Abstract
The aim of this study was to investigate the associations of accelerometer-assessed sedentary time and breaks in sedentary time with 24-h events and duration of hypoglycaemia (<3.9 mmol/l), euglycaemia (3.9–7.8 mmol/l), hyperglycaemia (>7.8 mmol/l) and above target glucose (>9 mmol/l). Thirty-seven participants with type 2 diabetes (age, 62.8 ± 10.5 years; body mass index, 29.6 ± 6.8 kg/m2) in Glasgow, United Kingdom were enrolled between February 2016 and February 2017. Participants wore an activity monitor (activPAL3) recording the time and pattern of sedentary behaviour and a continuous glucose monitoring (CGM, Abbott FreeStyle Libre) for up to 14 days. Linear regression analyses were used to investigate the associations. Participants spent 3.7%, 64.7%, 32.1% and 19.2% of recording h/day in hypoglycaemia, euglycaemia, hyperglycaemia and above target, respectively. There was a negative association between sedentary time and time in euglycaemia (β = −0.44, 95% CI −0.86; −0.03, p = 0.04). There was a trend towards a positive association between sedentary time and time in hyperglycaemia (β = 0.36, 95% CI −0.05; 0.78, p = 0.08). Breaks in sedentary time was associated with higher time in euglycaemia (β = 0.38, 95% CI 0.00; 0.75, p = 0.04). To conclude, in individuals with type 2 diabetes, more time spent in unbroken and continuous sedentary behaviour was associated with poorer glucose control. Conversely, interrupting sedentary time with frequent breaks appears to improve glycaemic control. Therefore, this should be considered as a simple adjunct therapy to improve clinical outcomes in type 2 diabetes.
Abbreviations: CGM, Continuous glucose monitoring; GLUT4, Glucose transporter 4; MET, Metabolic equivalent task; IL, Interleukin; TNF, Tumour necrosis factor
Keywords: Sedentary lifestyle, Physical activity, Glucose, Type 2 diabetes
Highlights
-
•
Glucose control is poor in people with type 2 diabetes spending 1/5 of their day above target range.
-
•
People with type 2 diabetes spent 1/3 of their day in hyperglycaemia.
-
•
Prolonged sedentary time was associated with poor glucose control.
-
•
Breaks in sedentary time were beneficially associated with glucose control.
1. Introduction
Type 2 diabetes is a chronic non-communicable disease affecting >90% of the global diabetes population (415 million) (International Diabetes Federation, 2015). The principal therapeutic goal of diabetes management is to achieve good glucose control in order to prevent diabetes-related complications (Bonora et al., 2001; Reid, 2010; Tancredi et al., 2015). However, daily glucose fluctuates widely outside the recommended range in people with type 2 diabetes even with diet management and oral anti-diabetes agents (Bonakdaran and Rajabian, 2009; Hay et al., 2003; Paing et al., 2017). This could be due to the heterogeneous and progressive nature of type 2 diabetes. Factors such as age, sex, body mass index (BMI), duration of diabetes and lifestyle all impact on glucose control (Franks et al., 2013; Hartz et al., 2006). It is therefore important to identify and target the modifiable lifestyle factors, in addition to oral anti-diabetes agents, to improve glucose control in type 2 diabetes.
Among lifestyle factors, sedentary time (time spent sitting or reclining) shows a consistent association with the risk of type 2 diabetes (Wilmot et al., 2012). Additionally, prolonged sedentary time is reported as a risk factor for high 2-h postprandial glucose and insulin resistance (Healy et al., 2007; Helmerhorst et al., 2009; Sardinha et al., 2017). In contrast, there is an emerging experimental evidence that breaks in sedentary time improve glucose metabolism through muscle contraction and insulin dependent and independent glucose uptakes (Bergouignan et al., 2016). A break in sedentary time is generally defined as a period of non-sedentary activity (e.g. standing or walking) in between two sedentary conditions (e.g. sitting or reclining posture) (Tremblay et al., 2017). In well-controlled laboratory settings and quasi-free-living settings, experimental studies showed that interrupting sedentary time with short frequent breaks reduces postprandial glucose, daily glucose and insulin resistance (Chastin et al., 2015; Dempsey et al., 2016; Duvivier et al., 2017). However, limited evidence is available that this is the case in actual free-living settings and that more frequent interruption of sedentary time in normal daily living is associated with better glucose control. Therefore, the present study aimed to explore the associations of sedentary time and breaks in sedentary time with glycaemic control measured as events and time in hypoglycaemia, euglycaemia, hyperglycaemia and above target glucose, using concurrent and continuous glucose and activity data in free-living settings.
2. Methods
The present study was a cross-sectional study and was approved by the University Ethics Committee (UEC) of University of Strathclyde. This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
2.1. Participants
Eligible participants were individuals with type 2 diabetes aged 18 years and over. Exclusion criteria were age < 18 years, pregnancy, insulin therapy, alcohol and substance abuse, liver and renal diseases and cancer. Recruitment was achieved through advertising within the staff of two universities, the Diabetes UK website, Diabetes Balance magazine and diabetes support groups in the Glasgow area.
