Key Points
Question
Is initiation of a basal insulin analog compared with human neutral protamine Hagedorn (NPH) insulin associated with a reduced risk of hypoglycemia-related emergency department (ED) visits or hospital admissions in patients with type 2 diabetes?
Findings
In this retrospective observational study of 25 489 patients with type 2 diabetes, initiation of basal insulin analogs compared with NPH insulin was not associated with a significant difference in hypoglycemia-related ED visits or hospital admissions among a propensity-score matched cohort of 4428 patients (hazard ratio, 1.16).
Meaning
Among patients with type 2 diabetes, the use of basal insulin analogs compared with NPH insulin was not associated with a reduced risk of hypoglycemia-related ED visits or hospital admissions.
Abstract
Importance
In clinical trials of patients with type 2 diabetes, long-acting insulin analogs modestly reduced the risk of nocturnal hypoglycemia compared with human neutral protamine Hagedorn (NPH) insulin, but cost 2 to 10 times more. Outcomes in clinical practice may differ from trial results.
Objective
To compare the rates of hypoglycemia-related emergency department (ED) visits or hospital admissions associated with initiation of long-acting insulin analogs vs human NPH insulin in patients with type 2 diabetes.
Design, Setting, and Participants
A retrospective observational study using data from Kaiser Permanente of Northern California from January 1, 2006, through September 30, 2015. Patients with type 2 diabetes who initiated a long-acting insulin analog or NPH insulin were included and censored at death, loss of health plan coverage, change in insulin treatment, or study end on September 30, 2015.
Exposure
Initiation of basal insulin analogs (glargine or detemir) vs NPH insulin.
Main Outcomes and Measures
The primary outcome was the time to a hypoglycemia-related ED visit or hospital admission and the secondary outcome was the change in hemoglobin A1c level within 1 year of insulin initiation.
Results
There were 25 489 patients with type 2 diabetes who initiated basal insulin therapy (mean age, 60.2 [SD, 11.8] years; 51.9% white; 46.8% female). During a mean follow-up of 1.7 years, there were 39 hypoglycemia-related ED visits or hospital admissions among 1928 patients who initiated insulin analogs (11.9 events [95% CI, 8.1 to 15.6] per 1000 person-years) compared with 354 hypoglycemia-related ED visits or hospital admissions among 23 561 patients who initiated NPH insulin (8.8 events [95% CI, 7.9 to 9.8] per 1000 person-years) (between-group difference, 3.1 events [95% CI, −1.5 to 7.7] per 1000 person-years; P = .07). Among 4428 patients matched by propensity score, the adjusted hazard ratio was 1.16 (95% CI, 0.71 to 1.78) for hypoglycemia-related ED visits or hospital admissions associated with insulin analog use. Within 1 year of insulin initiation, hemoglobin A1c level decreased from 9.4% (95% CI, 9.3% to 9.5%) to 8.2% (95% CI, 8.1% to 8.2%) after initiation of insulin analogs and from 9.4% (95% CI, 9.3% to 9.5%) to 7.9% (95% CI, 7.9% to 8.0%) after initiation of NPH insulin (adjusted difference-in-differences for glycemic control, −0.22% [95% CI, −0.09% to −0.37%]).
Conclusions and Relevance
Among patients with type 2 diabetes, initiation of a basal insulin analog compared with NPH insulin was not associated with a reduced risk of hypoglycemia-related ED visits or hospital admissions or with improved glycemic control. These findings suggest that the use of basal insulin analogs in usual practice settings may not be associated with clinical advantages for these outcomes.
This pharmacoepidemiology study estimates the risk of hypoglycemia-related emergency department visits or hospital admissions associated with initiation of long-acting insulin analogs vs human neutral protamine Hagedorn (NPH) insulin in patients with type 2 diabetes.
Introduction
Treatment of type 2 diabetes typically begins with lifestyle modification and initiation of metformin; however, 14% to 25% of patients eventually require initiation of insulin to reach recommended glycemic targets.1,2 The mainstay of insulin treatment has long been human synthetic insulin; however, insulin analogs have become increasingly popular in clinical practice during the past decade.3,4 Insulin analogs are molecularly altered forms of insulin that more closely mimic the pharmacokinetic profile of endogenous insulin.
In clinical trials, long-acting insulin analogs modestly reduce the risk of nocturnal hypoglycemia compared with human insulin, but have not been shown to reduce the risk of severe hypoglycemia or to improve glycemic control among patients with type 2 diabetes.5 Discrepancies between trial results and outcomes in clinical practice are common and highlight the importance of gathering additional evidence from usual care settings.6
Although human insulin products are still used preferentially within Kaiser Permanente of Northern California (KPNC), prior work demonstrated widespread adoption of insulin analogs among US patients during the past 2 decades.3,4,7 At the same time, the prices of insulin analogs have increased dramatically,8,9 Medicaid payments for insulin have increased substantially,10 and patients’ out-of-pocket spending on insulin analogs has doubled.4 In this setting, it is imperative to understand the differences in health outcomes associated with the use of the more expensive insulin analogs vs the more affordable human insulin products.
This study investigated the rates of hypoglycemia-related emergency department (ED) visits or hospital admissions and changes in levels of glycemic control after initiation of long-acting insulin analogs (glargine or detemir) compared with human neutral protamine Hagedorn (NPH) insulin among patients with type 2 diabetes in clinical practice.
Methods
Study Source
The institutional review boards of the Kaiser Foundation Research Institute and the University of Chicago approved the study. Participant informed consent was waived. A large, integrated health care delivery system, KPNC provides care for approximately 30% of the residents in the Northern California service area. The KPNC diabetes registry has been maintained since 1993. The registry now includes more than 350 000 adults with diabetes and is updated annually by identifying all health plan members with diabetes.
