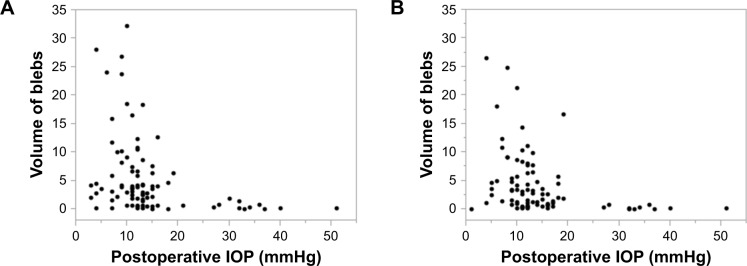
There was no case in which we could not analyze the volume of blebs with AS-OCT. For patients who needed additional glaucoma surgery, we used the volume of the blebs and IOP data immediately before additional surgery. The correlation between the volume of the blebs and postoperative IOP is shown in Figure 3. The higher postoperative IOP, the smaller the blebs. A significant correlation was observed between the volume of the blebs and the postoperative IOP after 1 year (P=0.0013, Spearman correlation coefficient =0.439) and 2 years (P=0.0014, Spearman correlation coefficient =0.419). The mean bleb volumes were 5.28±6.41 mm3 at 1 year after surgery and 4.02±5.26 mm3 at 2 years after surgery. The mean volume of the blebs with successful cases was 6.22±6.85 mm3 after 1 year and 4.71±5.61 mm3 after 2 years with Criterion A. The mean volume of the blebs with successful cases was 6.23±6.96 mm3 after 1 year and 4.84±5.79 mm3 after 2 years with Criterion B. The mean volume of the blebs with unsuccessful cases was 0.97±1.87 mm3 with Criterion A and 1.83±3.2 mm3 with Criterion B. The mean volume of blebs is significantly larger in success than in failure. (Figure 4) The mean bleb retention rate was reduced to 74.9%±29.9% between the first and second year postoperation.
Figure 3.
(A) The correlation between bleb volume and postoperative IOP after 1 year. (B) The correlation between bleb volume and postoperative IOP after 2 years. After 1 year and 2 years, the postoperative IOP and the volume of blebs showed a significant correlation. (1 year; P=0.0013, P=0.439 and 2 years; P=0.0014, P=0.419).
Abbreviation: IOP, intraocular pressure.
Figure 4.
(A) Comparison of the volume of the blebs at 1 year and 2 years after Ex-PRESS surgery and the volume of blebs of failure cases with Criterion A. Blebs of failure were significantly smaller. (1 year; P=0.004 and 2 years; P=0.0094) (B) Comparison of the volume of the blebs at 1 year and 2 years after Ex-PRESS surgery and the volume of blebs of failure cases with Criterion B. Blebs of failure were significantly smaller. (1 year; P=0.0085 and 2 years; P=0.0293).
We show the relationship between the volume of the bleb and the factors that may be affected in Tables 2 and 3. The factors that significantly influenced the bleb volume after 1 year were age (P=0.0458), postoperative IOP (P=0.034), presence of simultaneous cataract surgery (P=0.0096), and type of glaucoma (P=0.0202). After 2 years, the influencing factors were postoperative IOP (P=0.017) and presence of simultaneous cataract surgery (P=0.0064). Younger age, POAG, low postoperative IOP, and trabeculectomy with Ex-PRESS alone contributed to larger blebs. Presence of simultaneous cataract was the only factor influencing the bleb retention rate (P=0.0441): trabeculectomy with Ex-PRESS alone contributed to retention of the blebs.
Table 2.