2.2. Study protocol
This cross-sectional study was carried out between February 2016 and February 2017 and consisted of two short visits to the University laboratory or convenient location (e.g. participant's home). At the first visit, a continuous glucose monitoring (CGM, Abbott FreeStyle Libre) sensor was inserted into the subcutaneous tissue on the back of upper arm, and an activPAL3 activity monitor (PAL Technologies, Glasgow, UK) was attached to the anterior aspect of the right thigh, after the device was waterproofed. Demographic data were collected by the researcher. Participants were then requested to wear the CGM and activPAL3 for up to 14 days of normal daily living and to follow habitual diet. The activPAL3 and real-time glucose measurements provided by the CGM might influence participants' diet and physical activity patterns. To minimise this, they were reminded to maintain habitual diet and lifestyle throughout the study. To record diet, medication, bedtime and waking time; participants were provided with 24-h Dietary Recall Forms and sleep diary. Participants attended a second visit to remove the CGM and activPAL3.
2.3. Covariates
Demographic data included age, gender, body mass index (BMI), anti-diabetes medication, alcohol consumption and smoking status. Smoking status was classified as non-smoker and smoker, and alcohol consumption was classified as non-consumer, low consumer (≤14 units per week) and high consumer (>14 units per week). For each participant, carbohydrate intake in each day was calculated using 24-h Dietary Recall Form and Carbs & Cals Counter, and carbohydrate intake in each day was then averaged to estimate carbohydrate intake per day (Cheyette et al., 2013).
2.4. Glucose monitoring and glucose control measurements
The CGM (Freestyle Libre) used in this study measures interstitial glucose every 15 min for up to 2 weeks, and glucose data are retrieved wirelessly by the reader every 8 h. This is a well-tolerated consumer grade device, and the interstitial glucose measurements by this device are as accurate as capillary blood glucose (Bailey et al., 2015). The glucose data from the CGM were downloaded to a personal computer using FreeStyle Libre software (version 1.0). Global guideline for type 2 diabetes by the International Diabetes Federation was used to define thresholds for glucose control measures: events and time in hypoglycaemia (glucose < 3.9 mmol/l), euglycaemia (glucose 3.9–7.8 mmol/l), hyperglycaemia (glucose > 7.8 mmol/l) and above target (glucose > 9 mmol/l) (International Diabetes Federation Guideline Development Group, 2014). This is an evidence-based guideline targeting HbA1c < 53 mmol/mol (7%) to reduce diabetes-related complications, and 36% of national guidelines were also based on this guideline (Home et al., 2013). Daily events and time in hypoglycaemia, euglycaemia, hyperglycaemia and above target were computed using the glucose data from 00:00 to 00:00 h of two consecutive days. The first and final days, which do not have full 24-h recording, were excluded. Average daily events and time in hypoglycaemia, euglycaemia, hyperglycaemia and above target were then calculated. However, daily missing glucose data points can influence time in hypoglycaemia, euglycaemia, hyperglycaemia and above target because each missing glucose data point represents 15 min missing data time. Therefore, normalisation method was applied to deal with missing glucose data points and to calculate time spent in glucose control measures (e.g. Time in hypoglycaemia [% of recording h/day] = [Average daily time in hypoglycaemia / (24 h − Average daily missing data time)] × 100). Inclusion or exclusion criteria were not considered regarding missing glucose data points. HbA1c was self-reported by participants and it was based on their personal records from their last visits to diabetes clinic, diabetes specialist nurse (DSN) and general practitioner (GP).
2.5. Sedentary time, breaks in sedentary time and physical activity measurements
The activPAL3 was used to monitor sedentary time, breaks in sedentary time and physical activity of each participant. This is a small (53 × 35 × 7 mm) validated accelerometer and has been routinely used in clinical trials and epidemiological studies (Grant et al., 2006; Grant et al., 2008; Kozey-Keadle et al., 2011). This device records the start and duration of sitting, lying, standing and stepping for up to two weeks. The data were downloaded using the activPAL3™ software (version 7.2.32).
To determine daily sedentary time, time spent in sitting or lying posture between 00:00 to 00:00 h of two consecutive days was calculated, after sleeping time was removed using the sleep diary and activPAL events file (Chastin et al., 2014; Edwardson et al., 2016). The sleep diary, which still needs to be validated, was developed by our research group, and the sleep diary was used in conjunction with activPAL events file (Edwardson et al., 2016). A break in sedentary time was considered as a transition from sitting or lying condition to standing or stepping condition during waking hours. For each participant, daily sedentary time and number of breaks in sedentary time were first calculated, and average sedentary time and number of breaks per day were then computed. Average standing time, walking time and moderate to vigorous physical activity (MVPA) time per day were also calculated. A cadence greater or equal to 100 steps/min was considered as MVPA (Marshall et al., 2009).
2.6. Statistical analysis
Sample size calculations were based on a previous study, which reported the association between breaks in sedentary time and high 2-h plasma glucose (R2 = 0.21) (Healy et al., 2008). Assuming a statistical power of 85%, an alpha of 0.05 and six predictors, we estimated that 37 participants would be required to detect significant association between breaks in sedentary time and glucose control measures.
