Key Points
Question
What is the effect of intravenous recombinant human pentraxin 2 on change in forced vital capacity (FVC) for patients with idiopathic pulmonary fibrosis?
Findings
In this randomized clinical trial that included 117 patients with IPF, treatment with recombinant human pentraxin 2 every 4 weeks for 24 weeks resulted in a change in FVC percentage of predicted value of −2.5% compared with −4.8% with placebo, a difference that was statistically significant. Recombinant human pentraxin 2 infusions were well tolerated.
Meaning
These preliminary findings suggest that recombinant human pentraxin 2 may reduce the decline in lung function in patients with idiopathic pulmonary fibrosis, but more definitive research is required.
Abstract
Importance
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic lung disease with poor prognosis. Approved therapies do not halt disease progression.
Objective
To determine the effect of recombinant human pentraxin 2 vs placebo on change from baseline to week 28 in mean forced vital capacity (FVC) percentage of predicted value.
Design, Setting, and Participants
Phase 2, randomized, double-blind, placebo-controlled trial conducted at 18 sites in 7 countries of eligible patients with IPF (N = 117; aged 40-80 years; FVC ≥50% and ≤90% predicted; ratio of forced expiratory volume in the first second/FVC >0.70; diffusing capacity for carbon monoxide [Dlco] ≥25% and ≤90% predicted; and distance of ≥150 m on the 6-minute walk test). Study period was August 2015-May 2017.
Interventions
Patients were randomized to receive either recombinant human pentraxin 2 (10 mg/kg intravenous every 4 weeks, n = 77) or placebo (n = 39) for 24 weeks, and stratified by concurrent IPF treatment status.
Main Outcomes and Measures
The primary end point was the least-squares mean change in FVC percentage of predicted value from baseline to week 28 (minimal clinically important difference, decline of 2%-6%). Secondary end points included mean change in lung volumes (total, normal, and interstitial lung abnormalities) on high-resolution computed tomography (HRCT) and 6-minute walk distance (minimal clinically important difference, 24-45 m).
Results
Of 117 randomized patients, 116 received at least 1 dose of study drug (mean age, 68.6 years; 81.0% men; mean time since IPF diagnosis, 3.8 years), and 111 (95.7%) completed the study. The least-squares mean change in FVC percentage of predicted value from baseline to week 28 in patients treated with recombinant human pentraxin 2 was −2.5 vs −4.8 for those in the placebo group (difference, +2.3 [90% CI, 1.1 to 3.5]; P = .001). No significant treatment differences were observed in total lung volume (difference, 93.5 mL [90% CI, −27.7 to 214.7]), quantitative parenchymal features on HRCT (normal lung volume difference, −1.2% [90% CI, −4.4 to 1.9]; interstitial lung abnormalities difference, 1.1% [90% CI, −2.2 to 4.3]), or measurement of Dlco (difference, −0.4 [90% CI, −2.6 to 1.7]). The change in 6-minute walk distance was −0.5 m for patients treated with recombinant human pentraxin 2 vs −31.8 m for those in the placebo group (difference, +31.3 m [90% CI, 17.4 to 45.1]; P < .001). The most common adverse events in the recombinant human pentraxin 2 vs placebo group were cough (18% vs 5%), fatigue (17% vs 10%), and nasopharyngitis (16% vs 23%).
Conclusions and Relevance
In this preliminary study, recombinant human pentraxin 2 vs placebo resulted in a slower decline in lung function over 28 weeks for patients with idiopathic pulmonary fibrosis. Further research should more fully assess efficacy and safety.
Trial Registration
clinicaltrials.gov Identifier: NCT02550873
This randomized clinical trial compares the effects of recombinant human pentraxin 2, a monocyte differentiation inhibitor, vs placebo on changes in percent predicted forced vital capacity after 28 weeks in patients with idiopathic pulmonary fibrosis.
Introduction
Idiopathic pulmonary fibrosis (IPF), a progressive disease that leads to an irreversible loss of lung function, has a 5-year survival rate between 20% and 40%.1 In 2015, the incidence of IPF in North America and Europe was estimated at 3 to 9 cases per 100 000 person-years, with lower incidence in Asia and South America.2 IPF disproportionately affects older men.3 Pirfenidone4 and nintedanib5 are currently the only therapies approved for the treatment of IPF. Although the rate of decline in forced vital capacity (FVC) is slower in patients treated with pirfenidone and nintedanib, neither treatment halts disease progression or improves any objective measurements of disease status. Thus, a need for additional novel treatment approaches remains.
