Key Points
Question
What is the effect of 5-day nitrofurantoin, compared with single-dose fosfomycin, on clinical resolution of uncomplicated lower urinary tract infection (UTI) in women?
Findings
In this randomized clinical trial that included 513 women with uncomplicated UTI, clinical resolution at 28 days occurred in 70% of patients in the nitrofurantoin group vs 58% of patients in the fosfomycin group, a statistically significant difference.
Meaning
Five-day nitrofurantoin may be a better alternative to single-dose fosfomycin for treating uncomplicated UTI in women.
Abstract
Importance
The use of nitrofurantoin and fosfomycin has increased since guidelines began recommending them as first-line therapy for lower urinary tract infection (UTI).
Objective
To compare the clinical and microbiologic efficacy of nitrofurantoin and fosfomycin in women with uncomplicated cystitis.
Design, Setting, and Participants
Multinational, open-label, analyst-blinded, randomized clinical trial including 513 nonpregnant women aged 18 years and older with symptoms of lower UTI (dysuria, urgency, frequency, or suprapubic tenderness), a positive urine dipstick result (with detection of nitrites or leukocyte esterase), and no known colonization or previous infection with uropathogens resistant to the study antibiotics. Recruitment took place from October 2013 through April 2017 at hospital units and outpatient clinics in Geneva, Switzerland; Lodz, Poland; and Petah-Tiqva, Israel.
Interventions
Participants were randomized in a 1:1 ratio to oral nitrofurantoin, 100 mg 3 times a day for 5 days (n = 255), or a single 3-g dose of oral fosfomycin (n = 258). They returned 14 and 28 days after therapy completion for clinical evaluation and urine culture collection.
Main Outcomes and Measures
The primary outcome was clinical response in the 28 days following therapy completion, defined as clinical resolution (complete resolution of symptoms and signs of UTI without prior failure), failure (need for additional or change in antibiotic treatment due to UTI or discontinuation due to lack of efficacy), or indeterminate (persistence of symptoms without objective evidence of infection). Secondary outcomes included bacteriologic response and incidence of adverse events.
Results
Among 513 patients who were randomized (median age, 44 years [interquartile range, 31-64]), 475 (93%) completed the trial and 377 (73%) had a confirmed positive baseline culture. Clinical resolution through day 28 was achieved in 171 of 244 patients (70%) receiving nitrofurantoin vs 139 of 241 patients (58%) receiving fosfomycin (difference, 12% [95% CI, 4%-21%]; P = .004). Microbiologic resolution occurred in 129 of 175 (74%) vs 103 of 163 (63%), respectively (difference, 11% [95% CI, 1%-20%]; P = .04). Adverse events were few and primarily gastrointestinal; the most common were nausea and diarrhea (7/248 [3%] and 3/248 [1%] in the nitrofurantoin group vs 5/247 [2%] and 5/247 [1%] in the fosfomycin group, respectively).
Conclusions and Relevance
Among women with uncomplicated UTI, 5-day nitrofurantoin, compared with single-dose fosfomycin, resulted in a significantly greater likelihood of clinical and microbiologic resolution at 28 days after therapy completion.
Trial Registration
ClinicalTrials.gov Identifier: NCT01966653
This randomized clinical trial compares the clinical and microbiologic efficacy of 5-day nitrofurantoin vs single-dose fosfomycin in women with uncomplicated urinary tract infection (UTI).
Introduction
Given increasing antimicrobial resistance, guidelines for the treatment of uncomplicated lower urinary tract infections (UTIs) were modified in 2010 to recommend nitrofurantoin and fosfomycin as first-line agents1; their use has since increased exponentially.2 These antibiotics were commercialized in 1953 and 1971, respectively, in an era of less-stringent methodologic standards for drug testing and results reporting, which required neither randomization nor intention-to-treat (ITT) analyses. Thus, uncertainties regarding clinical efficacy persist, particularly for single-dose fosfomycin. While meta-analyses of randomized clinical trials evaluating nitrofurantoin for lower UTI suggest efficacy comparable with that of newer agents, such as fluoroquinolones,3,4 the same has not been observed for fosfomycin. In a randomized clinical trial conducted in the 1990s that, to our knowledge, has not yet been published (PubMed database searched on February 25, 2018), the efficacy rate for fosfomycin was 70% compared with rates of 96% and 94% for ciprofloxacin and trimethoprim/sulfamethoxazole, respectively.5
There may be a microbiologic and pharmacologic basis for decreased efficacy. Mechanisms of resistance to fosfomycin, some of which are plasmid encoded, were described soon after the drug’s approval in Europe.6 While reported Escherichia coli resistance rates have been low,7 most data were collected before the shift to its widespread use in 2011. In communities using fosfomycin regularly, significant increases in resistance have been observed, as demonstrated in Spain in 2008.8 The single-dose regimen may be insufficient1,5 for effective bactericidal activity given the drug’s at least partially time-dependent killing9,10 and the high interindividual variability observed in urinary concentrations.11
Thus far, nitrofurantoin and fosfomycin have been compared in few randomized clinical trials.12,13 The purpose of this study was to assess the comparative efficacy of 5-day nitrofurantoin and single-dose fosfomycin for clinical resolution of uncomplicated UTI.