Correlation of factors related to volume of blebs
| Factors | 1 year
|
2 years
|
Retention rate
|
|||
|---|---|---|---|---|---|---|
| Correlation coefficient (ρ) | P-value | Correlation coefficient (ρ) | P-value | Correlation coefficient (ρ) | P-value | |
| Age | 0.531 | 0.0458 | 0.411 | 0.0967 | 0.0564 | 0.667 |
| CCT | 0.108 | 0.207 | 0.143 | 0.165 | 0.155 | 0.159 |
| Pre-IOP | 0.168 | 0.579 | 0.142 | 0.0767 | 0.118 | 0.29 |
| Pre-Med | 0.0489 | 0.132 | 0.152 | 0.457 | 0.0889 | 0.166 |
| Post-IOP | 0.229 | 0.034 | 0.276 | 0.017 | 0.157 | 0.311 |
| Post-Med | 0.203 | 0.0737 | 0.173 | 0.131 | 0.0715 | 0.527 |
Notes: Correlation coefficient: Spearman correlation coefficient (ρ). Post-Med, postoperative glaucoma medications (bold numbers are significant); Pre-Med, preoperative glaucoma medications.
Abbreviations: CCT, central corneal thickness; Pre-IOP, preoperative intraocular pressure; Post-IOP, postoperative intraocular pressure.
Table 3.
Comparison of the bleb volume with influence factors (mean ± SD)
| 1 year | 2 years | Retention bleb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| The mean of volume of blebs | P-value | The mean of volume of blebs | P-value | Retention rate | P-value | ||||
| Gender | Male | 5.30±6.26 | 0.972 | Male | 4.23±5.66 | 0.669 | Male | 75.7±31.4 | 0.793 |
| Female | 5.25±6.70 | Female | 3.76±4.76 | Female | 73.9±28.3 | ||||
| Surgical methods | Single | 6.25±6.90 | 0.0096 | Single | 4.86±5.69 | 0.0064 | Single | 79.1±28.4 | 0.0441 |
| Triple | 2.22±3.08 | Triple | 1.40±2.04 | Triple | 62.8±32.7 | ||||
| History of trabeculotomy | A | 4.85±4.24 | 0.737 | A | 3.70±4.17 | 0.756 | A | 68.2±29.4 | 0.283 |
| N/A | 5.40±6.93 | N/A | 4.11±5.55 | N/A | 76.8±30.0 | ||||
| Glaucoma subtype | POAG | 7.18±7.70 | 0.0202 | POAG | 5.36±6.09 | 0.0733 | POAG | 72.7±32.0 | 0.262 |
| PEXG | 3.90±4.96 | PEXG | 3.24±4.61 | PEXG | 80.3±26.1 | ||||
Notes: Single, trabeculectomy with Ex-PRESS® alone; Triple, trabeculectomy with Ex-PRESS® and phacoemulsification and intraocular lens (bold numbers are significant).
Abbreviations: A, available; N/A, not available; PEXG, pseudo-exfoliation glaucoma; POAG, primary open-angle glaucoma.
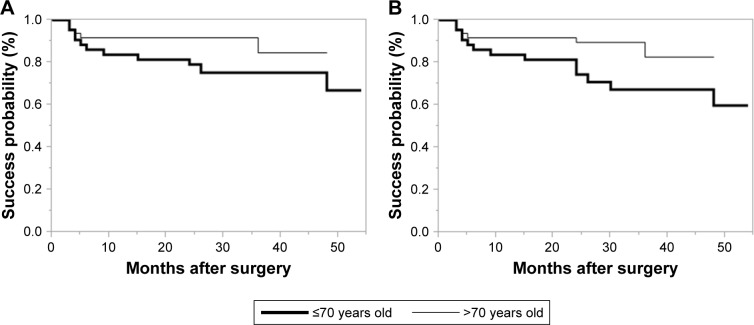
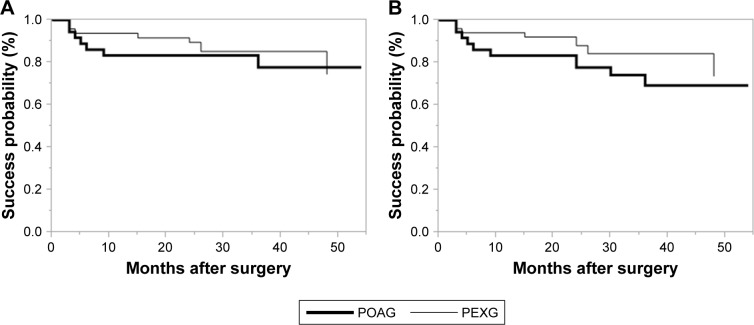
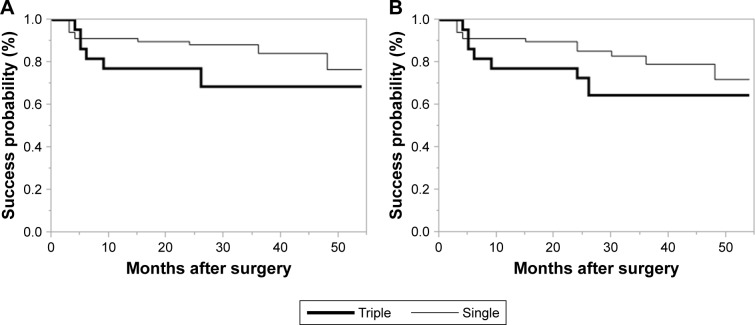