Evidence suggests that epithelial damage and abnormal wound repair contribute to the pathogenesis of IPF.6 Fibrocytes, usually inactive fibroblast-like cells derived from peripheral blood monocytes, have been implicated in this process.7 Purified serum amyloid P, also known as pentraxin 2, inhibits monocyte differentiation into profibrotic fibrocytes.8,9 Pentraxin 2 is also a potent inhibitor of monocyte differentiation into proinflammatory macrophages10 and production of transforming growth factor (TGF)-β1, which is a key mediator of pulmonary fibrosis.11 Plasma pentraxin 2 concentrations are reduced in patients with IPF and correlate with disease severity, further supporting its role in modulating fibrosis.11
A recombinant human pentraxin 2 protein decreased lung fibrosis in preclinical models of TGF-β1 overexpression and bleomycin-induced lung fibrosis; the effect lasted for as long as 30 days after dosing.11,12,13 In a phase 1 study in healthy participants and patients with pulmonary fibrosis, administration of recombinant human pentraxin 2 increased circulating pentraxin 2 concentrations 6- to 13-fold.14 An additional phase 1 study in patients with IPF showed improvements in predicted percentage of FVC value and 6-minute walk distance following treatment with recombinant human pentraxin 2.15 This placebo-controlled phase 2 trial (PRM-151-202) was conducted to assess the efficacy and adverse events of recombinant human pentraxin 2 in patients with IPF.
Methods
The trial protocol and statistical analysis plan are available in Supplement 1.
Study Oversight
The trial was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines and was approved by local ethical review boards. The clinical protocol was approved by an independent ethics committee or institutional review board at each participating center. All patients provided written informed consent prior to enrollment. An independent data and safety monitoring board provided oversight.
Study Sites and Patients
This study was conducted at 18 sites in 7 countries (United States, Germany, Italy, Czech Republic, the Netherlands, Spain, and Switzerland); all sites were interstitial lung disease specialty centers. Eligible patients were between the ages of 40 and 80 years with a diagnosis of IPF (established at the study sites in accordance with the inclusion criteria; eAppendix [Complete Patient Inclusion Criteria] in Supplement 2),16 FVC of 50% or greater and of 90% or less predicted, diffusing capacity for carbon monoxide (Dlco) of 25% or greater and of 90% or less predicted, distance of at least 150 m on the 6-minute walk test,17 and ratio of forced expiratory volume in the first second (FEV1)/FVC greater than 0.70. Concurrent therapy with pirfenidone or nintedanib was permitted if the dosage was stable for at least 3 months; patients not receiving concurrent pirfenidone or nintedanib must have stopped treatment for at least 4 weeks prior to baseline. Patients with emphysema (≥50% on chest high-resolution computed tomography [HRCT]) or greater extent of emphysema than fibrosis (see eAppendix [Complete Patient Exclusion Criteria] in Supplement 2 for further methodological details), cigarette smoking within the previous 3 months, or oxygen saturation by pulse oximetry less than 89% on room air or supplemental oxygen at rest were excluded. A comprehensive list of inclusion and exclusion criteria is provided in the eAppendix (Complete Patient Inclusion Criteria; Complete Patient Exclusion Criteria; in Supplement 2).
Study Design
This phase 2, randomized, double-blind, placebo-controlled trial included a 4-week screening period, a 24-week randomized treatment period, and a 4-week follow-up visit (week 28). Patient groups were assigned using a software-generated randomization list (Proc plan; SAS version 9.4) composed of a block of 3, balanced at a ratio of 2:1 in favor of recombinant human pentraxin 2 over placebo. Randomization was stratified according to concomitant IPF treatments (patients receiving pirfenidone or nintedanib; patients not receiving concurrent pirfenidone or nintedanib for IPF). Patients received either recombinant human pentraxin 2 (10 mg/kg intravenous [IV] every 4 weeks) or placebo (see eAppendix [Randomization] in Supplement 2) with a loading regimen of 3 doses at the start of treatment (days 1, 3, and 5). Patients who completed the trial were eligible to participate in an open-label treatment extension period.
Efficacy and Tolerability End Points
Researchers and the site study teams were blinded to study group assignment, as was the study sponsor; therefore, outcomes assessments were blinded.