Methods
Study Design and Population
This open-label/analyst-blinded, multicenter, randomized clinical trial was conducted from October 2013 to May 2017 at 3 sites (Geneva, Switzerland; Lodz, Poland; and Petah-Tiqva, Israel) and included adult women aged 18 years and older presenting with at least 1 symptom of acute lower UTI (dysuria, urgency, frequency, or suprapubic tenderness) and a urine dipstick test result positive for either nitrites or leukocyte esterase.14 The statistical analysis plan and full trial protocol are available in Supplement 1 and Supplement 2, respectively. Main exclusion criteria were pregnancy and lactation; suspected upper UTI (presence of fever, chills, or flank pain); antibiotic use or any symptoms consistent with UTI in the preceding 4 weeks; indwelling urinary catheter or otherwise complicated UTI; immunosuppression (untreated infection with HIV, ongoing chemotherapy or radiation therapy, use of high-dose corticosteroids or other immunosuppressive medication); and renal insufficiency (creatinine clearance <30 mL/min). Additional exclusion criteria and definitions are available in the eAppendix in Supplement 3.
Both hospitalized and ambulatory patients were recruited, with the former hospitalized for other medical reasons and recruited on development of urinary symptoms, and the latter attending walk-in clinics or their primary care physicians’ offices due to their symptoms. The study was reviewed and approved by the independent ethics committees of each site and by the Swiss Agency for Therapeutic Products (2013DR4095). All participants provided written informed consent before their inclusion.
Randomization
The randomization sequence was computer-generated and used randomly permuted blocks of 4 to 12 allocations within site. Assignments were concealed from study investigators via opaque sealed envelopes until patient enrollment, and allocated treatment to either nitrofurantoin or fosfomycin in a 1:1 ratio.
Intervention and Procedures
Participants were randomly assigned to either oral macrocrystalline nitrofurantoin, 100 mg 3 times a day for 5 days (the most commonly recommended regimen in Europe; eAppendix in Supplement 3) or a single 3-g dose of oral fosfomycin tromethamine and instructed to contact study investigators in the absence of clinical improvement. They attended 2 follow-up visits at 14 (±2) and 28 (±7) days after completion of antibiotic therapy; urine cultures were collected at all scheduled and unscheduled visits. Because of fosfomycin’s purported long half-life,15 antibiotic therapy was considered completed in both groups on day 5 after randomization.
Outcomes and Definitions
The primary outcome was clinical response in the 28 (±7) days following completion of therapy, defined as clinical resolution (complete resolution of symptoms and signs of UTI without prior failure), failure (need for additional or change in antibiotic treatment due to a UTI or discontinuation due to lack of efficacy), or indeterminate (either persistence of symptoms without objective evidence of infection or any extenuating circumstances precluding a classification of clinical resolution or failure). Clinical resolution was thus a lack of prior failure with complete resolution of symptoms at the date of the examination, indeterminate response was a lack of prior failure yet with persistence of symptoms without objective evidence of infection, and clinical failure at any time point was carried through to the end of the study.
A secondary outcome was bacteriologic response at 14 (±2) and 28 (±7) days after therapy completion, defined as resolution (eradication of the infecting strain with no recurrence of bacteriuria [<103 colony-forming units {cfu}/mL] during follow-up) or failure (bacteriuria ≥103 cfu/mL with the infecting strain). Other secondary outcomes included clinical response at 14 (±2) days after therapy completion, duration of symptoms after treatment initiation, incidence of progression to pyelonephritis or urosepsis, lost days of work due to hospital admission, incidence of adverse events (considered possibly, probably, or certainly related to the study antibiotic) throughout the study period, incidence of confirmed UTI at baseline, incidence of baseline resistance to either study antibiotic and other UTI-targeted antibiotics, and emergence of phenotypic bacterial resistance throughout the study period.
Confirmed UTI required the presence of at least 1 of the 4 urinary symptoms required for inclusion and a positive urine culture (≥103 cfu/mL).14 Positive cultures could be mixed (containing >1 uropathogen); laboratory reporting of culture growth is further detailed in the online supplement, as are other definitions (eAppendix in Supplement 3).
Women deemed at increased risk for carriage of resistant bacteria had at least 1 of the following: systemic antibiotic exposure (≥1 dose) or hospitalization in an acute or long-term care center in the previous 12 months, UTI fulfilling criteria for health care–associated infection,16 recent (in the preceding 12 months) carriage of resistant organisms, or stay of at least 1 month in a high-risk country (any country in the Mediterranean basin excluding France, South or Southeast Asia, the Middle East, Africa, and Central or South America).
Laboratory Methods
Voided midstream urine specimens were collected in sterile containers and transported within 24 hours to each site’s central laboratory, where specimens were cultured according to published recommendations.17 Cultures were reported to be positive when 103 cfu/mL or more of at least 1 bacterium was detected (see the eAppendix in Supplement 3 for more details). Resistances were determined in Poland and Israel via Clinical and Laboratory Standards Institute18 and in Switzerland via European Committee on Antimicrobial Susceptibility Testing19 break points.
Statistical Methods
Sample Size
The sample size calculation was driven by a superiority hypothesis based on previously reported clinical response rates with nitrofurantoin and fosfomycin of 90% and 80%, respectively.12,20,21 Assuming these rates and attrition of roughly 12%, 300 participants per group were necessary to show clinical superiority by 10% or more of nitrofurantoin with 90% power and 5% 2-sided significance in the treatment of this common infection. Delays in study launch and limited budgets curtailed the planned recruitment period, which was thus closed when 513 patients were enrolled, ensuring 80% power. A minimal clinically important difference of 10% was chosen in line with guidance provided by the Infectious Disease Society of America22 and given previous studies’ findings suggesting at least a 10% difference in clinical resolution between treatment groups.12,20,21
Study Populations
The ITT population consisted of all patients randomized to either study antibiotic and the per-protocol population of those who took the study antibiotic with at least 80% adherence and for whom no major protocol deviations were documented. There were no significant differences between the ITT and per-protocol populations in outcomes (eAppendix in Supplement 3); unless otherwise specified, ITT results are reported.