The identification of clinically recognized diabetes among health plan members is based on multiple sources of data including pharmacy use; laboratory results; and outpatient, emergency department, and hospitalization diagnoses of diabetes detailed further in a published algorithm.11 Race/ethnicity was measured because prior studies suggest it is associated with both hypoglycemia and glycemic control.12,13 Determination of race/ethnicity was based on self-reported race/ethnicity captured in the electronic medical record according to fixed categories. The study methods and a validation study of the KPNC diabetes registry (99% sensitivity for diabetes based on chart review registration) have been published.14
Study Population
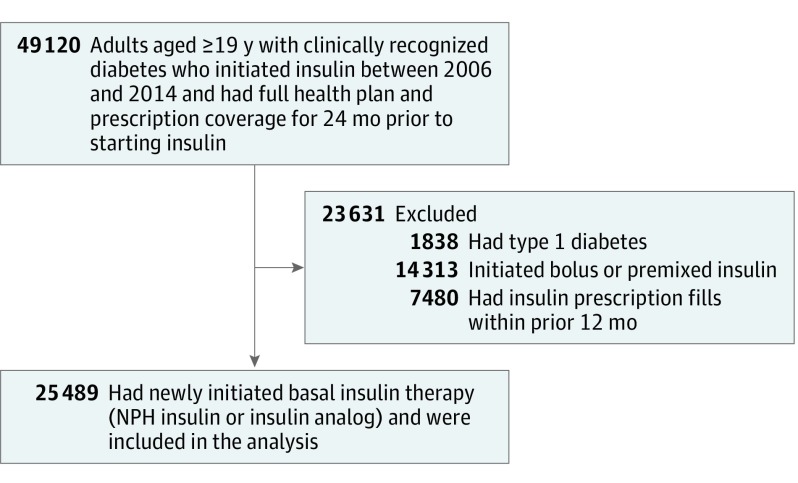
Using electronic medical records from KPNC, 49 190 adults (aged ≥19 years) with diabetes were identified. Each patient had full health plan and prescription coverage for 24 months prior to initiating insulin between January 1, 2006, and December 31, 2014. Patients with type 1 diabetes were excluded (n = 1838) based on a validated algorithm that uses self-report or age of diabetes onset and drug treatment history to determine diabetes type.15 Clinicians within KPNC can prescribe either NPH insulin or insulin analogs to patients with type 2 diabetes without obtaining prior approval; however, clinicians are encouraged to start with NPH insulin.
The analytic cohort consisted of patients who initiated basal insulin therapy and had no insulin prescription fills during the prior 12 months (Figure 1). Patients started with either NPH insulin or the insulin analog glargine or detemir. Patients using prandial insulin at baseline were excluded from the study. Patients who initiated prandial insulin during the study were censored at that time.
Figure 1. Derivation of the Study Cohort.
Adults with type 2 diabetes and full health plan and prescription coverage were included if they began basal insulin therapy (neutral protamine Hagedorn [NPH] or insulin analog) between January 1, 2006, and December 31, 2014.
Study Outcomes
The primary outcome was the time to hypoglycemia-related ED visit or hospital admission after initiation of insulin therapy based on a primary or principal discharge diagnosis of hypoglycemia using a validated algorithm (any of the following International Classification of Diseases, Ninth Revision codes: 251.0, 251.1, 251.2, 962.3, or 250.8 modified by 259.8, 272.7, 681, 682, 686.9, 707.1-707.9, 709.3, 730.0-730.2, or 731.8).16
The secondary outcome was the change in hemoglobin A1c level, which is a marker for the clinical effectiveness of insulin. For the baseline hemoglobin A1c level, the last measure during the 12 months prior to insulin initiation was used. The change from baseline to the last hemoglobin A1c level was assessed prior to censoring and within 3 to 12 months after insulin initiation. A change in hemoglobin A1c level of 0.5% or greater is typically considered to be clinically significant.17
Statistical Analysis
The analysis involved multiple steps. During the first step, a propensity score model was developed, predicting the binary outcome of initiating treatment with basal insulin analogs (compared with NPH insulin) using a flexible, data-adaptive model selection procedure called the deletion, substitution, and addition algorithm by Neugebauer and Bullard (available in R version 3.1.4; R Foundation for Statistical Computing).18 The deletion, substitution, and addition procedure made use of training and test data sets to select the estimator with the lowest cross-validated risk among a list of candidate estimators developed via machine learning (ie, deletion, substitution, and addition of potential covariates as well as interactions and higher-order parameters).
Potential covariates included: demographics, index year, clinical and comorbid characteristics, clinician specialty (primary care, endocrinology, or other specialty), KPNC service area, Charlson comorbidity index, chronic kidney disease stage, chronic liver disease, visual impairment, history of diabetic ketoacidosis, history of depression, glycemic control, the number of hypoglycemia-related ED visits or hospital admissions during the year prior to baseline, the number of ED visits or inpatient stays (for any reason) during the year prior to baseline, medication nonadherence (continuous measure of medication gaps19,20), outpatient medical visits (ie, the number of face-to-face visits with a clinician) during the 2 years prior to baseline, the patient co-pay for index insulin dispensed, and indicators of prevalent use for each of the diabetes therapeutic drug classes, statins, angiotensin-converting enzyme inhibitors, and β-blockers.
Missing data for continuous variables were imputed based on the within-group mean. Missing data for categorical variables were treated as a separate category. The C statistic (area under the receiver operating characteristic curve) for this model was 0.81, suggesting good discrimination.
During the second step, the predicted probability (ie, propensity score) of initiating treatment with long-acting insulin analogs was calculated for each patient. Quintiles of the propensity score were created based on the distribution of the propensity scores among the exposed patients (ie, patients who initiated insulin analogs). Using frequency matching (random sampling with replacement), 500 reference patients who initiated NPH insulin were selected from each of the quintiles defined by the exposed group.
This frequency matching created a population in which the distribution of covariates in the NPH insulin cohort was similar to those in the insulin analog cohort, thus minimizing observed confounders. Balance in the covariate distribution in each cohort was assessed by visually inspecting box plots and cumulative probability distributions of the propensity scores between exposed and reference patients and quantitatively through the calculation of the standardized difference, which compares the difference in means or prevalence of baseline covariates in units of the pooled SDs. A standardized difference with the absolute value of less than or equal to 0.1 indicates a negligible difference in the mean or prevalence of a covariate between groups.21
During the third step, a survival analysis was conducted for the outcome of hypoglycemia-related ED visits or hospital admissions. This approach examined time to first event of hypoglycemia-related ED visit or hospital admission. Patients were censored at the earliest event: death, end of KPNC membership, end of prescription drug benefits, discontinuation of NPH insulin or long-acting insulin, addition of any other insulin subtype, or end of follow-up (September 30, 2015). The hazard ratios (HRs) and 95% CIs were calculated from the results of the Cox proportional hazards analyses on 1000 bootstrap samples with replacement, and were created using the methods described above.