The primary efficacy end point of the trial was the least-squares mean change in FVC percentage of predicted value from baseline to week 28 (a decline of 2% to 6% represents a clinically important difference in an IPF population).18 Secondary end points included least-squares mean change from baseline to week 28 in FVC percentage of predicted value analyzed by concomitant therapy status (patients receiving concomitant pirfenidone or nintedanib or patients without concurrent IPF therapy), mean change from baseline to week 28 in total lung volume and volume of parenchymal features (in mL [not reported in this article] and percentage of total lung volume) on HRCT using quantitative imaging software (Lung Texture Analysis, Imbio; clinically important difference in an IPF population is not defined), the percentage of patients with categorical change in FVC (≥5% or ≥10% absolute change in percentage of the predicted value, <5% change, and ≥100 mL or ≥200 mL change), hemoglobin-corrected Dlco (clinically important difference in an IPF population is not defined; however, a change of 10% to 15% in predicted values is used clinically), and 6-minute walk distance (a difference of 24 to 45 m in the 6-minute walk test has been reported to be clinically important in the IPF population).19 All pulmonary function test readings were centralized. The correlation of the least-squares mean change of FVC percentage (predicted values) and measures of quantitative imaging (a secondary end point) is not reported in this article.
Adverse events and tolerability were assessed by means of scheduled physical examinations, vital signs evaluation, 12-lead electrocardiogram, clinical laboratory test results, any incidental testing, and recording of adverse events, including serious adverse events. The frequency and severity of adverse events were documented according to the Medical Dictionary for Regulatory Activities (MedDRA; version 19.0). Tolerability analyses were descriptive.
Additional adverse events of interest included respiratory decline events (any unscheduled visits to a health care professional or urgent care for deterioration in respiratory status, or hospitalization due to a worsening of respiratory symptoms; see eAppendix [Respiratory Decline Events] in Supplement 2). Drug-induced liver injury was assessed post hoc via evaluation of drug-induced serious hepatotoxicity (eDISH) plot. Serum levels of antibodies to human pentraxin 2 were measured to assess potential immune response to study drug every 4 weeks. All patients were observed for symptoms of infusion reaction, including allergic reactions or hypersensitivity.
Statistical Analysis
Assuming that it would not be possible to evaluate data for 15% of patients, the sample size was calculated as 117 patients (78 patients in the recombinant human pentraxin 2 group and 39 patients in the placebo group) to provide 80% power to demonstrate superiority with a 2-sided type I error rate of .10. The study was powered to detect a difference of 2.63 in FVC percentage (predicted value) favoring the recombinant human pentraxin 2 group, based on the following assumptions: (1) 75% of patients were receiving concurrent IPF therapy; (2) a decline of 3% in patients not receiving concurrent IPF therapy in the placebo group; and (3) a decline of 1.5% in patients receiving concurrent IPF therapy in the placebo group (resulting in an average decline of 1.88% in the placebo group) compared with an increase of 0.75% in the recombinant human pentraxin 2 group (with or without concurrent IPF treatment). The goal of the stratified randomization was to provide a similar proportion of patients in both treatment groups receiving concomitant pirfenidone or nintedanib, and patients not receiving concurrent IPF therapy.
The primary efficacy end point as well as secondary end points collected at each visit were analyzed using linear mixed models for repeated measures with the end point from week 4 to week 28 as a dependent variable, and time (days since first dose), treatment group, and the treatment × time interaction as explanatory variables. Post hoc analyses were performed to assess the potential effects of site (random model effect) and sex (fixed model effect) on the results. The statistical model was used without imputation of missing values and is valid under the assumption of missing at random. Multiple imputations technique (jump to reference method)20 was used to assess the robustness of the primary efficacy analysis.
To analyze data collected only at baseline and week 28, a linear model was fitted with the observed change from baseline to week 28 as the response variable and treatment group as the explanatory variable. Both models were adjusted for concurrent treatment status and corresponding baseline score. The comparison of recombinant human pentraxin 2 vs placebo was conducted by computing the difference in the estimated change from baseline at week 28, the corresponding 90% CI, and the P value of the difference estimate vs 0. Statistical significance was determined using a 2-sided type I error rate of .10. Given the multiplicity of secondary end points and risk of type I error, some end points may be considered exploratory in nature. Interaction tests were conducted to assess if background therapy status influenced the primary efficacy end point. All analyses were performed on all randomized patients having received at least 1 dose of the study medication. SAS version 9.4 was used for the statistical analyses.