Analyses
Analyses were performed with blinding to treatment allocation. Baseline characteristics are described by frequencies, medians, and interquartile ranges (IQRs). The incidence of clinical and bacteriologic resolution was compared between treatment groups using the χ2 test; incidence of adverse events and other outcome measures with events numbering less than 10 were compared using the Fisher exact test. Patients who had documented clinical failure and were then lost to follow-up were included in the primary outcome analysis of any failure by day 28, while those whose response status was unknown before dropout were considered missing; missing values were excluded from the primary analysis.
Post-hoc analyses were performed to quantify the potential of missing data for primary outcomes only: multiple imputation for missing at random was used,23 with M = 20 imputations and adjustment of the imputation model for site, age, number of urinary symptoms and signs, previous antibiotic use, and any previous UTI. Sensitivity analyses were conducted with worst- and best-case scenarios, indeterminate clinical responses treated alternatively as failures, or, among those with improvement, successes, and using the rctmiss command in Stata for missing-not-at-random assumptions. Post-hoc analyses were also conducted to evaluate clinical and microbiologic response among patients with confirmed E coli infections. Bivariable regression models were constructed to assess associations between clinical failure and baseline demographic, clinical, and microbiologic characteristics.
Post-hoc mixed effects logistic regression models were constructed to take into account potential site variability on the intercept of the model in this multicenter trial. An interaction term between the treatment effect and E coli baseline positivity was introduced to assess differences in treatment effects in patients with and without E coli infections. Statistical tests were 2-sided with a significance level of .05. Because there was no adjustment for multiple comparisons, secondary end point analyses should be interpreted as exploratory. All analyses were performed using Stata (StataCorp).
Results
Study Population
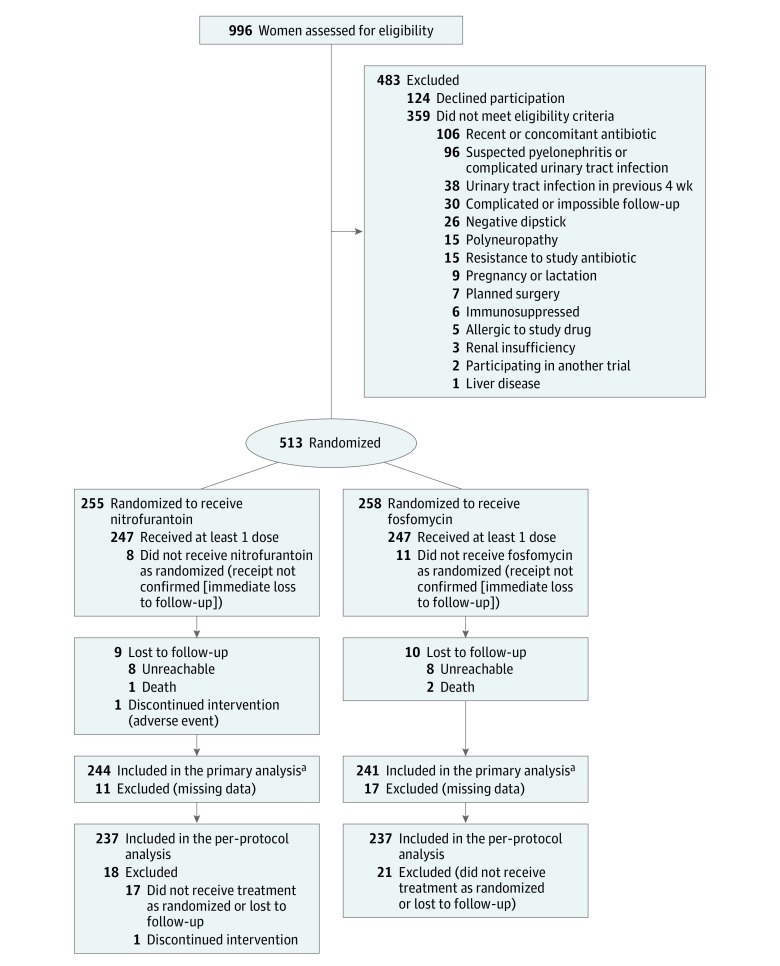
Of 996 patients screened, 513 were enrolled and randomized, 255 to nitrofurantoin and 258 to fosfomycin (ITT population; Figure). Within the ITT population, receipt of the study intervention was confirmed among 494 women (96%), with thereafter 20 patients lost to follow-up, so that ultimately 474 (92%) composed the per-protocol population.
Figure. Study Flowchart of the Nitrofurantoin and Fosfomycin Groups.
Both the intention-to-treat and per-protocol populations were analyzed. The intention-to-treat population included all patients randomized and the per-protocol population, those with at least 80% medication adherence, no major protocol deviations, and available primary outcome data.
aData on the primary outcome were available for 244 of 255 patients (96%) randomized to nitrofurantoin and 241 of 258 patients (93%) randomized to fosfomycin; 7 and 4 patients in these groups, respectively, had clinical failure before being lost to follow-up. The only major protocol deviation documented in either group was nonadherence to the study antibiotic.
Baseline Demographics
The median age was 44 years (IQR, 31-64; Table 1). Baseline characteristics were similar by treatment group. Women in Lodz tended to be older and to have taken antibiotics more frequently in the year prior to inclusion as compared with those in Geneva and Petah-Tiqva. Nearly 90% of all women were at risk for resistant organisms, more than half of them due at least in part to the consumption of antibiotics in the previous year.