We show Kaplan–Meier survival plots comparing between younger patients (≤70 years of age) and older ones (>70 years of age) in Figure 5. There was no significant difference between the 2 groups with Criterion A (P=0.0941), significantly difference with Criterion B (P=0.0408). We show Kaplan–Meier survival plots comparing between POAG and PEXG patients in Figure 6. There was no significant difference between the 2 groups with Criterion A (P=0.664) and Criterion B (P=0.250). We show Kaplan–Meier survival plots comparing between Triple surgery patients and Single surgery in Figure 7. There was no significant difference between the 2 groups with Criterion A (P=0.172) and Criterion B (P=0.183).
Figure 5.
(A) Kaplan–Meier survival plots comparing younger patients (≤70 years of age; bold line) and older ones (>70 years of age; normal line) with Criterion A. There was no significant difference between the two groups. (P=0.0941) (B) Kaplan–Meier survival plots comparing younger patients (≤70 years of age; bold line) and older ones (>70 years of age; normal line) with Criterion B. Elderly patients had significantly better outcomes than younger ones. (P=0.0408).
Figure 6.
(A) Kaplan–Meier survival plots comparing POAG patients (bold line) and PEXG patients (normal line) with Criterion A. There was no significant difference between the 2 groups (P=0.644). (B) Kaplan–Meier survival plots comparing POAG patients (bold line) and PEXG patients (normal line) with Criterion B. There was no significant difference between the 2 groups (P=0.250).
Abbreviations: PEXG, pseudo-exfoliation glaucoma; POAG, primary open-angle glaucoma.
Figure 7.
(A) Kaplan–Meier survival plots comparing Triple surgery (bold line) and Single surgery patients (normal line) with Criterion A. There was no significant difference between the 2 groups (P=0.172). (B) Kaplan–Meier survival plots comparing Triple surgery (bold line) and Single surgery (normal line) with Criterion B. There was no significant difference between the 2 groups (P=0.187).
Discussion
Trabeculectomy with Ex-PRESS significantly reduced the IOP from 26.3±9.7 mmHg to 11.6±3.7 mmHg after 2 years. The mean bleb volumes were 5.28±6.41 mm3 at 1 year, and 4.02±5.26 mm3 at 2 years after surgery. Elderly patients, PEXG, high postoperative IOP, and Triple surgery are factors for small blebs. In previous analyses using Ex-PRESS, it has been reported that the postoperative IOP after 2 years ranges from 12.1 to 14.7 mmHg.8,11–13 Our results were thus slightly lower than those reported previously. This was at least partly due to the greater number of postoperative medications in our patients. The previous studies reported that 0.17–0.9 medications were administered after 2 years, while in our study, the number was 1.9 medications after 2 years.