The proportional hazard assumption was tested by assessing independence between the Schoenfeld residuals and follow-up time. The primary analysis included the HR after adjusting for baseline covariates that remained unbalanced after propensity score matching (ie, those with the absolute value of the standardized difference >0.1), as well as additional adjustments for prior hypoglycemia-related ED visits or hospital admissions and for time-dependent indicators of diabetes medication use. The use of sulfonylureas, metformin, or thiazolidinediones was based on dispensing of a given medication within 6 months prior to the start of insulin; thereafter, it was based on monthly fills and days’ supply dispensed.
In a sensitivity analysis, the HR was additionally calculated using traditional regression adjustment for covariates that were significantly different at baseline for prior hypoglycemia-related ED visits or hospital admissions and for time-dependent indicators of diabetes medication use. Based on a post hoc estimate with a sample size of 25 489 patients, the study had 80% power to detect a HR of 2.1 or greater or of 0.5 or less for the outcome of hypoglycemia-related ED visits or hospital admissions associated with the initiation of insulin analogs vs NPH insulin.
During the fourth step, the change in hemoglobin A1c level following insulin initiation was estimated using a difference-in-differences approach. This approach measured the change in glycemic control associated with the initiation of long-acting insulin analogs (first difference) after subtracting the background change (second difference [eg, due to secular trends]) among patients who initiated NPH insulin.22 This model was based on the counterfactual assumption that if patients who initiated insulin analogs had instead initiated NPH insulin, their changes in hemoglobin A1c level would be similar to the changes observed in the NPH insulin reference group, who were frequency matched based on the propensity score quintile. The model was adjusted for baseline covariates that remained unbalanced after propensity score matching.
In the main secondary outcome analysis, participants with missing data for hemoglobin A1c level at baseline and those who were censored within 90 days of baseline were excluded. In a sensitivity analysis, patients also were excluded if the use of any class of diabetes medications changed from baseline until they were censored or until 12 months after initiation of insulin, whichever occurred first. The purpose of this analysis was to isolate the relationship between insulin initiation and change in hemoglobin A1c levels.
The difference-in-differences estimates and 95% CIs were calculated from the results of a least-squares regression analysis on 1000 bootstrap samples with replacement.23 We used R version 3.3.1 and SAS version 9.3 (SAS Institute Inc) statistical software for all analyses. A P value <.05 was considered statistically significant and all testing was 2-sided.
Results
Patient Characteristics at Baseline
Between 2006 and 2014, a total of 25 489 patients with type 2 diabetes initiated basal insulin therapy (Table 1). The mean age was 60.2 years (SD, 11.8 years) and 46.8% were female. The racial/ethnic makeup of the cohort consisted of 51.9% who were white, 9.2% who were black, 17.6% who were Hispanic, and 15.3% who were Asian. The Charlson comorbidity index value was 0 among 28.1%, 1 among 28.5%, 2 among 11.3%, and 3 or greater among 32.1%.
Table 1. Baseline Characteristics of 25 489 Patients With Type 2 Diabetes.
| Characteristic | Insulin Analog (n = 1928) | Before Frequency Matching | After Frequency Matchinga | ||
|---|---|---|---|---|---|
| NPH Insulin (n = 23 561) |
Standardized Differenceb |
NPH Insulin (n = 2500)c |
Standardized Differenceb |
||
| Age, mean (SD), y | 60.6 (12.8) | 60.2 (11.8) | 0.04 | 60.8 (11.8) | −0.01 |
| Female sex, No. (%) | 912 (47) | 11 105 (47) | 0.01 | 1140 (46) | 0.03 |
| Race/ethnicity, No. (%)d | |||||
| Asian | 332 (17) | 3534 (15) | 0.06 | 383 (15) | 0.05 |
| Black | 214 (11) | 2109 (9) | 0.07 | 231 (9) | 0.06 |
| White | 957 (50) | 12 136 (52) | −0.04 | 1265 (51) | −0.02 |
| Hispanic | 293 (15) | 4130 (18) | −0.06 | 446 (18) | −0.07 |
| Other | 114 (6) | 1390 (6) | −0.04 | 133 (5) | −0.04 |
| Neighborhood deprivation index by quartile, No. (%)d,e | |||||
| First (least deprived) | 374 (20) | 4643 (20) | −0.01 | 486 (19) | −0.002 |
| Second | 538 (28) | 6695 (29) | −0.01 | 702 (28) | −0.004 |
| Third | 572 (30) | 7030 (30) | −0.004 | 760 (30) | −0.02 |
| Fourth (most deprived) | 423 (22) | 4972 (21) | 0.02 | 532 (21) | 0.02 |
| Comorbidities, No. (%) | |||||
| Charlson comorbidity indexf | |||||
| 0 | 501 (26) | 6654 (28) | −0.05 | 690 (28) | −0.04 |
| 1 | 533 (28) | 6736 (29) | −0.02 | 735 (29) | −0.04 |
| 2 | 228 (12) | 2652 (11) | 0.02 | 256 (10) | 0.05 |
| ≥3 | 666 (35) | 7519 (32) | 0.06 | 819 (33) | 0.04 |
| Chronic kidney disease staged | |||||
| 0 | 202 (11) | 3121 (13) | −0.09 | 337 (14) | −0.09 |
| 1 | 468 (25) | 6024 (26) | −0.03 | 597 (24) | 0.01 |
| 2 | 656 (35) | 8348 (36) | −0.03 | 883 (35) | −0.03 |