Results
Patients
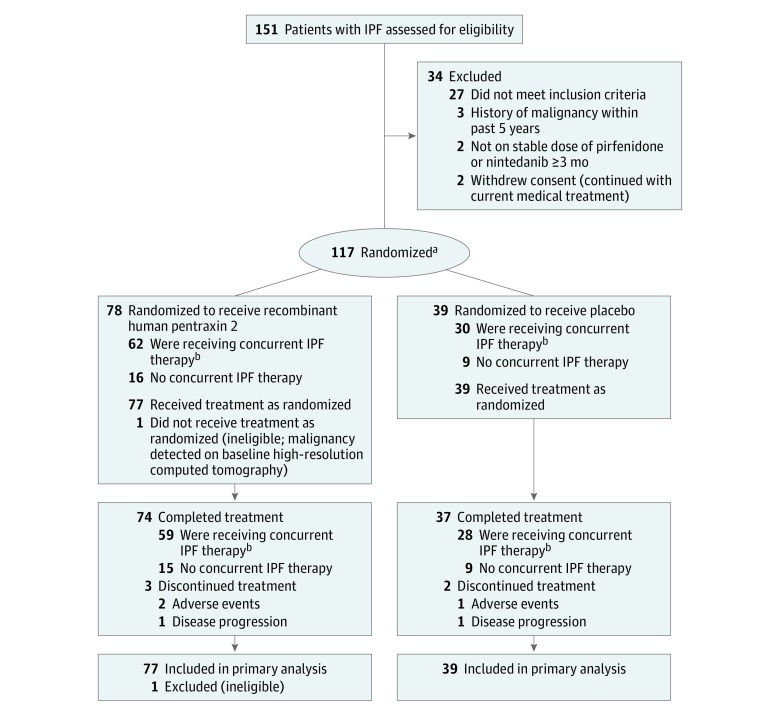
During the study period (August 2015 and May 2017), 151 patients were screened, 117 underwent randomization, and 116 received at least 1 dose of the study drug. A total of 111 patients completed the study and 5 patients discontinued from the study (Figure 1). The baseline characteristics of recombinant human pentraxin 2 and placebo-treated patients were similar (Table 1). Participants were predominantly male (81.0%). Patients’ mean age was 68.6 years and their mean time since IPF diagnosis was 3.8 years. A total of 91 patients (78.4%) were receiving concurrent therapy (pirfenidone or nintedanib) at baseline. Men were more likely to be receiving concurrent IPF therapy compared with women (36.0% vs 14.3%). A total of 15 patients (12.9%) noted not having received IPF treatment in the past.
Figure 1. Participant Flow in a Trial of Recombinant Human Pentraxin 2 vs Placebo for Idiopathic Pulmonary Fibrosis.
IPF indicates idiopathic pulmonary fibrosis.
aRandomization ratio of 2:1 for recombinant human pentraxin 2 to placebo.
bPatients receiving a stable dosage of pirfenidone or nintedanib.
Table 1. Baseline Characteristics of Patients.
| Characteristics | Recombinant Human Pentraxin 2 (n = 77) | Placebo (n = 39) |
|---|---|---|
| Male sex, No. (%) | 65 (84) | 29 (74) |
| Age, mean (SD), y | 69.0 (6.3) | 67.6 (7.1) |
| Weight, mean (SD), kg | 86.1 (15.2) | 87.5 (13.4) |
| Time since diagnosis of IPF | ||
| Mean (SD), y | 3.7 (2.2) | 3.9 (2.6) |
| Median (IQR), y | 3.0 (2.0-5.0) | 3.0 (2.0-4.0) |
| FVC | ||
| Mean (SD), mL | 2733 (630) | 2763 (654) |
| Median (IQR), mL | 2700 (2320-3140) | 2610 (2290-3250) |
| Percentage of predicted value, mean (SD) | 67.7 (10.9) | 67.4 (11.4) |
| FEV1/FVC, mean (SD), % | 81.2 (5.1) | 81.6 (4.7) |
| Hemoglobin-corrected Dlco, % of predicted values, mean (SD) | 40.1 (9.14) | 43.2 (10.5) |
| 6-min walk test distance | ||
| Mean (SD), m | 434.8 (92.5) | 457.7 (117.7) |
| Median (IQR), m | 436.0 (368.0-494.0) | 450.0 (370.0-535.0) |
| SpO2 at rest, mean (SD), % | 95.6 (2.1) | 95.5 (1.8) |
| Patients with SpO2 at rest <95%, No. (%) | 20 (26) | 9 (23) |
| IPF therapy status at baseline, No. (%) | ||
| Concurrent IPF therapy | 61 (79) | 30 (77) |
| Pirfenidone | 39 (64) | 22 (73) |
| Nintedanib | 22 (36) | 8 (27) |
| No concurrent IPF therapy | 16 (21) | 9 (23) |
| IPF therapy naive | 8 (10) | 7 (18) |
| Baseline pentraxin 2 concentrations | ||
| Mean (SD), ng/mL | 30 456 (13 567) | 30 962 (10 090) |
| Median (IQR), ng/mL | 29 500 (23 500-38 750) | 29 500 (26 250-33 500) |
| Comorbid conditions | ||
| GERD | 47 (61) | 16 (41) |
| Hypertension | 38 (49) | 14 (35) |
| Cardiac disorders | 29 (38) | 7 (18) |
| Coronary artery disorders | 15 (19) | 4 (10) |
| Emphysema | 2 (3) | 0 |
| Pulmonary hypertension | 1 (1) | 3 (8) |
Abbreviations: Dlco, diffusing capacity of the lungs for carbon monoxide; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; GERD, gastroesophageal reflux disease; IPF, idiopathic pulmonary fibrosis; IQR, interquartile range; SpO2, oxygen saturation of peripheral blood.