Several studies have reported that the volume of the blebs is related to the postoperative IOP.1–5 Kojima et al reported that larger blebs are a prognostic factor for long-term IOP control.4
In terms of risk factors for the failure of trabeculectomy, previous studies have identified 3 factors: the type of glaucoma (neovascular glaucoma, uveitic glaucoma, or PEXG), the number of preoperative glaucoma medications, and simultaneous cataract surgery.14–18
Lim et al reported that trabeculectomy for PEXG had a success rate similar to POAG at 1 year, but after 2 postoperative years, the eyes with PEXG showed worse long-term IOP control than those with POAG.18 In our study, the eyes with PEXG had significantly smaller blebs than those with POAG (P=0.0202). This may have been due to inflammation involving the extracellular matrix in the eyes of patients with PEXG. Browne et al reported that the aqueous humor of PEXG patients contained greater levels of a connective tissue growth factor that increases the production of fibrillin-1 than did the aqueous humor of POAG patients.19 Tognetto et al reported that scanning electron microscopic images of the Ex-PRESS revealed the presence of fibrosis or cellular adhesion on the device.20 In our study, there was no significant difference in the surgical success rate between the patients with POAG and PEXG (Figure 6). Further long-term follow-up of this issue may be warranted. The blebs in PEXG patients did not become significantly smaller than those in POAG ones between the first and second postoperative years (P=0.492). This may indicate that blebs in patients with PEXG tend to be smaller within the first year after Ex-PRESS surgery.
In our study, Triple surgery was one of the factors associated with a reduction in the bleb volume and a low bleb retention rate. Ogata-Iwao et al reported that Triple surgery had worse surgical outcome compared with Single surgery.17 Inoue et al reported that monocyte chemotactic protein-1, which is prognostic factor for the results of trabeculectomy, increased when cataract surgery was performed.21,22 In this way, the characteristic cytokines that are increased as a result of cataract surgery may cause smaller blebs in patients undergoing Triple surgery. It seems that the outcome of the Single surgery is slightly better; there was no significant difference between the 2 groups (Figure 7).
The younger patients had a significantly larger mean bleb volume (P=0.0458). Moreover, the volume of the blebs in younger patients did not decline significantly between the postoperative measurement at 1 year and that at 2 years (P=0.667). Our results thus appeared to deviate from the previous reports in which younger patients had lower rates of surgical success.2,3,16 However, we consider that the bleb volume and surgical success rate may not necessarily have corresponded in our cohort, since we excluded the patients who underwent needling or additional glaucoma surgeries. In our study, the success rate at 2 years was 90.9% in patients >70 years of age and 79.6% in those ≤70 years of age, and there was significant difference in the success rate with Criterion B between age groups by the log-rank test of the Kaplan–Meier curve (P=0.0408). Therefore, despite our present findings, there may indeed be fewer young patients with successful trabeculectomy. In addition, young patients with successful surgeries may tend to have larger blebs than their older counterparts. On the contrary, blebs of elderly patients may be characterized by lower postoperative IOP, even if they were small.
Issa de Fendi et al reported that the use of >4 preoperative glaucoma medications is a risk factor for trabeculectomy failure.15 In our study, the number of preoperative medications did not affect the volume of the blebs. In our study, most of the patients were using 4 or more glaucoma medications (81.8%). Unless there was a special reason not too, we freely prescribed tolerated topical treatments, and therefore, many of our patients were using multiple glaucoma medications. The number of postoperative glaucoma medications after 1 year might have influenced the volume of the bleb, with fewer medications being associated with larger blebs. Sherwood et al reported that the use of glaucoma medications affected the conjunctiva and Tenon’s capsule.23 For other reasons, patients with small blebs might require more glaucoma medications. We considered that small blebs were likely to be associated with high postoperative IOP. However, it could also be that glaucoma medications reduce the size of blebs. In this context, it is worth noting the findings of Liu et al, who reported that latanoprost influenced bleb scarring after trabeculectomy.24
Although there have been many reports on the factors influencing conventional trabeculectomy, there have been few on the factors influencing trabeculectomy with Ex-PRESS. Mariotti et al reported that the risk factors for failure of Ex-PRESS surgery are diabetes, non-Caucasian race, and previous glaucoma surgery.25 In our study, we measured hemoglobin-A1C by blood sampling test in some patients, but not all, and thus this parameter was not entered into the analysis. All patients were Japanese and underwent a trabeculectomy with Ex-PRESS for the first time. It is thus difficult to make direct comparisons between this previous report and our present one.