| 3A | 297 (15) | 3064 (13) | 0.04 | 316 (13) | 0.05 |
| 3B | 179 (9) | 2088 (9) | 0.01 | 237 (9) | −0.01 |
| 4 | 77 (4) | 627 (3) | 0.07 | 82 (3) | 0.04 |
| 5 or dialysis | 28 (1) | 115 (1) | 0.10 | 19 (1) | 0.07 |
| Elevated serum creatinine level, Nod,g | 266 (14) | 2664 (11) | 0.08 | 334 (13) | 0.01 |
| Chronic liver disease | 103 (5) | 1392 (6) | −0.02 | 141 (6) | −0.01 |
| Depression | 395 (20) | 5266 (22) | −0.05 | 527 (21) | −0.01 |
| Visual impairment or blindness | 95 (5) | 618 (3) | 0.12 | 93 (4) | 0.06 |
| Health Care Use, No. (%) | |||||
| Emergency department visit for any cause in prior year | 649 (34) | 6822 (29) | 0.10 | 780 (31) | 0.05 |
| Inpatient hospitalization for any cause in prior year | 379 (20) | 3069 (13) | 0.18 | 421 (17) | 0.07 |
| No. of outpatient medical visits in prior 2 y by quartile | |||||
| 0-6 | 423 (22) | 5931 (25) | −0.08 | 613 (25) | −0.06 |
| 7-11 | 435 (23) | 6148 (26) | −0.08 | 609 (24) | −0.04 |
| 12-19 | 480 (25) | 5769 (24) | 0.01 | 631 (25) | −0.01 |
| ≥20 | 590 (31) | 5713 (24) | −0.14 | 647 (26) | 0.11 |
| Diabetic ketoacidosis in prior year | 31 (2) | 206 (1) | 0.07 | 46 (2) | −0.02 |
| Emergency department or inpatient hospitalization for hypoglycemia within prior year | 16 (1) | 115 (1) | 0.04 | 22 (1) | −0.01 |
| No. of hypoglycemic events resulting in emergency department or inpatient stay in prior year, median (IQR) | 0 (0 to 2) | 0 (0 to 3) | 0.04 | 0 (0 to 3) | −0.0002 |
| Kaiser Permanente of Northern California service aread | |||||
| A | 114 (6) | 1989 (8) | −0.10 | 196 (8) | −0.08 |
| B | 209 (11) | 2392 (10) | 0.02 | 224 (9) | 0.06 |
| C | 123 (6) | 1631 (7) | −0.02 | 121 (5) | 0.07 |
| D | 144 (7) | 740 (3) | 0.19 | 172 (7) | 0.02 |
| E | 128 (7) | 1870 (8) | −0.05 | 164 (7) | 0.004 |
| F | 71 (4) | 1513 (6) | −0.13 | 123 (5) | −0.06 |
| G | 253 (13) | 2080 (9) | −0.14 | 272 (11) | 0.07 |
| H | 139 (7) | 3810 (16) | −0.28 | 244 (10) | −0.09 |
| I | 97 (5) | 1981 (8) | −0.14 | 166 (7) | −0.07 |
| J | 143 (7) | 581 (2) | 0.23 | 135 (5) | 0.08 |
| K | 65 (3) | 1831 (8) | −0.19 | 175 (7) | −0.16 |
| L | 139 (7) | 1600 (7) | 0.02 | 129 (5) | 0.09 |
| M | 272 (14) | 1513 (6) | 0.26 | 354 (14) | −0.001 |
| Prescribing clinician specialty | |||||
| Primary care | 1631 (85) | 21 595 (92) | −0.22 | 2120 (85) | −0.002 |
| Endocrinologist | 74 (4) | 667 (3) | 0.05 | 90 (4) | 0.01 |
| Other specialist | 223 (12) | 1299 (6) | 0.22 | 290 (12) | −0.01 |
| Clinical Characteristics of Diabetes | |||||
| Duration of diabetes, mean (SD), yd | 11.6 (7.9) | 10.6 (6.4) | 0.18 | 11.7 (7.4) | −0.01 |
| Age at diabetes onset, mean (SD), yd | 49.2 (11.2) | 50.0 (10.8) | −0.08 | 49.0 (9.3) | 0.03 |
| Body mass index, mean (SD)d | 32.2 (7.5) | 33.3 (7.5) | −0.15 | 32.7 (7.2) | −0.06 |
| Hemoglobin A1c level, mean (SD), %d | 9.41 (2.0) | 9.40 (1.8) | 0.01 | 9.39 (1.8) | 0.02 |
| Type of diabetes medication, No. (%) | |||||
| None | 166 (9) | 1142 (5) | 0.15 | 172 (7) | 0.06 |
| Metformin | 1330 (69) | 17 915 (76) | −0.16 | 1805 (72) | −0.07 |
| Sulfonylurea | 1590 (82) | 20 648 (88) | −0.15 | 2142 (86) | −0.09 |
| Thiazolidinedione | 540 (28) | 5533 (23) | 0.10 | 668 (27) | 0.03 |
| Dipeptidyl peptidase 4 inhibitors | 38 (2) | 248 (1) | 0.08 | 40 (2) | 0.03 |
| Glucagon-like peptide 1 receptor agonists | 23 (1) | 71 (<1) | 0.10 | 12 (1) | 0.07 |
| Otherh | 54 (3) | 322 (1) | 0.10 | 44 (2) | 0.07 |
| Types of cardiometabolic medications, No. (%) | |||||
| Statins | 1409 (73) | 18 553 (79) | −0.13 | 1922 (77) | −0.09 |
| Angiotensin-converting enzyme inhibitors | 912 (47) | 11 185 (47) | −0.003 | 1192 (48) | −0.01 |
| β-Blockers | 828 (43) | 9951 (42) | 0.01 | 1056 (42) | 0.01 |
| Medication nonadherence, %d,i | 432 (22) | 5473 (23) | 0.15 | 591 (24) | −0.03 |
| Year of index insulin prescription, No. (%) | |||||
| 2006 | 289 (15) | 1683 (7) | 0.25 | 313 (13) | 0.07 |
| 2007 | 310 (16) | 3277 (14) | 0.06 | 354 (14) | 0.06 |
| 2008 | 280 (15) | 2357 (10) | 0.14 | 373 (15) | −0.01 |
| 2009 | 243 (13) | 1947 (8) | 0.14 | 294 (12) | 0.03 |
| 2010 | 104 (5) | 2072 (9) | −0.13 | 176 (7) | −0.07 |
| 2011 | 169 (9) | 2667 (11) | −0.09 | 212 (8) | 0.01 |
| 2012 | 211 (11) | 3120 (13) | −0.07 | 289 (12) | −0.02 |
| 2013 | 214 (11) | 3227 (14) | −0.08 | 247 (10) | 0.04 |
| 2014 | 108 (6) | 3211 (14) | −0.27 | 242 (10) | −0.15 |
| Patient co-pay for index insulin dispensed, median (IQR), $ | 20 (10 to 35) | 10 (5 to 10) | 0.67 | 15 (10 to 45) | 0.05 |
Abbreviations: IQR, interquartile range; NPH, neutral protamine Hagedorn.
Patients who initiated NPH insulin were frequency matched with patients initiating insulin analogs based on propensity score quintile.
Compares characteristics for patients who initiated insulin analogs vs NPH insulin. An absolute value ≤0.1 indicates a negligible difference in the mean or prevalence of a covariate between groups.21
The number of patients in each category for the bootstrapped analysis (1000 samples of 2500 each) calculated based on the distributions in the 2.5 million observations and then applied to the 2500. Numbers reflect average across the 1000 samples.