Participants with minimum 3 days of concurrent and continuous glucose and activity data were included in final analysis. Linear regression models were used to investigate the associations of sedentary time (Model 1) and breaks in sedentary time (Model 2) with events and time in hypoglycaemia, euglycaemia, hyperglycaemia and above target glucose. Model 1 was adjusted for age, sex, BMI, carbohydrate intake, energy expenditure and anti-diabetes medication. Model 2 was adjusted for age, sex, energy expenditure, anti-diabetes medication and sedentary time. To illustrate with a visual presentation, quartiles of sedentary time and breaks in sedentary time were calculated. Average sedentary time and number of breaks in sedentary time per day were used to calculate quartiles. The cut-off points for quartiles of sedentary time were 8.3, 9.7 and 11.4 h/day and the cut-off points for quartiles of breaks in sedentary time were 43, 52 and 60 n/day. General linear model univariate analysis was used to assess differences in glucose control measures between quartiles of sedentary time and breaks in sedentary time, and pairwise comparisons were conducted using post hoc Fisher LSD tests to locate differences. The p value ≤ 0.05 was considered statistically significant. Standardised coefficient (β) with 95% confidence interval (CI), mean/number with standard deviation (SD) and estimated marginal mean with standard error (SE) are used to report the results. Data were prepared with Microsoft Excel 2016, and statistical analyses were conducted using IBM SPSS Statistics software (version 24.0).
Post hoc power analyses were conducted using GPower 3.1.9.2 to assess observed statistical power of both significant and non-significant associations between sedentary time and breaks in sedentary time and glucose control measures with our current sample.
3. Results
3.1. Participant characteristics
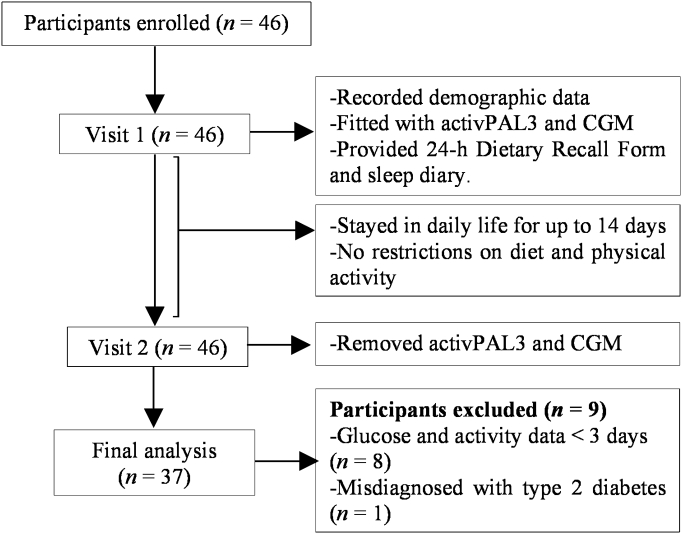
Study procedures and flow of participants are described in Fig. 1. Of the 46 participants, 37 participants were included in final analyses after excluding 9 participants for reasons: 1 participant with misdiagnosed type 2 diabetes and 8 participants with <3 days of glucose and activity data. Table 1 reports characteristics of the study population. The study sample has average 10 ± 3.4 days of the CGM and activPAL3 recording time.
Fig. 1.
Flow of participants.
Table 1.
Characteristics of the study population.
| Characteristics | |
|---|---|
| Number of participants (n) | 37 |
| Participants with 3 days data, n (%) | 1 (2.7%) |
| Participants with >3–<7 days data, n (%) | 9 (24.3%) |
| Participants with ≥7 days data, n (%) | 27 (72.9%) |
| Age (years) | 62.8 ± 10.5 |
| Sex, male/female (n) | 14/23 |
| BMI (kg/m2) | 29.6 ± 6.8 |
| HbA1c (mmol/mol), (n = 15 missing) | 47.7 ± 10.6 |
| HbA1c (%), (n = 15 missing) | 6.6 ± 0.9 |
| Diabetes management (n) | |
| No medication/diet modification alone | 12 |
| Metformin | 18 |
| Metformin + sulfonylurea | 5 |
| Metformin + gliptin | 1 |
| Metformin + sulfonylurea + gliptin | 1 |
| Alcohol consumption (n) | |
| Non-consumer | 18 |
| Low consumer (≤14 units per week) | 18 |
| High consumer (>14 units per week) | 1 |
| Smoking status (n) | |
| Non-smoker | 34 |
| Smoker | 3 |
| Carbohydrate intake (g/day) | 125.3 ± 21.1 |
| Energy expenditure (MET × h/day) | 33.6 ± 1.3 |
| Sedentary time (h/day) | 9.8 ± 1.8 |
| Breaks in sedentary time (n/day) | 52 ± 13 |
| Standing time (h/day) | 4.0 ± 1.5 |
| Walking time (h/day) | 1.6 ± 0.6 |
| MVPA time (min/day) | 32.1 ± 22.7 |
| CGM and activPAL3 recording time (days) | 10 ± 3.4 |
| CGM recording h/day | 22.4 ± 1.8 |
| Time in hypoglycaemia (% of recording h/day) | 3.7 ± 6.9 |
| Time in euglycaemia (% of recording h/day) | 64.7 ± 25.5 |
| Time in hyperglycaemia (% of recording h/day) | 32.1 ± 27.4 |
| Time above target (% of recording h/day) | 19.2 ± 20.8 |
| Hypoglycaemic events (n/day) | 0.7 ± 1.0 |
| Euglycaemic events (n/day) | 3.9 ± 1.1 |
| Hyperglycaemic events (n/day) | 2.9 ± 1.3 |
| Events above target (n/day) | 2.3 ± 1.4 |
Data are means ± SD or number (n). BMI, body mass index; MET, metabolic equivalent task.
3.2. Habitual sedentary time, breaks in sedentary time and glucose
Average daily sedentary time and number of breaks in sedentary time of participants were 9.8 ± 1.8 h/day and 52 ± 13 n/day. On average, the study population spent 3.7 ± 6.9% of recording h/day in hypoglycaemia, 64.7 ± 25.5% in euglycaemia, 32.1 ± 27.4% in hyperglycaemia and 19.2 ± 20.8% in above target; with 0.7 ± 1.0 hypoglycaemic events, 3.9 ± 1.1 euglycaemic events, 2.9 ± 1.3 hyperglycaemic events and 2.3 ± 1.4 above target events per day.