Our study has several limitations. The most important was that the retrospective, non-comparative design had some bias. The small number of patients was another limitation; a larger prospective comparative study will be needed to fully investigate the volume of blebs. Research on the reproducibility of volume measurement of the blebs with AS-OCT has not been reported and may not be sufficient. There is no other way to objectively measure the volume of the blebs at the present time and it is a future task. We could not take into account the data of dropped out cases, they might be influencing our results. Finally, our results might have been affected by the learning curve involved in trabeculectomy surgery and postoperative interventions.
In conclusion, the factors influencing the volume of the blebs in our patients were PEXG, high postoperative IOP, Triple surgery (cataract surgery simultaneously), and advanced age. For patients with these factors, it may be necessary to take care to ensure that the blebs do not become smaller after trabeculectomy with Ex-PRESS.
Footnotes
Disclosure
The authors report no conflict of interests in this work.
References
- 1.Hamanaka T, Omata T, Sekimoto S, Sugiyama T, Fujikoshi Y. Bleb analysis by using anterior segment optical coherence tomography in two different methods of trabeculectomy. Invest Ophthalmol Vis Sci. 2013;54(10):6536–6541. doi: 10.1167/iovs.13-12439. [DOI] [PubMed] [Google Scholar]
- 2.Matlach J, Panidou E, Grehn F, Klink T. Large-area versus small-area application of mitomycin C during trabeculectomy. Eur J Ophthalmol. 2013;23(5):670–677. doi: 10.5301/ejo.5000287. [DOI] [PubMed] [Google Scholar]
- 3.Park HY, Ahn MD. Imaging of trabeculectomy blebs with Visante anterior segment optical coherence tomography after digital ocular compression. Jpn J Ophthalmol. 2012;56(1):38–45. doi: 10.1007/s10384-011-0101-8. [DOI] [PubMed] [Google Scholar]
- 4.Kojima S, Inoue T, Nakashima K, Fukushima A, Tanihara H. Filtering blebs using 3-dimensional anterior-segment optical coherence tomography: a prospective investigation. JAMA Ophthalmol. 2015;133(2):148–156. doi: 10.1001/jamaophthalmol.2014.4489. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda S, Beheregaray S, Kasaragod D, et al. Noninvasive evaluation of phase retardation in blebs after glaucoma surgery using anterior segment polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2014;55(8):5200–5206. doi: 10.1167/iovs.14-14474. [DOI] [PubMed] [Google Scholar]
- 6.Miura M, Kawana K, Iwasaki T, et al. Three-dimensional Anterior Segment Optical Coherence Tomography of Filtering Blebs After Trabeculectomy. J Glaucoma. 2008;17(3):193–196. doi: 10.1097/IJG.0b013e31815a34cd. [DOI] [PubMed] [Google Scholar]
- 7.Kawana K, Kiuchi T, Yasuno Y, Oshika T. Evaluation of trabeculectomy blebs using 3-dimensional cornea and anterior segment optical coherence tomography. Ophthalmology. 2009;116(5):848–855. doi: 10.1016/j.ophtha.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Netland PA, Sarkisian SR, Moster MR, et al. Randomized, prospective, comparative trial of EX-PRESS glaucoma filtration device versus trabeculectomy (XVT study) Am J Ophthalmol. 2014;157(2):433–440. doi: 10.1016/j.ajo.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Li W, Jiang F, Mao S, Tong Y. Ex-PRESS implantation versus trabeculectomy in open-angle glaucoma: a meta-analysis of randomized controlled clinical trials. PLoS One. 2014;9(1):e86045. doi: 10.1371/journal.pone.0086045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Zhang X. Meta-analysis of randomized controlled trials comparing EX-PRESS implantation with trabeculectomy for open-angle glaucoma. PLoS One. 2014;9(6):e100578. doi: 10.1371/journal.pone.0100578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Rodriguez JM, Trope GE, Drori-Wagschal L, Jinapriya D, Buys YM. Comparison of trabeculectomy versus Ex-PRESS: 3-year follow-up. Br J Ophthalmol. 2016;100(9):1269–1273. doi: 10.1136/bjophthalmol-2015-307161. [DOI] [PubMed] [Google Scholar]