Missing data: race/ethnicity (n = 280), neighborhood deprivation index (n = 242), chronic kidney disease stage (n = 213), elevated serum creatinine (n = 33), Kaiser Permanente of Northern California service area (n = 61), duration of diabetes (n = 6641), age at diabetes onset (n = 6641), body mass index (n = 1429), hemoglobin A1c (n = 402), and medication nonadherence (n = 5474).
Created by a principal components analysis of 8 census-derived variables at the census tract level (% of men in management and professional occupations, living in crowded housing, households in poverty, female-headed households with dependents, households receiving public assistance, households earning <$30 000/year, individuals with less than a high school education, and unemployment).24,25 Negative scores = less deprivation.
Based on the modified version of the Deyo Charlson Score.26 Possible scores ranged from 0-17 and represent the number of selected comorbid conditions (other than diabetes) during the 2 years prior to baseline.
Defined as >1.5 mg/dL in men and >1.4 mg/dL in women.
Including α-glucosidase inhibitors, amylin, and meglitinides.
Based on the continuous measure of medication gaps.19,20 Possible values ranged from 0-100 and represent the percentage of time a patient did not have sufficient medication supply based on electronic pharmacy dispensing data. Medication nonadherence was defined as lacking medication >20% of the time within the year prior to baseline.
In this cohort, data were missing for race/ethnicity (n = 280), chronic kidney disease stage (n = 213), duration of diabetes (n = 6641), age at diabetes onset (n = 6641), body mass index (n = 1429), elevated serum creatinine level (n = 33), neighborhood deprivation index (n = 242), hemoglobin A1c level (n = 402), KPNC service area (n = 61), and medication nonadherence (n = 5474).
Among the patients who initiated insulin, 23 561 (92%) started with NPH insulin and 1928 (8%) started with insulin analogs. Patients who initiated insulin analogs were more likely to have a greater number of comorbid conditions and had more ED or hospital use events (for any cause) within the prior year, but the magnitude of the differences was small (Table 1). One substantive difference was that the median co-payments for insulin analogs ($20) were significantly higher than for NPH insulin ($10). The mean baseline hemoglobin A1c levels for the 2 groups were 9.41% [SD, 2.0%] among patients who started insulin analogs and 9.40% [SD, 1.8%] among patients who started NPH insulin.
In the propensity score–matched cohort (n = 4428), the differences in the characteristics of patients who initiated insulin analog vs NPH insulin were minimized; however, statistical differences persisted for outpatient medical visits, KPNC service area, and year of index prescription. These differences were not substantive.
Primary Outcome
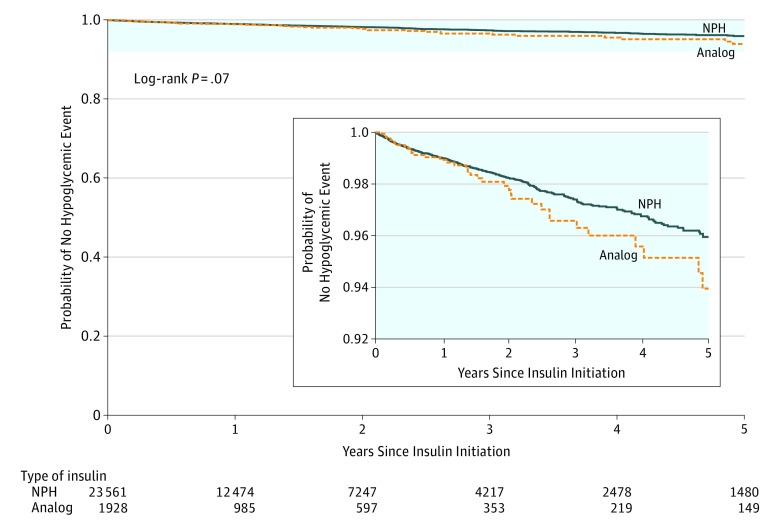
Among patients who initiated insulin analogs (n = 1928; 3289.8 person-years), there were 32 ED visits and 7 hospital admissions related to hypoglycemia (11.9 events [95% CI, 8.1 to 15.6] per 1000 person-years) during a mean follow-up of 1.71 years (95% CI, 1.62 to 1.79) and a median follow-up of 1.03 years (interquartile range, 0.36 to 2.37). Among patients who initiated NPH insulin (n = 23 561; 40 060.0 person-years), there were 309 ED visits and 45 hospital admissions related to hypoglycemia (8.8 events [95% CI, 7.9 to 9.8] per 1000 person-years) during a mean follow-up of 1.70 years (95% CI, 1.68 to 1.72) and a median follow-up of 1.09 years (interquartile range, 0.41 to 2.38). The between-group difference was 3.1 events (95% CI, −1.5 to 7.7) per 1000 person-years (P = .07).
The Kaplan-Meier curve appears in Figure 2. Among all censoring events, 2.8% were due to death, 31.9% were due to discontinuation of insulin, and 31.6% were due to initiation of an additional type of insulin. The proportional hazard assumption was met because the Schoenfeld residuals for the exposure were independent of time (Pearson correlation coefficient, 0.06; P = .20).
Figure 2. Time to First Hypoglycemia-Related Emergency Department Visit or Hospital Admission Among Patients With Type 2 Diabetes.
The median follow-up was 1.03 years (interquartile range, 0.36-2.37 years) among patients who initiated an insulin analog and 1.09 years (interquartile range, 0.41-2.38 years) among patients who initiated neutral protamine Hagedorn (NPH) insulin.
After frequency matching the patients who initiated insulin analogs with those who initiated NPH insulin, and after additional adjustment for unbalanced covariates, prior hypoglycemia-related ED visits or hospital admissions, and time-dependent indicators of diabetes medication use, there was no significant difference in hypoglycemia-related ED visits or hospital admissions (HR, 1.16 [95% CI, 0.71 to 1.78]; Table 2).
Table 2. Survival Analysis.
| Hazard Ratio (95% CI)a | |
|---|---|
| Full Cohort (N = 25 489) | |
| Unadjusted | 1.35 (0.97-1.88) |
| Model 1: adjusts for prior severe hypoglycemia-related ED visits or hospital admissions | 1.33 (0.95-1.84) |
| Model 2: adjusts for prior severe hypoglycemia-related ED visits or hospital admissions and time-dependent indicators for oral diabetes therapy use | 1.31 (0.94-1.82) |
| Model 3: adjusts for prior severe hypoglycemia-related ED visits or hospital admissions, time-dependent indicators for oral diabetes therapy use, and additional unbalanced baseline covariatesb | 1.22 (0.86-1.75) |
| Propensity Score–Matched Sample (n = 4428) | |
| Unadjusted | 1.17 (0.73-1.75) |
| Model 1: adjusts for prior severe hypoglycemia-related ED visits or hospital admissions | 1.18 (0.74-1.78) |
| Model 2: adjusts for prior severe hypoglycemia-related ED visits or hospital admissions and time-dependent indicators for oral diabetes therapy use | 1.21 (0.75-1.84) |
| Model 3: adjusts for prior severe hypoglycemia-related ED visits or hospital admissions, time-dependent indicators for oral diabetes therapy use, and additional unbalanced baseline covariatesc | 1.16 (0.71-1.78) |
Abbreviation: ED, emergency department.