3.3. Association of sedentary time and breaks in sedentary time with glucose control measures
Table 2 shows standardised regression coefficients of sedentary time against glucose control measures. After adjustment for age, sex, BMI, carbohydrate intake, energy expenditure and anti-diabetes medication (Model 1), sedentary time was significantly associated with less time in euglycaemia (β = −0.44, 95% CI −0.86; −0.03, p = 0.04). There was a borderline statistically significant association of sedentary time with more time in hyperglycaemia (β = 0.36, 95% CI −0.05; 0.78, p = 0.08). But sedentary time showed no significant association with time in hypoglycaemia, time above target, and number of daily events in hypoglycaemic, euglycaemic, hyperglycaemic and above target.
Table 2.
Standardised regression of sedentary time with glucose control measures (Model 1).
| Glucose control measures | β (95% CI) | p value | Adjusted R2 |
|---|---|---|---|
| Time in hypoglycaemia (% of recording h/day) | 0.09 (−0.31, 0.47) | 0.68 | 0.03 |
| Time in euglycaemia (% of recording h/day) | −0.44 (−0.86, −0.03) | 0.04 | 0.14 |
| Time in hyperglycaemia (% of recording h/day) | 0.36 (−0.05, 0.78) | 0.08 | 0.14 |
| Time above target (% of recording h/day) | 0.33 (−0.08, 0.75) | 0.11 | 0.15 |
| Hypoglycaemic events (n/day) | −0.05 (−0.51, 0.40) | 0.81 | −0.05 |
| Euglycaemic events (n/day) | −0.07 (−0.51, 0.36) | 0.72 | 0.05 |
| Hyperglycaemic events (n/day) | 0.08 (−0.74, 0.51) | 0.72 | 0.05 |
| Events above target (n/day) | 0.29 (−0.12, 0.71) | 0.17 | 0.12 |
Data was adjusted for age, sex, BMI, carbohydrate intake, energy expenditure and anti-diabetes medication. β, standardised coefficient; CI, confidence interval.
Standardised regression coefficients of breaks in sedentary time with glucose measures are shown in Table 3. After adjustment for age, sex, energy expenditure, anti-diabetes medication and sedentary time (Model 2), there was a significant association of breaks in sedentary time with more time in euglycaemia (β = 0.38, 95% CI 0.00; 0.75, p = 0.04), but not with other glucose control measures. The association between breaks in sedentary time and time in euglycaemia was no longer significant (data not shown) when the model was additionally adjusted for BMI and carbohydrate intake.
Table 3.
Standardised regression of breaks in sedentary time with glucose control measures (Model 2).
| Glucose control measures | β (95% CI) | p value | Adjusted R2 |
|---|---|---|---|
| Time in hypoglycaemia (% of recording h/day) | −0.15 (−0.45, 0.15) | 0.39 | 0.02 |
| Time in euglycaemia (% of recording h/day) | 0.38 (0.00, 0.75) | 0.04 | 0.07 |
| Time in hyperglycaemia (% of recording h/day) | −0.30 (−0.70, 0.05) | 0.11 | −0.001 |
| Time above target (% of recording h/day) | −0.30 (−6.62, 0.06) | 0.11 | −0.03 |
| Hypoglycaemic events (n/day) | −0.16 (−0.56, 0.22) | 0.39 | −0.06 |
| Euglycaemic events (n/day) | −0.12 (−0.49, 0.26) | 0.52 | −0.09 |
| Hyperglycaemic events (n/day) | −0.15 (−0.49, 0.20) | 0.39 | 0.07 |
| Events above target (n/day) | −0.25 (−0.61, 0.11) | 0.16 | 0.09 |
Data was adjusted for age, sex, energy expenditure, anti-diabetes medication and sedentary time. β, standardised coefficient; CI, confidence interval.