Table 1. Baseline Demographics and Clinical Characteristics.
| Characteristic by Site | Nitrofurantoin (n = 255) | Fosfomycin (n = 258) |
|---|---|---|
| Age, median (IQR) [range], y | 43 (31-63) [18-101] | 46 (31-66) [18-93] |
| Geneva | 43 (31-58) [18-101] | 37 (26-54) [18-91] |
| Lodz | 51 (33-65) [19-90] | 58 (40-68) [18-88] |
| Petah-Tiqva | 37 (27-59) [18-83] | 42 (30-60) [19-93] |
| Outpatient at the time of inclusion, No. (%) | 237 (93) | 238 (92) |
| Geneva | 77 (82) | 74 (80) |
| Lodz | 98 (100) | 102 (100) |
| Petah-Tiqva | 62 (98) | 62 (97) |
| No. of symptoms, median (IQR)a | 3 (2-4) | 3 (2-4) |
| Geneva | 3 (2-4) | 3 (2-4) |
| Lodz | 3 (2-4) | 3 (2-4) |
| Petah-Tiqva | 4 (3-5) | 4 (3-5) |
| Urine culture positive at inclusion, No. (%)b | 194 (76) | 183 (71) |
| Geneva | 88 (94) | 81 (91) |
| Lodz | 68 (70) | 68 (68) |
| Petah-Tiqva | 38 (73) | 34 (62) |
| At risk for resistant organisms, No. (%)c | 220 (86) | 232 (90) |
| Geneva | 60 (65) | 68 (74) |
| Lodz | 97 (99) | 100 (98) |
| Petah-Tiqva | 63 (100) | 64 (100) |
| Antibiotic therapy for any reason in the past year, No. (%) | 131 (51) | 137 (53) |
| Geneva | 31 (33) | 39 (42) |
| Lodz | 97 (99) | 95 (93) |
| Petah-Tiqva | 3 (5) | 3 (5) |
| Any previous UTI, No. (%)d | 41 (16) | 43 (17) |
| Geneva | 9 (10) | 20 (21) |
| Lodz | 6 (6) | 8 (9) |
| Petah-Tiqva | 26 (41) | 15 (23) |
Abbreviations: IQR, interquartile range; UTI, urinary tract infection.
A total of 9 symptoms were assessed; they included at least 1 of the following: dysuria, frequency, urgency, or suprapubic tenderness; and they may have additionally included flank and/or lower back pain, nausea, subjective fever, or a subjective feeling of chills.
Positive culture was defined as the growth of 103 colony-forming unites/mL or more of at least 1 uropathogen; laboratory reporting of culture growth is described in detail in the eAppendix in Supplement 3. All patients were symptomatic at baseline, thus those with a positive culture had confirmed UTI.
Systemic antibiotic exposure (≥1 dose) or hospitalization in an acute or long-term care center in the previous 12 months, UTI fulfilling criteria for health care–associated infection, recent (in the preceding 12 months) carriage of resistant organisms, or stay of at least 1 month in a high-risk country (any country in the Mediterranean basin excluding France, South or Southeast Asia, the Middle East, Africa, and Central or South America).
Self-reported.
Baseline Urine Cultures
Of the 487 baseline urine cultures obtained, 377 (77%) were positive (Table 2; eTable 1 in Supplement 3). Among these, E coli (230/377 [61%]), Klebsiella spp (27/377 [7%]), Enterococcus spp (27/377 [7%]), and Proteus spp (17/377 [5%]) predominated. There were no significant differences in epidemiology in the 2 treatment groups; baseline antibiotic resistance among E coli isolates in Petah-Tiqva was increased but differences were not statistically significant. Patients randomized there had higher rates of E coli either producing extended-spectrum beta-lactamase (3/25 [12%] vs 4/112 [4%] and 2/93 [2%] in Geneva and Lodz, respectively) and/or fluoroquinolone resistance (5/25 [20%] vs 13/112 [12%] and 9/93 [10%]). Yet only in Lodz, Poland (where nitrofuran derivatives are sold over the counter24), was baseline resistance among E coli isolates to nitrofurantoin detected (6/93 [7%]; P = .004). The Lodz site also had the study’s only E coli strain resistant to fosfomycin at baseline (1/93 [1%]). Conversely, only in Geneva was any baseline resistance to study drugs detected among Klebsiella spp, with 3 of 10 (30%) and 2 of 10 (20%) strains resistant to nitrofurantoin and fosfomycin, respectively.
Table 2. Baseline Urinary Isolates and Their Susceptibilities by Treatment Allocation.
| No. (%) | ||
|---|---|---|
| Nitrofurantoin (n = 255) |
Fosfomycin (n = 258) |
|
| Baseline cultures obtained | 243 (95) | 244 (95) |
| Positive culturesa | 194 (80) | 183 (75) |
| Escherichia colib | 111 (57) | 119 (65) |
| Nitrofurantoin resistant | 2 (1) | 4 (3) |
| Fosfomycin resistant | 0 (0) | 1 (1) |
| Co-trimoxazole resistant | 26 (23) | 25 (21) |
| Fluoroquinolone resistant | 13 (12) | 14 (12) |
| Extended-spectrum beta-lactamase | 7 (6) | 2 (1) |
| Klebsiella sppb | 20 (10) | 7 (4) |
| Nitrofurantoin resistant | 3 (15) | 0 (0) |
| Fosfomycin resistant | 2 (10) | 0 (0) |
| Co-trimoxazole resistant | 4 (20) | 2 (29) |
| Fluoroquinolone resistant | 1 (5) | 1 (14) |
| Extended-spectrum beta-lactamase | 2 (10) | 1 (14) |
| Proteus sppb | 7 (4) | 10 (5) |
| Nitrofurantoin resistant | 6 (86) | 9 (90) |
| Fosfomycin resistant | 0 (0) | 2 (20) |
| Co-trimoxazole resistant | 3 (42) | 2 (20) |
| Fluoroquinolone resistant | 2 (29) | 0 (0) |
| Extended-spectrum beta-lactamase | 0 (0) | 0 (0) |
| Enterococcus sppb | 13 (7) | 14 (7) |
| Group B Streptococcusb | 7 (4) | 6 (3) |
| Enterobacter sppb | 5 (3) | 4 (2) |
| Mixed florab | 51 (26) | 40 (21) |
| Otherb,c | 10 (5) | 7 (4) |
Positive culture was defined as the growth of 103 colony-forming units/mL or more of at least 1 uropathogen; laboratory reporting of culture growth is described in detail in the eAppendix in Supplement 3. All patients were symptomatic at baseline, thus those with a positive culture had confirmed urinary tract infection. Patients with mixed flora at baseline could not be analyzed for microbiologic outcomes.