The hazard ratios in the full cohort used traditional regression adjustment. The hazard ratios in the frequency-matched sample used 1000 bootstrap regressions. Risk conferred by initiating long-acting insulin analog vs neutral protamine Hagedorn insulin. Hazard ratio >1 favors NP insulin.
The covariates were baseline diabetes treatment regimen, statin use, visual impairment, hospital use, outpatient medical visits, duration of diabetes, body mass index, year of index prescription, patient insulin co-pay, Kaiser Permanente of Northern California (KPNC) service area, prescribing clinician specialty, and medication nonadherence.
The covariates were outpatient medical visits, KPNC service area, and year of index prescription.
Secondary Outcome
In the main secondary outcome analysis of change in glycemic control, participants with missing data for hemoglobin A1c level at baseline (n = 402) and those who were censored within 90 days of baseline (n = 3665) were excluded (n = 4067). Within 1 year of initiation of insulin analogs, hemoglobin A1c level decreased by 1.26 percentage points (95% CI, 1.16 to 1.36 percentage points) from 9.41% (95% CI, 9.34% to 9.50%) to 8.16% (95% CI, 8.09% to 8.24%).
Within 1 year of initiation of NPH insulin, hemoglobin A1c level decreased by 1.48 percentage points (95% CI, 1.39 to 1.57 percentage points) from 9.39% (95% CI, 9.32% to 9.47%) to 7.92% (95% CI, 7.85% to 7.99%). Between the baseline and postbaseline measures, the mean number of days was 298 (SD, 103 days) among patients who initiated insulin analogs and 288 days (SD, 98 days) among patients who initiated NPH (standardized difference, 0.10). After adjustment, the difference-in-differences for glycemic control was −0.22% (95% CI, −0.09% to −0.37%), indicating that the use of NPH insulin was associated with a statistically significant greater decrease in hemoglobin A1c level (Table 3). However, this difference is not considered clinically significant.17
Table 3. Changes in Glycemic Control.
| Hemoglobin A1c Level, Mean (95% CI), %a | ||
|---|---|---|
| Insulin Analog | NPH Insulin | |
| Baselineb | 9.41 (9.34 to 9.50) | 9.39 (9.32 to 9.47) |
| Postbaselinec | 8.16 (8.09 to 8.24) | 7.92 (7.85 to 7.99) |
| No. of days between baseline and postbaseline measure, mean (SD) | 298 (103) | 288 (98) |
| Pre-post change | 1.26 (1.16 to 1.36) | 1.48 (1.39 to 1.57) |
| Unadjusted difference-in-differences estimated | −0.22 (−0.09 to −0.37) | |
| Adjusted difference-in-differences estimatee | −0.22 (−0.09 to −0.37) | |
Abbreviation: NPH, neutral protamine Hagedorn.
The results are from 1000 bootstrap samples of the frequency-matched cohort representing 21 422 of the 24 489 patients in the cohort who met the inclusion criteria for the hemoglobin A1c analysis (prebaseline hemoglobin A1c level collected within the 12 months prior to insulin initiation and at least 90 days of follow-up after insulin initiation).
Indicates the last measure within the 12 months prior to insulin initiation.
The last hemoglobin A1c level was assessed prior to censoring and within 3 to 12 months after insulin initiation.
Calculated as the pre-post change in hemoglobin A1c level for the insulin analog group minus the pre-post change in hemoglobin A1c level for the NPH insulin group.
Adjusted for outpatient medical visits, Kaiser Permanente of Northern California service area, and year of index prescription.
Sensitivity Analyses
For hypoglycemia-related ED visits or hospital admissions after initiation of insulin analogs vs NPH insulin, the HR was 1.22 (95% CI, 0.86 to 1.75) using traditional regression adjustment for covariates that were significantly different at baseline, for prior hypoglycemia-related ED visits or hospital admissions, and for time-dependent indicators of diabetes medication use (Table 2).
Within 1 year of insulin initiation, hemoglobin A1c levels decreased by 1.27 percentage points (95% CI, 1.14 to 1.39 percentage points) among patients who started insulin analogs and by 1.43 percentage points (95% CI, 1.33 to 1.53 percentage points) among patients who started NPH insulin after excluding patients with changes in the use of diabetes medications (n = 5199). The difference-in-differences in hemoglobin A1c levels was −0.16% (95% CI, −0.005% to 0.34%), which was not a clinically significant change.
Discussion
In this observational study of patients with type 2 diabetes in a large integrated health care system, initiation of basal insulin analogs compared with NPH insulin was not associated with a lower rate of ED visits or hospital admissions related to hypoglycemia. Moreover, initiation of NPH insulin was associated with a slightly greater, but not clinically meaningful, decline in hemoglobin A1c level from baseline. These results suggest that the use of basal insulin analogs among patients with type 2 diabetes in usual practice settings may not be associated with clinical advantages with respect to these outcomes compared with NPH insulin.
Randomized clinical trials suggest that long-acting insulin analogs may offer some advantages for patients with type 2 diabetes, but the benefits appear relatively modest.5 In several meta-analyses,5,27,28 benefits in terms of reduced hypoglycemia risk have been reported for both types of insulin analog (glargine and detemir) compared with NPH insulin, but only with respect to nocturnal hypoglycemia. Benefits with respect to severe hypoglycemia (defined as requiring assistance from another person to administer carbohydrates, glucagon, or other resuscitative treatment) were not significant.