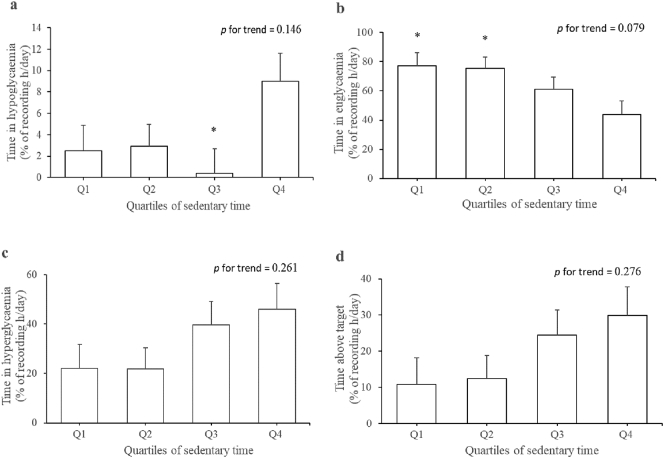
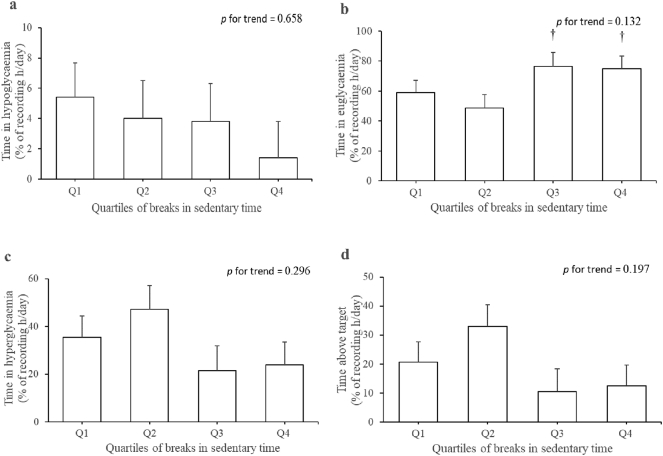
To describe the effect size pictorially, estimated marginal means for the associations of sedentary time and breaks in sedentary time with time in hypoglycaemia, euglycaemia, hyperglycaemia and above target are respectively shown in Fig. 2, Fig. 3. Compared with participants in the first and second quartiles of sedentary time, time in euglycaemia was 43.3% and 42.1% lower in those in the highest quartile (p < 0.03). Time in hypoglycaemia was 72.2%, 67.8% and 95.6% lower in participants in the first, second and third quartiles of sedentary time relative to those in the fourth quartile. However, significant difference was only observed between the fourth quartile and third quartile (p < 0.03). Time in euglycaemia was 36.2% and 34.8% higher in those in the third and fourth quartiles of breaks in sedentary time compared to those in the second quartile (p < 0.05).
Fig. 2.
Quartiles of sedentary time with glucose control measures: (a) time in hypoglycaemia, (b) time in euglycaemia, (c) time in hyperglycaemia and (d) time above target. Estimated marginal means (SE) were adjusted for age, sex, BMI, carbohydrate intake, energy expenditure and anti-diabetes medication. Cut-off points for quartiles of sedentary time were 8.3, 9.7 and 11.4 h/day. *p < 0.03 compared to quartile 4.
Fig. 3.
Quartiles of breaks in sedentary time with glucose control measures: (a) time in hypoglycaemia, (b) time in euglycaemia, (c) time in hyperglycaemia and (d) time above target. Estimated marginal means (SE) were adjusted for age, sex, energy expenditure and sedentary time. Cut-off points for quartiles of breaks in sedentary time were 43, 52 and 60 n/day. †p < 0.05 compared to quartile 2.
Post hoc power analyses showed that the sample size (n = 37) in this study provided 97% power to detect significant association between sedentary time and reduced time in euglycaemia and 88% power to detect significant association between breaks in sedentary time and increased time in euglycaemia, while adopting an alpha of 0.05 and six predictors. However, limited statistical power was observed for non-significant associations between sedentary time and hypoglycaemic events (69%) and non-significant associations between breaks in sedentary time and time in hyperglycaemia (75%), time above target (68%), hypoglycaemic events (56%) and euglycaemic events (45%). The recommended statistical power (≥80%) was observed for non-significant associations between sedentary time and breaks in sedentary and the remaining glucose control measures.
4. Discussion
In this study, the CGM revealed that people with type 2 diabetes considered well-controlled by diet modification and oral anti-diabetes agents according to HbA1c criteria can experience poor daily glucose control. This has also been suggested by other studies using CGM (Bonakdaran and Rajabian, 2009; Hay et al., 2003; Paing et al., 2017). In this study, 1/3 of the day was spent in hyperglycaemia and 1/5 of the day was spent above target range. Moreover, a short period of daily hypoglycaemia (3.7% of recording h/day) was observed in participants. We found that sedentary time was associated with this poor glucose control, reflected in reduced time in euglycaemia and a potential trend towards increased time in hyperglycaemia. On the other hand, breaks in sedentary time showed beneficial association with time in euglycaemia. The results suggest that reduction of sedentary time and increased breaks in sedentary time could improve daily glucose control.
The negative effect of sedentary time on increased time in hyperglycaemia in this study was in agreement with a previous cross-sectional study (Fritschi et al., 2016). The findings from our study extends the observation of this previous cross-sectional study by demonstrating the association of sedentary time with reduced time in euglycaemia. The present study showed that excessive sedentary time > 11.4 h/day (the fourth quartile) could be associated with increased time in hypoglycaemia. However, six out of nine participants in the fourth quartile of sedentary time were taking sulfonylurea whereas participants in the first, second and third quartiles were taking metformin ± gliptin (data not shown). There is evidence that hypoglycaemia is very common in people with type 2 diabetes taking sulfonylurea compared with those taking metformin (Van Dalem et al., 2016). We therefore suggest that increased time in hypoglycaemia in participants in the fourth quartile of sedentary time could be due to sulfonylurea. In addition, cause-effect relationship could not be confirmed by cross-sectional nature of this study. We suggest future studies to investigate cause-effect relationship and temporal relationship of sedentary time and breaks in sedentary time with glucose control measures, including glycaemic variability, postprandial glucose excursions and nocturnal glucose, in participants taking the same anti-diabetes medication in free-living settings.
Our results reiterate the importance of breaking sedentary behaviour, and it seems that the effect observed in the laboratory settings can be seen in free-living settings. The beneficial associations between breaks in sedentary time and fasting glucose and 2-h postprandial glucose after a test meal was observed in previous cross-sectional studies (Healy et al., 2008; Sardinha et al., 2017). The present study also extends the observation of these studies by showing that breaks in sedentary time is beneficially associated with increased time in euglycaemia in people with type 2 diabetes with habitual diet and physical activity patterns. The association between breaks in sedentary time and glucose control measures observed in this study might be influenced by the nature of breaks in free-living settings, such as standing breaks and walking breaks (Pulsford et al., 2017), and this should be investigated in future studies.