Overall, the mean (SD) baseline plasma pentraxin 2 concentration was 30 626 ng/mL (12 463 ng/mL) and was similar between treatment groups. No patients had antidrug antibodies at baseline. Almost all randomized patients received the 9 planned infusions (73 patients [94.8%]) in the recombinant human pentraxin 2 group and 37 patients [94.9%] in the placebo group. The volume of study drug administered vs intended administration volume was above 96%.
Primary End Point
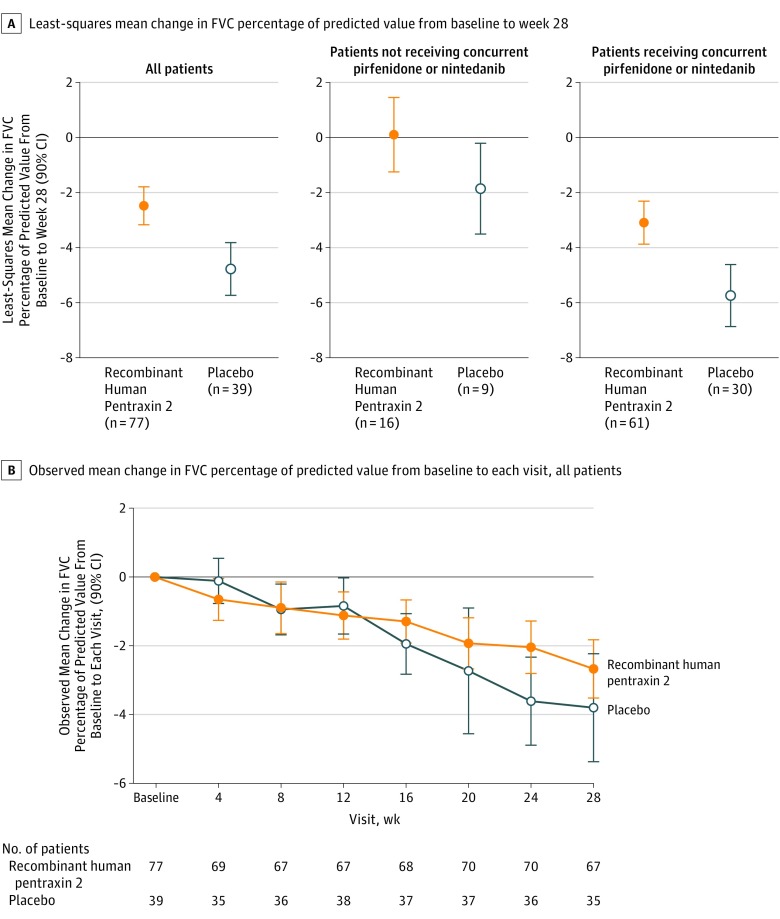
The between-group difference in least-squares mean change in FVC percentage of predicted value from baseline to week 28 in patients treated with recombinant human pentraxin 2 (−2.5) compared with placebo (−4.8) was 2.3 (90% CI, 1.1 to 3.5; P = .001; Figure 2A). This effect was independent of concurrent IPF therapy status (P = .65). In separate subgroup analyses, the between-group difference in least-squares mean change of FVC percentage of predicted value in patients treated with recombinant human pentraxin 2 (0.1) compared with placebo (−1.9) without concurrent IPF therapy was 2.0 (90% CI, −0.2 to 4.1; P = .13), and the between-group difference in least-squares mean change of FVC percentage of predicted value in patients treated with recombinant human pentraxin 2 (−3.1) compared with placebo (−5.7) in the subgroup who received concomitant pirfenidone or nintedanib was 2.6 (90% CI, 1.3 to 4.0; P = .002; Figure 2A). Patients treated with recombinant human pentraxin 2 had less decline in observed mean change in FVC percentage of predicted value over time compared with patients treated with placebo (Figure 2B). Additional analyses showing the distribution in observed change in FVC percentage of predicted value were performed and are shown in eFigure 1 in Supplement 2.
Figure 2. Least-Squares Mean Change (Primary End Point) and Observed Mean Change in Forced Vital Capacity Percentage of Predicted Value From Baseline to Week 28.