One meta-analysis29 found that glargine use in combination with oral agents was associated with a higher probability of reaching the target hemoglobin A1c level without nocturnal hypoglycemia compared with the use of NPH insulin in combination with oral agents. This difference was largely driven by a reduction in nocturnal hypoglycemia with the use of glargine. In a patient-level meta-analysis,30 the incidence of overall and nocturnal hypoglycemia was modestly lower in patients with type 2 diabetes treated with glargine compared with NPH insulin. Differences in glycemic control between NPH insulin and basal insulin analogs in the trials were minimal and there were not consistently greater decreases in hemoglobin A1c levels associated with insulin analog use in patients with type 2 diabetes.30
In 1 meta-analysis,28 compared with NPH insulin, the difference in hemoglobin A1c level was −0.05% (95% CI, −0.13% to 0.04%) for insulin glargine and 0.13% (95% CI, 0.03% to 0.22%) for insulin detemir. Because allocation to insulin analogs vs NPH insulin was not concealed in most trials, the potential for ascertainment bias exists, especially for subjective outcomes such as patient-reported hypoglycemia. In addition, clinical trials typically include specific algorithms to achieve strict glycemic control targets and, as a result, trial participants achieve tighter glycemic control compared with patients encountered in clinical practice.
Prior observational studies have shown reduced hypoglycemia risk and improved glycemic control with the use of insulin analogs compared with NPH insulin, but they did not adequately account for confounding factors. For example, 2 large observational studies31,32 using national registries in Finland showed a significantly increased risk of hospitalization related to severe hypoglycemia with the use of NPH insulin compared with either the insulin analog detemir or glargine, but the studies lacked information about hemoglobin A1c level and major risk factors for hypoglycemia.
In a related study also conducted in Finland, NPH insulin use was associated with increased mortality compared with basal insulin analogs, but again, the study did not adjust for important confounders.33 Similarly, other studies did not use the more rigorous techniques for balancing covariates, such as propensity score matching.32,34
One study from the US Department of Veterans Affairs compared insulin analogs with NPH insulin using clinician practice pattern as an instrumental variable to address confounding by indication.35 This study examined hospitalizations for ambulatory care–sensitive conditions and mortality, and found no consistent difference in these outcomes when comparing use of long-acting insulin analogs and NPH insulin.35
The findings of the present study support the use of NPH insulin in many patients with type 2 diabetes to reduce the costs of care. Insulin prices in the United States have increased 3-fold between 2002 and 2013, particularly for insulin analogs.8 In 2013, the estimated per-patient expenditures on insulin were greater than for all other diabetes medications combined.8 A prior study found an increase in the use of insulin analogs for the management of type 2 diabetes with a resultant increase in inflation-adjusted out-of-pocket costs.4
The rising cost of insulin may directly affect the health outcomes of patients with diabetes because the associated increased cost share is known to contribute to nonadherence.36,37,38,39 In contrast to insulin analogs, NPH insulin can be purchased for as little as $25 per vial, about one-tenth the price of either insulin analog glargine or detemir.40 It is likely that only select patients with type 2 diabetes benefit from insulin analogs vs human insulin preparations. To contain health care costs, decisions to use more expensive insulin should be made by informed patients and clinicians, and driven by convincing data about the benefits, harms, and tradeoffs.
Limitations
This study has several limitations. First, this was an observational study and is thus subject to confounding. Specifically, patients with type 2 diabetes who initiated insulin analogs may be different from those who started NPH insulin. Despite matching on propensity score quintiles, some substantive differences between the 2 groups remained (standardized difference >0.1).
However, the results did not change after additional adjustment for these unbalanced factors. Nonetheless, it is possible that the study did not completely account for confounding by indication due to unmeasured or missing confounders.
Second, the primary outcome was based on ED or hospital use related to hypoglycemia. Therefore, differences in nocturnal and self-reported hypoglycemia, or adverse events treated by emergency medical services but not transported to the ED could not be examined.
Third, the 95% CIs ranged from 0.71 to 1.78 for the HR for hypoglycemia-related ED visits or hospital admissions, which may include a clinically important difference. Therefore, this study may have been underpowered to detect a benefit or harm of that magnitude.
Fourth, these findings come from an integrated health care delivery system and may not necessarily be generalizable to other types of health care settings.
Fifth, the comparisons between NPH insulin and basal insulin analogs did not include convenience, number of injections required, or mode of delivery (vial vs pen). It is possible that basal insulin analogs may confer these and other advantages to patients with type 2 diabetes.
Conclusions
Among patients with type 2 diabetes, initiation of a basal insulin analog compared with NPH insulin was not associated with a reduced risk of hypoglycemia-related ED visits or hospital admissions or with improved glycemic control. These findings suggest that the use of basal insulin analogs in usual practice settings may not be associated with clinical advantages for these outcomes.
References
- 1.Best JD, Drury PL, Davis TM, et al. ; Fenofibrate Intervention and Event Lowering in Diabetes Study Investigators . Glycemic control over 5 years in 4,900 people with type 2 diabetes: real-world diabetes therapy in a clinical trial cohort. Diabetes Care. 2012;35(5):1165-1170. doi: 10.2337/dc11-1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006-2013. Diabetes Care. 2017;40(4):468-475. doi: 10.2337/dc16-0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landon BE, Zaslavsky AM, Souza J, Ayanian JZ. Trends in diabetes treatment and monitoring among Medicare beneficiaries. J Gen Intern Med. 2018;33(4):471-480. doi: 10.1007/s11606-018-4310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipska KJ, Ross JS, Van Houten HK, Beran D, Yudkin JS, Shah ND. Use and out-of-pocket costs of insulin for type 2 diabetes mellitus from 2000 through 2010. JAMA. 2014;311(22):2331-2333. doi: 10.1001/jama.2014.6316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2007;(2):CD005613. [DOI] [PubMed] [Google Scholar]
- 6.Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40(11):1425-1432. doi: 10.2337/dc16-1974 [DOI] [PubMed] [Google Scholar]