Breaking sedentary behaviour could be used as an adjunct therapy to oral anti-diabetes agents to improve glucose control in people with type 2 diabetes. The feasibility of short frequent habitual activity breaks in sedentary time in overweight and obese adults was previously reported (Bond et al., 2014; Graham Thomas and Bond, 2015). In addition, breaks in sedentary time intervention in free-living settings have been shown in subjects without type 2 diabetes to improve triglycerides and fasting glucose (Mailey et al., 2016). However, how often breaks in sedentary time should be performed during waking hours in order to achieve good glucose control in type 2 diabetes remains unknown. Dose-response of frequency of breaks in sedentary time and duration of sedentary bouts on glucose control measures needs to be investigated in type 2 diabetes.
The underlying mechanisms and effects of sedentary time and breaks in sedentary time could be explained by changes in muscle physiology. Skeletal muscle is one of the major sites for glucose metabolism (Sinacore and Gulve, 1993; DeFronzo and Tripathy, 2009; Dunstan et al., 2007). Reduced skeletal muscle contraction during prolonged sedentary time may contribute to poor glucose uptake peripherally (Bergouignan et al., 2016; Tremblay et al., 2010). It is suggested that even minimal contraction of skeletal muscle during breaks in sedentary time could increase blood flow and concentration of glucose transporter 4 (GLUT4) at muscle cell surface (Bergouignan et al., 2016; Tremblay et al., 2010; Richter and Hargreaves, 2013). As a result, habitual activity breaks in sedentary time could improve glucose metabolism and glucose control with or without the help of insulin. In addition, even minimal standing activity breaks in sedentary time have been shown experimentally to increase daily energy expenditure (Hawari et al., 2016), and this may also reflect improved glucose metabolism and glucose control in free-living settings (Assah et al., 2009).
Exposure to hyperglycaemia and hypoglycaemia diminish potent intracellular anti-oxidant, glutathione (GSH) but trigger oxidative stress and inflammatory biomarkers such as IL-1β (Interleukin), IL-8 and TNF-α (Tumour necrosis factor), which can induce endothelial dysfunction and vascular complications (Butkowski and Jelinek, 2016; Giacco and Brownlee, 2010; Gonzalez et al., 2012; Uberos Fernández et al., 2009; Razavi Nematollahi et al., 2009). In contrast, good glucose control closer to euglycaemic state has been shown to reduce diabetes-related complications and mortality in type 2 diabetes (Turner, 1998). The findings from this study suggest that reducing prolonged sedentary time and promoting breaks in sedentary time could be effective in achieving euglycaemia and reducing diabetes-related complications.
4.1. Study strengths and limitations
The main strength of the present study is the use of CGM and activPAL3. With the use of the activPAL3, accurate habitual sedentary time was assessed instead of self-reported sedentary time, and it was previously described that habitual sedentary time could be underreported with self-reported measures (Godfrey et al., 2007; Kozey-Keadle et al., 2011; Healy et al., 2011). With the use of the CGM (Abbott FreeStyle Libre), we assessed glucose throughout the study rather than periodic plasma glucose levels, therefore providing real-time accurate glucose control measures for up to 14 days (Bailey et al., 2015).
However, the present study has some limitations. First, cross-sectional design used in this study does not confirm causal inference. Nonetheless, the present study applied multiple linear regression analyses, which allow to adjust for possible causative factors such as age, sex and energy expenditure (Hartz et al., 2006; Assah et al., 2009). Second, the sample size was relatively small, which is often the case in CGM studies due to cost and burden. This means that we might be underpowered to detect some of the associations. For example, a sample size of n = 49 would be needed to detect association between breaks in sedentary time and time above target to reach a statistical power of 80% and an alpha of 0.05. Despite the small sample size, sedentary behaviour of our participants is similar to sedentary behaviour of participants in much larger study (van der Berg et al., 2016). Third, it is suggested that minimum 7 days of the activPAL data should be deployed to assess free-living sedentary and physical activity patterns (Edwardson et al., 2016), but ten participants with 3–6 days of glucose and activity data were included in this study. Nevertheless, the majority of participants (72.9%) reported ≥7 days of glucose and activity data. Fourth, analyses were not adjusted for types of food (glycaemic index) and meal timing, which could influence the relationship of sedentary time and breaks in sedentary time with glucose control measures (Morgan et al., 2012).
5. Conclusions
Less sedentary time and more frequent interruption in sedentary behaviour are associated with better glucose control in type 2 diabetes cross-sectionally in free-living settings. This suggests that reducing and breaking sedentary time could be a simple adjunct therapy to improve glycaemic control and reduce diabetes-related complications.
Acknowledgments
Acknowledgments
The authors thank KAM who put significant effort on participant recruitment. The authors would like to thank participants who devoted their time to the study.
Funding
This work was supported by School of Psychological Sciences and Health, University of Strathclyde; PAL technologies Ltd. (Glasgow, UK) and School of Health and Life-Sciences, Glasgow Caledonian University. The funding agencies did not play any role in the study design; collection, analysis and interpretation of data; preparing and writing up manuscript and making a decision to submit manuscript.
Duality of interest
No duality of interest associated with this manuscript was reported.
