Abstract
This cohort study uses electronic health record data to examine associations between maternal diabetes (preexisting type 1, type 2, and gestational) and autism spectrum disorder in children.
Maternal preexisting type 2 diabetes (T2D) and gestational diabetes mellitus (GDM) diagnosed by 26 weeks’ gestation have been associated with increased risk of autism spectrum disorder (ASD) in offspring.1 However, little is known about ASD risk associated with maternal preexisting type 1 diabetes (T1D). We extend previous observations by examining the risk of ASD in offspring associated with maternal T1D, T2D, and GDM.
Methods
This retrospective cohort study included singleton children born at 28 to 44 weeks’ gestation in Kaiser Permanente Southern California (KPSC) hospitals from January 1, 1995, through December 31, 2012. Children were tracked through electronic health records from age 1 year until the first date of the following: clinical diagnosis of ASD, last date of continuous KPSC membership, death, or study end date (December 31, 2017). The KPSC institutional review board approved this study and provided waiver of participant consent.
Methods to identify ASD, T2D, and GDM exposure were described previously.1 GDM exposure was divided into diagnosis by or after 26 weeks’ gestation based on a prior finding that risk of ASD was elevated for exposure to GDM by 26 weeks.1 T1D was identified using the algorithm developed for electronic health records data2 and confirmed by prescription of insulin during pregnancy. Potential confounders were birth year, maternal age at delivery, parity, education, self-reported race/ethnicity, median family household income based on residence census tract, history of comorbidity (≥1 diagnosis of heart, lung, kidney, or liver disease; cancer), and child’s sex. Data on maternal smoking and obesity (measured by prepregnancy body mass index [calculated as weight in kilograms divided by height in meters squared]) were available beginning after 2006 (on 36% of the cohort). The missing indicator method was used to additionally adjust for these covariates. Cox regression was used to estimate hazard ratios (HRs) adjusting for potential confounders. All mothers with T1D and T2D, but only 29% of those with GDM, were dispensed antidiabetic medications during pregnancy. Potential risk associated with antidiabetic medication exposure was assessed in the GDM group, adjusting for potential confounders plus the gestational age at GDM diagnosis. SAS Enterprise Guide (SAS Institute), version 5.1, and R (R Foundation), version 3.4.3 (64 bit), were used for data analysis. A 2-sided P value less than .05 was considered significant.
Results
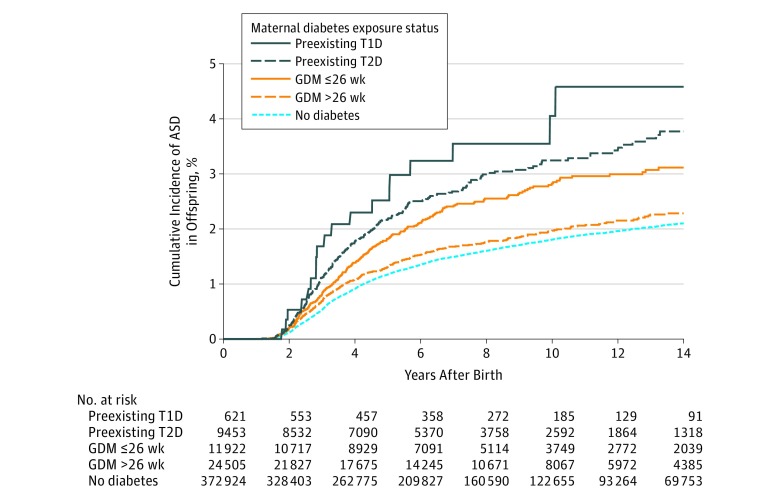
Of 419 425 children (boys, 51%) meeting study criteria, 621 were exposed to maternal T1D, 9453 to maternal T2D, 11 922 to GDM diagnosed by 26 weeks’ gestation, and 24 505 to GDM diagnosed after 26 weeks’ gestation. During a median follow-up of 6.9 years (interquartile range, 3.4-11.9), 5827 children were diagnosed with ASD. Unadjusted average annual ASD incidence rates per 1000 children were 4.4 for exposure to T1D; 3.6 for T2D; 2.9 for GDM by 26 weeks; 2.1 for GDM after 26 weeks; and 1.8 for no diabetes. The Figure depicts the cumulative incidence rates by maternal diabetes exposure groups. Relative to no diabetes exposure, the adjusted HRs for exposure to maternal diabetes were 2.36 (95% CI, 1.36-4.12) for T1D, 1.45 (95% CI, 1.24-1.70) for T2D, 1.30 (95% CI, 1.12-1.51) for GDM by 26 weeks’ gestation, and 0.99 (95% CI, 0.88-1.12) for GDM after 26 weeks. Additional adjustment for maternal smoking during pregnancy and prepregnancy BMI changed results only slightly (Table). Risks were not statistically significantly different between those with vs without antidiabetic medication exposure during pregnancy within the GDM group (adjusted HR, 1.18 [95% CI, 0.97-1.43]; P = .10).