For panel A, the between-group difference was 2.3 (90% CI, 1.1 to 3.5; P = .001) for all patients, 2.0 (90% CI, −0.2 to 4.1; P = .13) for patients not receiving concurrent pirfenidone or nintedanib, and 2.6 (90% CI, 1.3 to 4.0; P = .002) for patients receiving concurrent pirfenidone or nintedanib.
FVC indicates forced vital capacity.
There was no indication of a sex or site effect when added to the model because a post hoc analysis showed a significant treatment effect for recombinant human pentraxin 2 vs placebo on FVC percentage of predicted value (P<.01). Imputation of missing data using a conservative method (jump to reference method)20 still provided a significant treatment × time interaction for FVC percentage of predicted (P = .02).
Secondary End Points
In the quantitative HRCT analysis, no significant differences in least-squares mean changes were observed in the recombinant human pentraxin 2 group compared with the placebo group from baseline to week 28 for total lung volume (93.5 mL [90% CI, −27.7 to 214.7]; P = .20), percentage of normal lung volume (−1.2% [90% CI, −4.4% to 1.9%]; P = .52), or percentage of interstitial lung abnormalities (1.1% [90% CI, −2.2% to 4.3%]; P = .58) (see eFigure 2 in Supplement 2 for data by background therapy status, as well as additional analyses showing the distribution of the observed changes). Categorical changes in FVC are presented in eTable 1 (Supplement 2). Increases of 5% or greater in FVC percentage of predicted value were observed in 2 patients (3.0%) treated with recombinant human pentraxin 2 and none in the placebo group (P = .29); 7 patients (10.4%) in the recombinant human pentraxin 2 group and none in the placebo group experienced an increase of 100 mL or greater in FVC (P = .05). A total of 20 patients (29.9%) in the recombinant human pentraxin 2 group and 13 patients (37.1%) in the placebo group experienced a 5% or greater decrease in FVC percentage of predicted value (P = .42). Thirty-seven patients (55.2%) in the recombinant human pentraxin 2 group and 18 patients (51.4%) in the placebo group experienced a decrease of 100 mL or greater in FVC (P = .78).
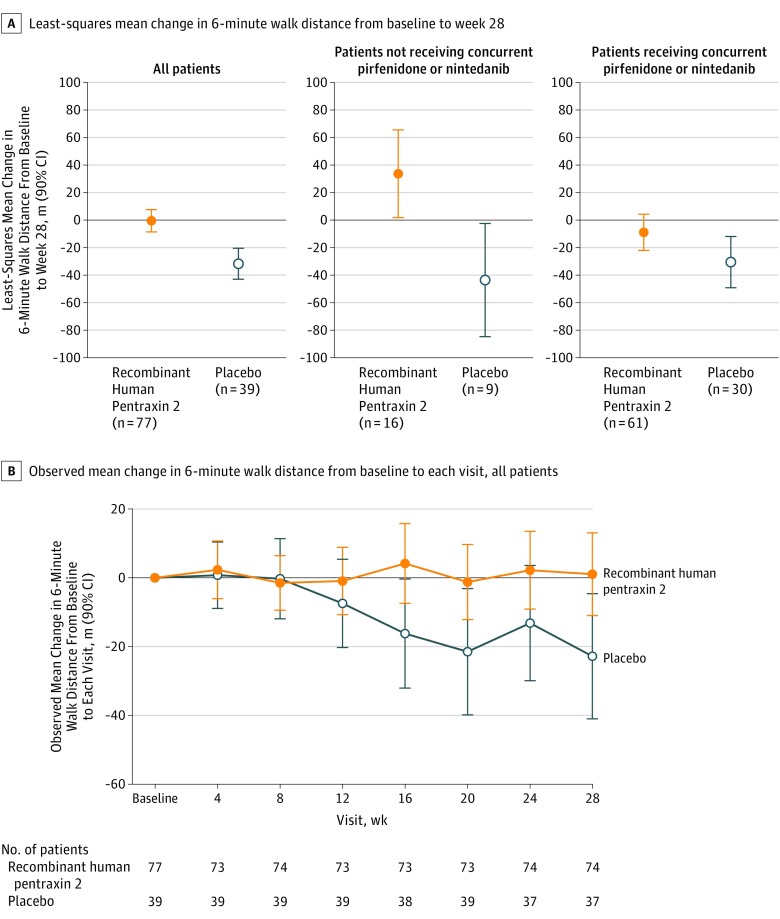
The difference in least-squares mean change in Dlco between patients in the recombinant human pentraxin 2 group and the placebo group from baseline to week 28 was −0.4 (90% CI, −2.6 to 1.7; P = .74) (see eFigure 3 in Supplement 2 for data by background therapy status, as well as an additional analysis showing the distribution of the observed changes). In the 6-minute walk test, the difference between patients treated with recombinant human pentraxin 2 (−0.5 m) and placebo (−31.8 m) in least-squares mean change from baseline to week 28 was 31.3 m (90% CI, 17.4 to 45.1; P < .001; Figure 3A). This effect was dependent on concurrent IPF therapy status (P=.002). The between-group difference in least-squares mean change from baseline to week 28 in the 6-minute walk test distance in patients treated with recombinant human pentraxin 2 (33.7 m) compared with placebo (−43.6 m) in the subgroup without concurrent IPF therapy was 77.2 m (90% CI, 25.2 to 129.3; P = .02 Figure 3A); the between-group difference in patients treated with recombinant human pentraxin 2 (−8.9 m) compared with placebo (−30.5 m) in the subgroup that received concurrent pirfenidone or nintedanib was 21.6 m (90% CI, −1.2 to 44.4; P = .12; Figure 3A). Patients treated with recombinant human pentraxin 2 experienced stabilization in the observed mean change in the 6-minute walk test over time, whereas patients treated with placebo experienced a decline (Figure 3B). Additional analyses showing the distribution of observed change in the 6-minute walk test were performed and are shown in eFigure 4 in Supplement 2.