Some cultures had polymicrobial growth.
Citrobacter koseri, Morganella spp, Staphylococcus aureus, Staphylococcus saprophyticus, and Streptococcus anginosus.
Clinical Outcomes
Clinical Response
At 28 days after therapy completion, 171 of 244 patients (70%) receiving nitrofurantoin had maintained clinical resolution vs 139 of 241 (58%) receiving fosfomycin (difference, 12% [95% CI, 4%-21%]; P = .004; Table 3). Data were missing for 28 randomized patients (6%), and 15 patients (3%) completing follow-up had “indeterminate” clinical response. Post-hoc multiple imputation and sensitivity analyses for both missing data (worst- and best-case scenarios, missing not at random) and indeterminate responses (treated alternatively as failures or, among those with some clinical improvement, successes) confirmed the robustness of this outcome in all scenarios (eTables 2-5 and eFigure in Supplement 3). Mixed effects models with site as a random effect provided an odds ratio for failure by day 28 after fosfomycin therapy of 1.76 (95% CI, 1.19-2.60; P = .004).
Table 3. Clinical and Microbiologic Outcomes.
| Clinical and Bacteriologic Outcome | No./Total No. (%) | Difference, % (95% CI) | P Valuea | |
|---|---|---|---|---|
| Nitrofurantoin (n = 255) |
Fosfomycin (n = 258) |
|||
| Primary Outcome | ||||
| Clinical response at 28 db | ||||
| Clinical resolution | 171/244 (70) | 139/241 (58) | 12 (4-21) | .004 |
| Clinical failure | 66/244 (27) | 94/241 (39) | ||
| Indeterminate | 7/244 (3) | 8/241 (3) | ||
| Missingc | 11 (4) | 17 (7) | ||
| Secondary Outcomes | ||||
| Clinical response at 14 d | ||||
| Clinical resolution | 184/247 (75) | 162/247 (66) | 9 (1-17) | .03 |
| Clinical failure | 56/247 (23) | 75/247 (30) | ||
| Indeterminate | 7/247 (3) | 10/247 (4) | ||
| Missingc | 8 (3) | 11 (4) | ||
| Microbiologic response at 28 db | ||||
| Culture obtained/baseline culture positive | 175/194 (90) | 163/183 (89) | ||
| Bacteriologic success through 28 d | 129/175 (74) | 103/163 (63) | 11 (1-20) | .04 |
| Bacteriologic success failure by 28 d | 46/175 (26) | 60/163 (37) | ||
| Microbiologic response at 14 d | ||||
| Culture obtained/baseline culture positive | 177/194 (91) | 165/183 (90) | ||
| Bacteriologic success through 14 d | 146/177 (82) | 121/165 (73) | 9 (0.4-18) | .04 |
| Bacteriologic success failure by 14 d | 31/177 (18) | 44/165 (27) | ||
Calculated using χ21 test.
Clinical response was defined as clinical resolution (complete resolution of symptoms and signs of urinary tract infection without prior failure), failure (need for additional or change in, antibiotic treatment due to a urinary tract infection, or discontinuation due to lack of efficacy), or indeterminate (either persistence of symptoms without objective evidence of infection or any extenuating circumstances precluding a classification of clinical resolution/failure). Microbiologic response was defined as resolution (eradication of the infecting strain with no recurrence of bacteriuria [<103 colony-forming units/mL] during follow-up) or failure (bacteriuria ≥103 colony-forming units/mL with the infecting strain).
Number of patients with missing data on this outcome measure and thus not included in this analysis (see eTables 2 and 3 in Supplement 3 for multiple imputation and sensitivity analyses for missing data).
Clinical response at the earlier point of 14 days after therapy completion also differed significantly between groups, with 184 of 247 patients (75%) receiving nitrofurantoin experiencing clinical resolution vs 162 of 247 (66%) receiving fosfomycin (difference, 9% [95% CI, 1%-17%]; P = .03; Table 3). Patients in either treatment group with early clinical failure (due to persistence of symptoms rather than recurrence after initial improvement) returned for additional antibiotic therapy at the same point after inclusion (mean [SD] of 6.3 [3.8] and 6.5 [3.6] days in the nitrofurantoin and fosfomycin groups, respectively).
In post-hoc analyses of the subgroup of patients with E coli infections, the difference in clinical response between treatment groups was more pronounced: through day 28, 80 of 103 (78%) vs 55 of 111 (50%) patients in the nitrofurantoin and fosfomycin groups, respectively, had clinical success (difference, 28% [95% CI, 15%-40%]; P < .001); there were 4 and 2 indeterminate outcomes, respectively (eTables 6 and 7 in Supplement 3). The test of interaction was significant (P < .001) when comparing treatment effect sizes in patients with E coli infections vs those without (odds ratios for failure after fosfomycin therapy: 4.48 [95% CI, 1.99-10.10] and 0.92 [95% CI 0.55-1.54], respectively).