Contribution statement
ACP, KAM, AC, AFK, AH and SFMC were involved in the conception and design of the study and study question. ACP participated in data collection and analysis, interpreted the results, drafted and revised the manuscript. KAM contributed to data collection and processing. SFMC contributed to data analysis and interpretation of results and revised the manuscript. AC, AFK, KAM and AH contributed to the revision of the manuscript. The final manuscript was approved by all authors.
References
- Assah F.K., Ekelund U., Brage S., Mbanya J.C., Wareham N.J. Free-living physical activity energy expenditure is strongly related to glucose intolerance in cameroonian adults independently of obesity. Diabetes Care. 2009;32:367–369. doi: 10.2337/dc08-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T., Bode B.W., Christiansen M.P., Klaff L.J., Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol. Ther. 2015;17:787–794. doi: 10.1089/dia.2014.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergouignan A., Latouche C., Heywood S. Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci. Rep. 2016;6 doi: 10.1038/srep32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdaran S., Rajabian R. Prevalence and extent of glycemic excursions in well-controlled patients with type 2 diabetes mellitus using continuous glucose-monitoring system. Indian J. Med. Sci. 2009;63:66–71. [PubMed] [Google Scholar]
- Bond D.S., Thomas J.G., Raynor H.A. B-MOBILE - a smartphone-based intervention to reduce sedentary time in overweight/obese individuals: a within-subjects experimental trial. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonora E., Calcaterra F., Lombardi S., Formentini N.B.G., Bonadonna R.C., Muggeo M. Plasma glucose levels throughout the day and HbA1c interrelationships in type 2 diabetes: implications for treatment and monitoring of metabolic control. Diabetes Care. 2001;24:2023–2029. doi: 10.2337/diacare.24.12.2023. [DOI] [PubMed] [Google Scholar]
- Butkowski E.G., Jelinek H.F. Hyperglycaemia, oxidative stress and inflammatory markers. Redox Rep. 2016;0:1–8. doi: 10.1080/13510002.2016.1215643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastin S.F.M., Culhane B., Dall P.M. Comparison of self-reported measure of sitting time (IPAQ) with objective measurement (activPAL) Physiol. Meas. 2014;35:2319–2328. doi: 10.1088/0967-3334/35/11/2319. [DOI] [PubMed] [Google Scholar]
- Chastin S.F.M., Egerton T., Leask C., Stamatakis E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity. 2015;23:1800–1810. doi: 10.1002/oby.21180. [DOI] [PubMed] [Google Scholar]
- Cheyette C., Balolia Y., Diabetes U.K. fifth ed. Chello Publishing Limited; Great Britain: 2013. Carbs & Cals: Count Your Carbs Calories With Over 1,700 Food Drink Photos. [Google Scholar]
- DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32:S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey P.C., Larsen R.N., Sethi P. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016;39:964–972. doi: 10.2337/dc15-2336. [DOI] [PubMed] [Google Scholar]
- van der Berg J.D., Stehouwer C.D.A., Bosma H. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: the Maastricht study. Diabetologia. 2016;59:709–718. doi: 10.1007/s00125-015-3861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstan D.W., Salmon J., Healy G.N. Association of television viewing with fasting and 2-h postchallenge plasma glucose levels in adults without diagnosed diabetes. Diabetes Care. 2007;30:516–522. doi: 10.2337/dc06-1996. [DOI] [PubMed] [Google Scholar]
- Duvivier B.M.F.M., Schaper N.C., Hesselink M.K.C. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia. 2017;60:490–498. doi: 10.1007/s00125-016-4161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson C.L., Winkler E.A.H., Bodicoat D.H. Considerations when using the activPAL monitor in field based research with adult populations. J. Sport Health Sci. 2016;6:162–178. doi: 10.1016/j.jshs.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks P.W., Pearson E., Florez J.C. Gene-environment and gene-treatment interactions in type 2 diabetes: progress, pitfalls, and prospects. Diabetes Care. 2013;36:1413–1421. doi: 10.2337/dc12-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi C., Park H., Richardson A. Association between daily time spent in sedentary behavior and duration of hyperglycemia in type 2 diabetes. Biol. Res. Nurs. 2016;18:160–166. doi: 10.1177/1099800415600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A., Culhane K.M., Lyons G.M. Comparison of the performance of the activPAL professional physical activity logger to a discrete accelerometer-based activity monitor. Med. Eng. Phys. 2007;29:930–934. doi: 10.1016/j.medengphy.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez Y., Herrera M.T., Soldevila G. High glucose concentrations induce TNF-α production through the down-regulation of CD33 in primary human monocytes. BMC Immunol. 2012;13 doi: 10.1186/1471-2172-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham Thomas J., Bond D.S. Behavioral response to a just-in-time adaptive intervention (JITAI) to reduce sedentary behavior in obese adults: implications for JITAI optimization. Health Psychol. 2015;34:1261–1267. doi: 10.1037/hea0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P.M., Ryan C.G., Tigbe W.W., Granat M.H. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br. J. Sports Med. 2006;40:992–997. doi: 10.1136/bjsm.2006.030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P.M., Dall P.M., Mitchell S.L., Granat M.H. Activity-monitor accuracy in measuring step number and cadence in community-dwelling older adults. J. Aging Phys. Act. 2008;16:201–214. doi: 10.1123/japa.16.2.201. [DOI] [PubMed] [Google Scholar]