Figure. Unadjusted Cumulative Incidence of ASD in Offspring by Maternal Diabetes Exposure In Utero.
ASD indicates autism spectrum disorder; GDM, gestational diabetes mellitus; T1D, type 1 diabetes; T2D, type 2 diabetes. ASD includes autistic disorders, Asperger syndrome, or pervasive developmental disorder not otherwise specified.
Table. Adjusted Risk of ASD in Offspring Associated With In Utero Exposure to Maternal Preexisting T1D, T2D, and Gestational Diabetes During the Index Pregnancya.
| Maternal Diabetes Status During Pregnancy | No. of ASD Cases/No. of Children | Adjusted HR (95% CI)b | P Value | Adjusted HR (With Smoking and Prepregnancy BMI) (95% CI)b,c |
P Value |
|---|---|---|---|---|---|
| No diabetes | 4950/372 924 | 1 [Reference] | 1 [Reference] | ||
| Preexisting | |||||
| Type 1 | 19/621 | 2.36 (1.36-4.12) | .003 | 2.33 (1.29-4.21) | .005 |
| Type 2 | 233/9453 | 1.45 (1.24-1.70) | <.001 | 1.39 (1.18-1.62) | <.001 |
| Gestational diagnosed at ≤26 wk | 253/11 922 | 1.30 (1.12-1.51) | <.001 | 1.26 (1.08-1.47) | <.001 |
| Gestational diagnosed at >26 wk | 372/24 505 | 0.99 (0.88-1.12) | .89 | 0.98 (0.87-1.10) | .72 |
Abbreviations: ASD, autism spectrum disorder; BMI, body mass index; HR, hazard ratio; T1D, type 1 diabetes; T2D, type 2 diabetes.
ASD includes autistic disorders, Asperger syndrome, or pervasive developmental disorder not otherwise specified.
Estimated by Cox regression models in which family was specified as a random effect to control for potential correlation due to multiple siblings born to the same mother. Potential confounders were birth year, maternal age, parity, education, median household income based on census tract of residence, self-reported race/ethnicity, history of comorbidity (≥1 diagnosis of heart, lung, kidney, or liver disease; cancer); and sex of the child. Race/ethnicity was self-reported. Race/ethnicity was included in this study as a potential confounder because it was associated with maternal diabetes and child’s ASD risk.
Additionally adjusted for smoking and prepregnancy BMI. About 36% of the cohort had data on smoking during pregnancy and prepregnancy BMI because these variables were routinely recorded in the electronic medical records after 2006. To preserve power for the primary exposures, a “missing” category was used for individuals with missing smoking information and a “missing” indicator variable was created for individuals with missing prepregnancy BMI in the multivariable adjusted analysis. Prepregnancy BMI was treated as a continuous variable in the adjustment.
Discussion
Among the 3 main types of diabetes complicating pregnancy, the risk of ASD in offspring was elevated in mothers with T1D, T2D, and GDM diagnosed by 26 weeks’ gestation compared with no diabetes. These results add new information on T1D and extend previous findings1 for preexisting T2D and GDM. GDM diagnosed after 26 weeks’ gestation was not associated with excess risk compared with no diabetes.
These results suggest that the severity of maternal diabetes and the timing of exposure (early vs late in pregnancy) may be associated with the risk of ASD in offspring of diabetic mothers. The potential role of maternal glycemia; other features of T1D such as autoimmunity and genetic factors3,4; prematurity; and neonatal hypoglycemia5 remains to be explored. Confounding due to paternal risk factors and other intrauterine and postnatal exposures could not be assessed.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Xiang AH, Wang X, Martinez MP, et al. Association of maternal diabetes with autism in offspring. JAMA. 2015;313(14):1425-1434. doi: 10.1001/jama.2015.2707 [DOI] [PubMed] [Google Scholar]
- 2.Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care. 2013;36(4):914-921. doi: 10.2337/dc12-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atladóttir HO, Pedersen MG, Thorsen P, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124(2):687-694. doi: 10.1542/peds.2008-2445 [DOI] [PubMed] [Google Scholar]
- 4.Keil A, Daniels JL, Forssen U, et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology. 2010;21(6):805-808. doi: 10.1097/EDE.0b013e3181f26e3f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens LA, Sedar J, Carmody L, Dunne F. Comparing type 1 and type 2 diabetes in pregnancy—similar conditions or is a separate approach required? BMC Pregnancy Childbirth. 2015;15:69. doi: 10.1186/s12884-015-0499-y [DOI] [PMC free article] [PubMed] [Google Scholar]