- 12.Salim S, du H, Boonyaleephan S, Wan J. Surgical outcomes of the Ex-PRESS glaucoma filtration device in African American and white glaucoma patients. Clin Ophthalmol. 2014;6:955–962. doi: 10.2147/OPTH.S32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman J, Ferri S. Long-term comparison using Ex-PRESS glaucoma shunt in black and white patients. Can J Ophthalmol. 2014;49(2):200–204. doi: 10.1016/j.jcjo.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Landers J, Martin K, Sarkies N, Bourne R, Watson P. A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012;119(4):694–702. doi: 10.1016/j.ophtha.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 15.Issa de Fendi L, Cena de Oliveira T, Bigheti Pereira C, Pereira Bigheti C, Viani GA. Additive Effect of Risk Factors for Trabeculectomy Failure in Glaucoma Patients: A Risk-group From a Cohort Study. J Glaucoma. 2016;25(10):e879–e883. doi: 10.1097/IJG.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 16.Okimoto S, Kiuchi Y, Akita T, Tanaka J. Using the early postoperative intraocular pressure to predict pressure control after a trabeculectomy. J Glaucoma. 2014;23(6):410–414. doi: 10.1097/IJG.0b013e318285fd7d. [DOI] [PubMed] [Google Scholar]
- 17.Ogata-Iwao M, Inatani M, Takihara Y, Inoue T, Iwao K, Tanihara H. A prospective comparison between trabeculectomy with mitomycin C and phacotrabeculectomy with mitomycin C. Acta Ophthalmol. 2013;91(6):e500–e501. doi: 10.1111/aos.12133. [DOI] [PubMed] [Google Scholar]
- 18.Lim SH, Cha SC. Long-term Outcomes of Mitomycin-C Trabeculectomy in Exfoliative Glaucoma Versus Primary Open-Angle Glaucoma. J Glaucoma. 2017;26(4):303–310. doi: 10.1097/IJG.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 19.Browne JG, Ho SL, Kane R, et al. Connective tissue growth factor is increased in pseudoexfoliation glaucoma. Invest Ophthalmol Vis Sci. 2011;52(6):3660–3666. doi: 10.1167/iovs.10-5209. [DOI] [PubMed] [Google Scholar]
- 20.Tognetto D, Cecchini P, D’Aloisio R, Vattovani O, Turco G. Scanning Electron Microscopy Evaluation of an EX-PRESS Mini Glaucoma Shunt After Explantation. J Glaucoma. 2017;26(1):e1–e4. doi: 10.1097/IJG.0000000000000521. [DOI] [PubMed] [Google Scholar]
- 21.Inoue T, Kawaji T, Tanihara H. Monocyte chemotactic protein-1 level in the aqueous humour as a prognostic factor for the outcome of trab-eculectomy. Clin Exp Ophthalmol. 2014;42(4):334–341. doi: 10.1111/ceo.12204. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Kawaji T, Inatani M, Kameda T, Yoshimura N, Tanihara H. Simultaneous increases in multiple proinflammatory cytokines in the aqueous humor in pseudophakic glaucomatous eyes. J Cataract Refract Surg. 2012;38(8):1389–1397. doi: 10.1016/j.jcrs.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Sherwood MB, Grierson I, Millar L, Hitchings RA. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96(3):327–335. doi: 10.1016/s0161-6420(89)32888-0. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Liu Y, Xu D, Li J. Latanoprost-induced Cytokine and Chemokine Release From Human Tenon’s Capsule Fibroblasts: Role of MAPK and NF-κB Signaling Pathways. J Glaucoma. 2015;24(9):635–641. doi: 10.1097/IJG.0000000000000140. [DOI] [PubMed] [Google Scholar]
- 25.Mariotti C, Dahan E, Nicolai M, Levitz L, Bouee S. Long-term outcomes and risk factors for failure with the EX-press glaucoma drainage device. Eye (Lond) 2014;28(1):1–8. doi: 10.1038/eye.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]