- Hartz A., Kent S., James P., Xu Y., Kelly M., Daly J. Factors that influence improvement for patients with poorly controlled type 2 diabetes. Diabetes Res. Clin. Pract. 2006;74:227–232. doi: 10.1016/j.diabres.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Hawari N.S.A., Al-Shayji I., Wilson J., Gill J.M.R. Frequency of breaks in sedentary time and postprandial metabolic responses. Med. Sci. Sports Exerc. 2016;48:2495–2502. doi: 10.1249/MSS.0000000000001034. [DOI] [PubMed] [Google Scholar]
- Hay L.C., Wilmshurst E.G., Fulcher G. Unrecognized hypo- and hyperglycemia in well-controlled patients with type 2 diabetes mellitus: the results of continuous glucose monitoring. Diabetes Technol. Ther. 2003;5:19–26. doi: 10.1089/152091503763816427. [DOI] [PubMed] [Google Scholar]
- Healy G.N., Dunstan D.W., Salmon J. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care. 2007;30:1384–1389. doi: 10.2337/dc07-0114. [DOI] [PubMed] [Google Scholar]
- Healy G.N., Dunstan D.W., Salmon J. Breaks in sedentary time. Diabetes Care. 2008;31:661–666. doi: 10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- Healy G.N., Clark B.K., Winkler E.A.H., Gardiner P.A., Brown W.J., Matthews C.E. Measurement of adults' sedentary time in population-based studies. Am. J. Prev. Med. 2011;41:216–227. doi: 10.1016/j.amepre.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst H.J.F., Wijndaele K., Brage S., Wareham N.J., Ekelund U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58:1776–1779. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home P., Haddad J., Latif Z.A. Comparison of National/Regional Diabetes Guidelines for the management of blood glucose control in non-western countries. Diab. Ther. 2013;4:91–102. doi: 10.1007/s13300-013-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Diabetes Federation . International Diabetes Federation; 2015. IDF Diabetes Atlas. Retrieved from. [Google Scholar]
- International Diabetes Federation Guideline Development Group Global guideline for type 2 diabetes. Diabetes Res. Clin. Pract. 2014;104:1–52. doi: 10.1016/j.diabres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Kozey-Keadle S., Libertine A., Lyden K., Staudenmayer J., Freedson P.S. Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sports Exerc. 2011;43:1561–1567. doi: 10.1249/MSS.0b013e31820ce174. [DOI] [PubMed] [Google Scholar]
- Mailey E.L., Rosenkranz S.K., Casey K., Swank A. Comparing the effects of two different break strategies on occupational sedentary behavior in a real world setting: a randomized trial. Prev. Med. Rep. 2016;4:423–428. doi: 10.1016/j.pmedr.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S.J., Levy S.S., Tudor-Locke C.E. Translating physical activity recommendations into a pedometer-based step goal. 3000 steps in 30 minutes. Am. J. Prev. Med. 2009;36:410–415. doi: 10.1016/j.amepre.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Morgan L.M., Shi J.W., Hampton S.M., Frost G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012;108:1286–1291. doi: 10.1017/S0007114511006507. [DOI] [PubMed] [Google Scholar]
- Paing A.C., Kirk A., Collier A., Chastin S.F.M. Basic and clinical science posters: type 2 diabetes. Diabet. Med. 2017;34:74–83. [Google Scholar]
- Pulsford R.M., Blackwell J., Hillsdon M., Kos K. Intermittent walking, but not standing, improves postprandial insulin and glucose relative to sustained sitting: a randomised cross-over study in inactive middle-aged men. J. Sci. Med. Sport. 2017;20:278–283. doi: 10.1016/j.jsams.2016.08.012. [DOI] [PubMed] [Google Scholar]
- Razavi Nematollahi L., Kitabchi A.E., Stentz F.B. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metab. Clin. Exp. 2009;58:443–448. doi: 10.1016/j.metabol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Reid T.S. The importance and treatment of postprandial hyperglycemia. J. Fam. Pract. 2010;59:S9–S14. [PubMed] [Google Scholar]
- Richter E.A., Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- Sardinha L.B., Magalhães J.P., Santos D.A., Júdice P.B. Sedentary patterns, physical activity, and cardiorespiratory fitness in association to glycemic control in type 2 diabetes patients. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinacore D.R., Gulve E.A. The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: implications for physical therapy. Phys. Ther. 1993;73:878–891. doi: 10.1093/ptj/73.12.878. [DOI] [PubMed] [Google Scholar]
- Tancredi M., Rosengren A., Svensson A.M. Excess mortality among persons with type 2 diabetes. N. Engl. J. Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- Tremblay M.S., Colley R.C., Saunders T.J., Healy G.N., Owen N. Physiological and health implications of a sedentary lifestyle. Appl. Physiol. Nutr. Metab. 2010;35:725–740. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- Tremblay M.S., Aubert S., Barnes J.D. Sedentary Behavior Research Network (SBRN) - terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017;14 doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- Uberos Fernández J., Fernández-García J.M., Molina-Carballo A., Muñoz-Hoyos A. Effects on the glutathione pool of the insulin-induced hypoglycaemia test. J. Biol. Regul. Homeost. Agents. 2009;23:149–154. [PubMed] [Google Scholar]
- Van Dalem J., Brouwers M.C.G.J., Stehouwer C.D.A. Risk of hypoglycaemia in users of sulphonylureas compared with metformin in relation to renal function and sulphonylurea metabolite group: population based cohort study. BMJ. 2016;354 doi: 10.1136/bmj.i3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot E., Edwardson C., Achana F. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]