Duration of Symptoms
The median duration of initial symptoms was 1 day longer in women receiving nitrofurantoin (4 days [IQR, 2-14] vs 3 days [IQR, 2-14]; P = .30).
Pyelonephritis and Other Morbidities
The development of pyelonephritis was rare. It occurred in 1 of 246 (0.4%) and 5 of 247 (2%) women in the nitrofurantoin and fosfomycin groups, respectively (difference, 1.6% [95% CI, −0.5% to 4.2%]; P = .22). There were no urosepsis events, and no lost days of work due to hospital admission (5/7 patients with pyelonephritis were retired; the remaining 2 were treated as outpatients).
Adverse Events
Adverse events were reported relatively infrequently and occurred with similar proportions in both treatment groups (eTable 8 in Supplement 3). Among patients with follow-up of at least 1 week following randomization, 21 of 248 (8%) and 16 of 247 (6%) in the nitrofurantoin and fosfomycin groups, respectively, reported at least 1 event. All events occurring with 1% or more frequency were gastrointestinal in nature and were of mild or moderate intensity (eTable 8 in Supplement 3). Six serious adverse events (eAppendix in Supplement 3) occurred within the study period; none were deemed related to the study antibiotic or the UTI at inclusion.
Bacteriologic Outcomes
Bacteriologic Success
As with clinical response, patients receiving nitrofurantoin had significantly more bacteriologic success: among those with positive baseline cultures, 146 of 177 (82%) and 121 of 165 (73%) saw no recurrence on day 14 in the nitrofurantoin and fosfomycin groups, respectively (P = .04; Table 3). The difference remained at day 28, when both groups saw an overall decrease in success, with 129 of 175 (74%) and 103 of 163 (63%), respectively (difference, 11% [95% CI, 1%-20%]; P = .04).
Again in post-hoc analyses restricted to patients with E coli infections, the difference was wider, with the nitrofurantoin group achieving 72% bacteriologic success (71/98) vs a rate of 58% in the fosfomycin group (63/109) (difference, 14% [95% CI, 2%-27%]; P = .03) by day 28 (eTable 7 in Supplement 3).
Emergence of Resistance Among Patients With Bacteriologic Recurrence
Within 14 days after nitrofurantoin therapy, 1 of 109 patients (1%) with initially nitrofurantoin-susceptible E coli had recurrence with a newly nitrofurantoin-resistant strain and by day 28, another saw recurrence of an E coli strain with new intermediate resistance. Neither experienced clinical failure, and the first was treated with fosfomycin. Her recurrent strain was also resistant to fosfomycin, but the initial strain’s susceptibility to this antibiotic was not tested. No emergence of resistance to fosfomycin could be documented.
Discussion
Among women with uncomplicated UTI, a 5-day course of nitrofurantoin compared with single-dose fosfomycin resulted in a significantly greater likelihood of clinical and microbiologic resolution at 28 days after therapy completion.
A purely clinical response was chosen as the primary outcome because it is the most meaningful to patients and, by extension, determines further medical pathways (ie, additional medical encounters with or without further laboratory work-up or additional antibiotic consumption). The use of a composite primary outcome incorporating both clinical and microbiologic responses would have excluded all patients without an available positive culture at baseline (up to 30% of patients in earlier studies,25 and 27% of randomized patients in this one).
In earlier nondynamic studies (which do not assess potential bacterial regrowth over time), fosfomycin was reported to possess high levels of in vitro activity against E coli and other uropathogens,7 yet in this trial’s post-hoc analysis of women with E coli infections, only 50% of those receiving fosfomycin experienced and maintained clinical resolution. The gap between those laboratory observations and fosfomycin’s in vivo efficacy5 suggests that pharmacokinetics, pharmacodynamics, or both play a major role in its ability to achieve clinical and in vivo microbiologic success. There is doubt that a single 3-g dose can reach adequate, or adequately durable, concentrations in urine.11
New evidence from more contemporary laboratory studies appears to confirm the role of pharmacodynamics: in an in vitro dynamic bladder infection model, significant rates of rapid regrowth of E coli, Klebsiella spp, and other uropathogens were observed following single-dose fosfomycin administration, with apparent amplification of resistant subpopulations.26
The perception that there is little baseline resistance to fosfomycin may be inaccurate: regions with increasing consumption have proportionately increasing resistance. A longitudinal study conducted in Spain documented an increase in fosfomycin resistance from 4% to 11% between 1997 and 2009, corresponding to a 340% increase in the drug’s consumption during that time.8 Fosfomycin is an antibiotic with a wide spectrum of activity, particularly against multidrug-resistant organisms,27 and a favorable safety profile, but its continued oral administration at an insufficient dose may rapidly diminish its usefulness, especially for invasive infections requiring intravenous administration of this drug.
Although patients taking nitrofurantoin experienced superior clinical and microbiologic outcomes, the drug’s overall response rate in this trial was lower than anticipated given earlier reported success rates of up to 90%.3,12,28 A few factors may explain the discrepancy, among them the relatively high incidence of non–E coli infections in this population. Nearly 80% of E coli infections were treated successfully with nitrofurantoin; Proteus spp have intrinsic resistance and Klebsiella spp resistance of up to 50%, depending on the region.29 And in earlier trials comparing nitrofurantoin with other UTI agents, definitions of “clinical” success often allowed for improvement in symptoms without full resolution30 or used microbiologic success with high bacterial counts as the cutoff. Two other trials comparing these drugs in the 1990s found clinical and microbiologic cure rates of 82% to 95% and 87% to 96%, respectively.12,13 Both trials defined clinical cure as symptomatic improvement and reported per-protocol results only; patients withdrawing because of clinical failure were excluded from analyses. In both trials, a reduction in urine culture counts from 105 to 104 cfu/mL qualified as microbiologic success.