Figure 3. Least-Squares Mean Change (Secondary End Point) and Observed Mean Change in 6-Minute Walk Distance From Baseline to Week 28.
For panel A, the between-group difference was 31.3 (90% CI, 17.4 to 45.1; P<.001) for all patients, 77.2 (90% CI, 25.2 to 129.3; P = .02) for patients not receiving concurrent pirfenidone or nintedanib, and 21.6 (90% CI, −1.2 to 44.4; P = .12) for patients receiving concurrent pirfenidone or nintedanib.
Adverse Events
Of 108 patients (93.1%) who experienced an adverse event during the trial, 107 patients (92.2%) experienced an event after receiving treatment (Table 2). Most events were mild or moderate in intensity. Cough was the most frequently reported adverse event (18% in the recombinant human pentraxin 2 group and 5% in the placebo group). Serious adverse events occurred in 6 patients (7.8%) in the recombinant human pentraxin 2 group and 4 patients (10.3%) in the placebo group. None of the serious adverse events were considered related to the study treatment. Three patients (2.6%) experienced adverse events that led to discontinuation from the study drug (2 patients in the recombinant human pentraxin 2 group and 1 patient in the placebo group). One patient in the placebo group died from pneumonia.
Table 2. Adverse Eventsa.
| Events | No. (%) of Patients With Event | |
|---|---|---|
| Recombinant Human Pentraxin 2 (n = 77) | Placebo (n = 39) | |
| Any adverse event | 71 (92) | 36 (92) |
| Most frequent adverse eventsb | ||
| Cough | 14 (18) | 2 (5) |
| Fatigue | 13 (17) | 4 (10) |
| Nasopharyngitis | 12 (16) | 9 (23) |
| Headache | 11 (14) | 3 (8) |
| Idiopathic pulmonary fibrosis | 11 (14) | 5 (13) |
| Diarrhea | 9 (12) | 2 (5) |
| Bronchitis | 8 (10) | 5 (13) |
| Dyspnea | 7 (9) | 4 (10) |
| Upper respiratory tract infection | 7 (9) | 5 (13) |
| Back pain | 3 (4) | 4 (10) |
| Severe adverse eventsc | 7 (9) | 2 (5) |
| Serious adverse eventsd | 6 (8) | 4 (10) |
| Fatal adverse events | 0 | 1 (3) |
| Adverse events leading to discontinuation | 2 (3) | 1 (3) |
| Pneumonia | 0 | 1 (3) |
| Lung carcinoma cell type unspecified stage II | 1 (1) | 0 |
| Idiopathic pulmonary fibrosis | 1 (1) | 0 |
Events occurring after the patient received treatment.
The most frequent adverse events were defined as those with an incidence greater than 10% in any study group ordered by frequency of occurrence in the recombinant human pentraxin 2 group. eTable 2 in Supplement 2 shows adverse events with an incidence greater than 5%.
Adverse events were graded as follows: grade 1 (mild) asymptomatic or mild symptoms and clinical or diagnostic observations only; grade 2 (moderate) minimal, local, or noninvasive intervention indicated; grade 3 (severe) medically significant but not immediately life threatening; grade 4 (life threatening) life threatening consequences with urgent intervention indicated; and grade 5 (fatal), death related to adverse event.