- 7.Turner LW, Nartey D, Stafford RS, Singh S, Alexander GC. Ambulatory treatment of type 2 diabetes in the US, 1997-2012. Diabetes Care. 2014;37(4):985-992. doi: 10.2337/dc13-2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua X, Carvalho N, Tew M, Huang ES, Herman WH, Clarke P. Expenditures and prices of antihyperglycemic medications in the United States: 2002-2013. JAMA. 2016;315(13):1400-1402. doi: 10.1001/jama.2016.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cefalu WT, Dawes DE, Gavlak G, et al. ; Insulin Access and Affordability Working Group . Insulin Access and Affordability Working Group: Conclusions and Recommendations. Diabetes Care. 2018;41(6):1299-1311. doi: 10.2337/dci18-0019 [DOI] [PubMed] [Google Scholar]
- 10.Luo J, Avorn J, Kesselheim AS. Trends in Medicaid reimbursements for insulin from 1991 through 2014. JAMA Intern Med. 2015;175(10):1681-1686. doi: 10.1001/jamainternmed.2015.4338 [DOI] [PubMed] [Google Scholar]
- 11.Karter AJ, Schillinger D, Adams AS, et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: the Diabetes Study of Northern California (DISTANCE). Diabetes Care. 2013;36(3):574-579. doi: 10.2337/dc12-0722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karter AJ, Lipska KJ, O’Connor PJ, et al. ; SUPREME-DM Study Group . High rates of severe hypoglycemia among African American patients with diabetes: the Surveillance, Prevention, and Management of Diabetes Mellitus (SUPREME-DM) network. J Diabetes Complications. 2017;31(5):869-873. doi: 10.1016/j.jdiacomp.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirk JK, D’Agostino RB Jr, Bell RA, et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2006;29(9):2130-2136. doi: 10.2337/dc05-1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519-2527. doi: 10.1001/jama.287.19.2519 [DOI] [PubMed] [Google Scholar]
- 15.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34(6):1329-1336. doi: 10.2337/dc10-2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginde AA, Blanc PG, Lieberman RM, Camargo CA Jr. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. doi: 10.1186/1472-6823-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little RR, Rohlfing CL, Sacks DB; National Glycohemoglobin Standardization Program (NGSP) Steering Committee . Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57(2):205-214. doi: 10.1373/clinchem.2010.148841 [DOI] [PubMed] [Google Scholar]
- 18.Sinisi SE, van der Laan MJ. Deletion/substitution/addition algorithm in learning with applications in genomics. Stat Appl Genet Mol Biol. 2004;3:e18. doi: 10.2202/1544-6115.1069 [DOI] [PubMed] [Google Scholar]
- 19.Steiner JF, Koepsell TD, Fihn SD, Inui TS. A general method of compliance assessment using centralized pharmacy records: description and validation. Med Care. 1988;26(8):814-823. doi: 10.1097/00005650-198808000-00007 [DOI] [PubMed] [Google Scholar]
- 20.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105-116. doi: 10.1016/S0895-4356(96)00268-5 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer BD. Natural and quasi-experiments in economics. J Bus Econ Stat. 1995;13(2):151-161. [Google Scholar]
- 23.Cheung YB. A modified least-squares regression approach to the estimation of risk difference. Am J Epidemiol. 2007;166(11):1337-1344. doi: 10.1093/aje/kwm223 [DOI] [PubMed] [Google Scholar]
- 24.Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041-1062. doi: 10.1007/s11524-006-9094-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laraia BA, Karter AJ, Warton EM, Schillinger D, Moffet HH, Adler N. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE). Soc Sci Med. 2012;74(7):1082-1090. doi: 10.1016/j.socscimed.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 27.Freemantle N, Chou E, Frois C, et al. Safety and efficacy of insulin glargine 300 u/mL compared with other basal insulin therapies in patients with type 2 diabetes mellitus: a network meta-analysis. BMJ Open. 2016;6(2):e009421. doi: 10.1136/bmjopen-2015-009421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh SR, Ahmad F, Lal A, Yu C, Bai Z, Bennett H. Efficacy and safety of insulin analogues for the management of diabetes mellitus: a meta-analysis. CMAJ. 2009;180(4):385-397. doi: 10.1503/cmaj.081041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and meta-analysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus. Acta Diabetol. 2015;52(4):649-662. doi: 10.1007/s00592-014-0698-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owens DR, Traylor L, Mullins P, Landgraf W. Patient-level meta-analysis of efficacy and hypoglycaemia in people with type 2 diabetes initiating insulin glargine 100U/mL or neutral protamine Hagedorn insulin analysed according to concomitant oral antidiabetes therapy. Diabetes Res Clin Pract. 2017;124:57-65. doi: 10.1016/j.diabres.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 31.Haukka J, Hoti F, Erästö P, Saukkonen T, Mäkimattila S, Korhonen P. Evaluation of the incidence and risk of hypoglycemic coma associated with selection of basal insulin in the treatment of diabetes: a Finnish register linkage study. Pharmacoepidemiol Drug Saf. 2013;22(12):1326-1335. doi: 10.1002/pds.3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strandberg AY, Khanfir H, Mäkimattila S, Saukkonen T, Strandberg TE, Hoti F. Insulins NPH, glargine, and detemir, and risk of severe hypoglycemia among working-age adults. Ann Med. 2017;49(4):357-364. doi: 10.1080/07853890.2016.1278302 [DOI] [PubMed] [Google Scholar]
- 33.Strandberg AY, Hoti FJ, Strandberg TE, Christopher S, Haukka J, Korhonen P. All-cause and cause-specific mortality among users of basal insulins NPH, detemir, and glargine. PLoS One. 2016;11(3):e0151910. doi: 10.1371/journal.pone.0151910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang T, Ji L, Gao Y, et al. Observational registry of basal insulin treatment in patients with type 2 diabetes in China: safety and hypoglycemia predictors. Diabetes Technol Ther. 2017;19(11):675-684. doi: 10.1089/dia.2017.0111 [DOI] [PubMed] [Google Scholar]
- 35.Prentice JC, Conlin PR, Gellad WF, Edelman D, Lee TA, Pizer SD. Long-term outcomes of analogue insulin compared with NPH for patients with type 2 diabetes mellitus. Am J Manag Care. 2015;21(3):e235-e243. [PubMed] [Google Scholar]
- 36.Randall L, Begovic J, Hudson M, et al. Recurrent diabetic ketoacidosis in inner-city minority patients: behavioral, socioeconomic, and psychosocial factors. Diabetes Care. 2011;34(9):1891-1896. doi: 10.2337/dc11-0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spain CV, Wright JJ, Hahn RM, Wivel A, Martin AA. Self-reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin Ther. 2016;38(7):1653-1664.e1. doi: 10.1016/j.clinthera.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 38.Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care. 2004;27(2):384-391. doi: 10.2337/diacare.27.2.384 [DOI] [PubMed] [Google Scholar]
- 39.Karter AJ, Parker MM, Solomon MD, et al. Effect of out-of-pocket cost on medication initiation, adherence, and persistence among patients with type 2 diabetes: the Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2018;53(2):1227-1247. doi: 10.1111/1475-6773.12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipska KJ, Hirsch IB, Riddle MC. Human insulin for type 2 diabetes: an effective, less-expensive option. JAMA. 2017;318(1):23-24. doi: 10.1001/jama.2017.6939 [DOI] [PubMed] [Google Scholar]