Baseline resistance rates to nitrofurantoin are also increasing; participants with E coli infections in Poland, where nitrofuran derivatives have been sold over the counter for the past 5 years, had significantly higher baseline resistance to nitrofurantoin than those at the other sites where the drug is obtained by prescription only (6% vs 0%).24 Nonetheless, currently these rates are low relative to those of other antibiotics, and nitrofurantoin’s role in guidelines and clinical practice as a fluoroquinolone-sparing agent remains essential.31,32
Both drugs led to few adverse events, with no serious adverse events occurring in relation to either. The more serious adverse effects of nitrofurantoin, pulmonary and hepatotoxicity, were neither expected in this trial of short-course therapy33 nor observed.
Limitations
This study has several limitations. First, it was conducted open label. The open design became inevitable in light of the costs of sachet-packaged fosfomycin dummies, which would have totaled more than a quarter of the trial’s budget. The open design may have introduced some level of measurement bias given a subjective primary end point. However, there was consistency between clinical and microbiologic outcomes. Second, laboratory analyses were not centralized, potentially introducing heterogeneity in microbiologic methods. Third, recruitment and follow-up in Israel did not reach original targets due to political events occurring soon after the site’s launch. Fourth, the 3 sites had some differences among patients (age and local resistance prevalences with, in Poland, over-the-counter sales of nitrofuran derivatives coincident with the trial’s launch).
Conclusions
Among women with uncomplicated UTI, 5-day nitrofurantoin, compared with single-dose fosfomycin, resulted in a significantly greater likelihood of clinical and microbiologic resolution at 28 days after therapy completion.
Statistical Analysis Plan
Trial Protocol
eAppendix. Additional Study Exclusion Criteria and Definitions
eTable 1. Baseline Urine Culture Results by Site (Intention-to-Treat Population)
eTable 2. Multiple Imputation of Missing Data
eTable 3. Sensitivity Analyses for Missing Primary Outcome Data for Nitrofurantoin (n=11) and Fosfomycin (n=17)
eTable 4. Post-Hoc Analyses: Clinical Response When Indeterminate Cases With Clinical Improvement are Considered Clinical Successes in (A) Intention-to-Treat and (B) Per-Protocol Populations
eTable 5. Post-Hoc Analyses: Clinical Response When Indeterminate Cases are Considered Failures in (A) Intention-to-Treat and (B) Per-Protocol Populations
eTable 6. Clinical Response Among Patients With E. coli in Baseline Urine Cultures
eTable 7. Bacteriologic Response Among Patients With E. coli in Baseline Urine Cultures
eTable 8. Adverse Events by Treatment Group in the Intention-to-Treat Population
eFigure. Sensitivity of the Fosfomycin-Versus-Nitrofurantoin Comparison for Failure by Day 28 to Missing Data Not-at-Random Using a Pattern Mixture Model Approach
eReferences
References
- 1.Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. [DOI] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control Trend of antimicrobial consumption by country. https://ecdc.europa.eu/en/antimicrobial-consumption/database/trend-country. Accessed February 25, 2018.
- 3.Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother. 2015;70(9):2456-2464. [DOI] [PubMed] [Google Scholar]
- 4.Zalmanovici Trestioreanu A, Green H, Paul M, Yaphe J, Leibovici L. Antimicrobial agents for treating uncomplicated urinary tract infection in women. Cochrane Database Syst Rev. 2010;(10):CD007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fosfomycin for urinary tract infections. Med Lett Drugs Ther. 1997;39(1005):66-68. [PubMed] [Google Scholar]
- 6.Kahan FM, Kahan JS, Cassidy PJ, Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974;235(0):364-386. [DOI] [PubMed] [Google Scholar]
- 7.Maraki S, Samonis G, Rafailidis PI, Vouloumanou EK, Mavromanolakis E, Falagas ME. Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob Agents Chemother. 2009;53(10):4508-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oteo J, Bautista V, Lara N, et al. ; Spanish ESBL-EARS-Net Study Group . Parallel increase in community use of fosfomycin and resistance to fosfomycin in extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli. J Antimicrob Chemother. 2010;65(11):2459-2463. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis. 2011;15(11):e732-e739. [DOI] [PubMed] [Google Scholar]
- 10.Mazzei T, Cassetta MI, Fallani S, Arrigucci S, Novelli A. Pharmacokinetic and pharmacodynamic aspects of antimicrobial agents for the treatment of uncomplicated urinary tract infections. Int J Antimicrob Agents. 2006;28(suppl 1):S35-S41. [DOI] [PubMed] [Google Scholar]
- 11.Wijma RA, Koch BCP, van Gelder T, Mouton JW. High interindividual variability in urinary fosfomycin concentrations in healthy female volunteers [published online September 1, 2017]. Clin Microbiol Infect. doi: 10.1016/j.cmi.2017.08.023 [DOI] [PubMed] [Google Scholar]
- 12.Stein GE. Comparison of single-dose fosfomycin and a 7-day course of nitrofurantoin in female patients with uncomplicated urinary tract infection. Clin Ther. 1999;21(11):1864-1872. [DOI] [PubMed] [Google Scholar]
- 13.Van Pienbroek E, Hermans J, Kaptein AA, Mulder JD. Fosfomycin trometamol in a single dose versus seven days nitrofurantoin in the treatment of acute uncomplicated urinary tract infections in women. Pharm World Sci. 1993;15(6):257-262. [DOI] [PubMed] [Google Scholar]
- 14.Nicolle LE. Uncomplicated urinary tract infection in adults including uncomplicated pyelonephritis. Urol Clin North Am. 2008;35(1):1-12, v. v. [DOI] [PubMed] [Google Scholar]
- 15.Borsa F, Leroy A, Fillastre JP, Godin M, Moulin B. Comparative pharmacokinetics of tromethamine fosfomycin and calcium fosfomycin in young and elderly adults. Antimicrob Agents Chemother. 1988;32(6):938-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332. [DOI] [PubMed] [Google Scholar]
- 17.Garcia LS, Isenberg HD. Clinical Microbiology Procedures Handbook. 3rd ed Washington, DC: ASM Press; 2010. [Google Scholar]
- 18.CLSI M100-S25 Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. [Google Scholar]
- 19.European Committee on Antimicrobial Susceptibility Testing http://www.eucast.org/. Accessed November 28, 2017.
- 20.Gupta K, Hooton TM, Stamm WE. Isolation of fluoroquinolone-resistant rectal Escherichia coli after treatment of acute uncomplicated cystitis. J Antimicrob Chemother. 2005;56(1):243-246. [DOI] [PubMed] [Google Scholar]
- 21.Ernst EJ, Ernst ME, Hoehns JD, Bergus GR. Women’s quality of life is decreased by acute cystitis and antibiotic adverse effects associated with treatment. Health Qual Life Outcomes. 2005;3:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Infectious Diseases Society of America White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis. 2012;55(8):1031-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377-399. [DOI] [PubMed] [Google Scholar]
- 24.Huttner A, Brossier C, von Dach E, et al. Increasing nitrofurantoin resistance in Escherichia coli urinary isolates in a country with over-the-counter antibiotic sales. Presented at: 27th European Congress on Clinical Microbiology and Infection; April 22, 2017; Vienna, Austria. [Google Scholar]
- 25.Giesen LG, Cousins G, Dimitrov BD, van de Laar FA, Fahey T. Predicting acute uncomplicated urinary tract infection in women: a systematic review of the diagnostic accuracy of symptoms and signs. BMC Fam Pract. 2010;11:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott IJ, Meletiadis J, Belghanch I, et al. Fosfomycin efficacy and emergence of resistance among Enterobacteriaceae in an in vitro dynamic bladder infection model [published online December 14, 2017]. J Antimicrob Chemother. doi: 10.1093/jac/dkx441 [DOI] [PubMed] [Google Scholar]
- 27.Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother. 2012;56(11):5744-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iravani A, Klimberg I, Briefer C, Munera C, Kowalsky SF, Echols RM. A trial comparing low-dose, short-course ciprofloxacin and standard 7 day therapy with co-trimoxazole or nitrofurantoin in the treatment of uncomplicated urinary tract infection. J Antimicrob Chemother. 1999;43(suppl A):67-75. [PubMed] [Google Scholar]
- 29.Mezzatesta ML, La Rosa G, Maugeri G, et al. In vitro activity of fosfomycin trometamol and other oral antibiotics against multidrug-resistant uropathogens. Int J Antimicrob Agents. 2017;49(6):763-766. [DOI] [PubMed] [Google Scholar]
- 30.Spencer RC, Moseley DJ, Greensmith MJ. Nitrofurantoin modified release versus trimethoprim or co-trimoxazole in the treatment of uncomplicated urinary tract infection in general practice. J Antimicrob Chemother. 1994;33(suppl A):121-129. [DOI] [PubMed] [Google Scholar]
- 31.Slekovec C, Leroy J, Huttner A, et al. When the precautionary principle disrupts 3 years of antibiotic stewardship: nitrofurantoin in the treatment of urinary tract infections. J Antimicrob Chemother. 2014;69(1):282-284. [DOI] [PubMed] [Google Scholar]
- 32.Stewardson AJ, Vervoort J, Adriaenssens N, et al. ; SATURN WP1 Study Group; SATURN WP3 Study Group . Effect of outpatient antibiotics for urinary tract infections on antimicrobial resistance among commensal Enterobacteriaceae: a multinational prospective cohort study. Clin Microbiol Infect. 2018;S1198-743X(18)30031-4. [DOI] [PubMed] [Google Scholar]
- 33.Muller AE, Verhaegh EM, Harbarth S, Mouton JW, Huttner A. Nitrofurantoin’s efficacy and safety as prophylaxis for urinary tract infections: a systematic review of the literature and meta-analysis of controlled trials. Clin Microbiol Infect. 2017;23(6):355-362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Analysis Plan
Trial Protocol
eAppendix. Additional Study Exclusion Criteria and Definitions
eTable 1. Baseline Urine Culture Results by Site (Intention-to-Treat Population)
eTable 2. Multiple Imputation of Missing Data
eTable 3. Sensitivity Analyses for Missing Primary Outcome Data for Nitrofurantoin (n=11) and Fosfomycin (n=17)
eTable 4. Post-Hoc Analyses: Clinical Response When Indeterminate Cases With Clinical Improvement are Considered Clinical Successes in (A) Intention-to-Treat and (B) Per-Protocol Populations
eTable 5. Post-Hoc Analyses: Clinical Response When Indeterminate Cases are Considered Failures in (A) Intention-to-Treat and (B) Per-Protocol Populations
eTable 6. Clinical Response Among Patients With E. coli in Baseline Urine Cultures
eTable 7. Bacteriologic Response Among Patients With E. coli in Baseline Urine Cultures
eTable 8. Adverse Events by Treatment Group in the Intention-to-Treat Population
eFigure. Sensitivity of the Fosfomycin-Versus-Nitrofurantoin Comparison for Failure by Day 28 to Missing Data Not-at-Random Using a Pattern Mixture Model Approach
eReferences