A serious adverse event was defined as any adverse event that resulted in death, was immediately life threatening, resulted in persistent or clinically significant disability or incapacity, required or prolonged hospitalization, was related to a congenital anomaly or birth defect, or was deemed serious for any other reason.
Four infusion-related reactions (IRRs) occurred in 3 patients. One patient in the recombinant human pentraxin 2 group experienced dizziness and another experienced a hypertensive crisis event; 1 patient in the placebo group experienced 2 hypertensive crisis events. None of the IRR events were deemed serious. There were no IRR events among the 7 patients with antidrug antibodies (6 patients in the recombinant human pentraxin 2 group; 1 patient in the placebo group) by week 28.
Of the 17 adverse events coded as IPF, 3 were IPF exacerbation events (1 event in 1 patient [1.3%] in the recombinant human pentraxin 2 group and 2 events in 1 patient [2.6%] in the placebo group that led to hospitalization). The remaining 14 events corresponded to IPF progression (10 patients in the recombinant human pentraxin 2 group [13.0%] and 4 patients in the placebo group [10.3%]); 1 patient in the placebo group (1.3%) required hospitalization. Four patients (10.3%) in the placebo group experienced a total of 8 respiratory decline events; 7 events were deemed serious. In the recombinant human pentraxin 2 group, 11 patients (14.3%) experienced a total of 14 respiratory decline events, of which 4 events were deemed serious.
Clinically significant elevations were observed for aspartate aminotransferase (2 patients in the recombinant human pentraxin 2 group [2.6%]) and liver function test (3 patients in the placebo group [7.7%]), as determined by an eDISH plot. Few abnormal findings from physical examinations or vital signs were observed. Heart rate, blood pressure, respiratory rate, and oxygen saturation did not change appreciably over the course of the study.
Discussion
In this trial, a significant treatment effect for recombinant human pentraxin 2 vs placebo on the primary end point, least-squares mean change in FVC percentage of predicted value, was observed; the effect was independent of patients receiving concurrent pirfenidone or nintedanib, or no concurrent therapy for IPF. The effect was robust to statistical modeling assumptions and aligned with values considered to be clinically important.18
The subset of patients who received placebo and concurrent IPF therapy experienced considerable decline in FVC during the trial compared with patients receiving IPF therapy in earlier studies. However, in contrast to patients enrolled in most other IPF clinical trials, the duration since IPF diagnosis prior to enrollment in the recombinant human pentraxin 2 trial was longer. Use of standard-of-care treatments (pirfenidone or nintedanib), reported in 87% of patients in this trial, as well as perceived response to these drugs over time, may have contributed to the time point when patients elected to enroll in this study. This observation curtails the direct comparability of these data to those of earlier IPF trials.
There was no appreciable decline from baseline to week 28 in the mean 6-minute walking distance for patients in the recombinant human pentraxin 2 group. However, there was a statistically significant mean decline of 32 m in patients in the placebo group. For context, results from a pivotal study of pirfenidone showed that all patients experienced a decline in the 6-minute walk test, with less decline experienced by patients who were treated using pirfenidone compared with those who received placebo.4 Furthermore, results from a phase 2 study of nintedanib compared with placebo showed that there was no significant difference in decline between treatment groups in the 6-minute walk test.21
Although the 6-minute walk findings should be interpreted as exploratory, the lack of decline in the treatment group may suggest a potential benefit for overall functional decline. This difference was also noteworthy because a difference of 24 to 45 m in the 6-minute walk test has been reported to be clinically important in the IPF population.19 Furthermore, a decline of more than 25 m has been independently associated with 1-year all-cause mortality in IPF,22 which suggests that the 6-minute walk distance may serve as an important measure of prognosis. The literature suggests that the 6-minute walk distance correlates weakly with FVC, Dlco, and dyspnea in patients with IPF.19
There was no notable difference in rate of adverse events between treatment groups. The most frequently reported adverse event was cough. The types of adverse events reported in this trial are consistent with symptoms common in patients with IPF. The percentage of patients in both treatment groups who experienced disease progression and respiratory decline were similar. Although approximately 9% of patients experienced serious adverse events, none were related to study medication. Infusion-related reactions were infrequent and none were serious.
Limitations
This study had several limitations. First, the sample size used to demonstrate superiority of recombinant human pentraxin 2 treatment over placebo was not appropriate to explore additional hypotheses beyond the prespecified primary analyses; secondary end points should be considered exploratory in nature. Second, the diagnosis of IPF was made in accordance with current guidelines, but allowing for “possible usual interstitial pneumonia,” which has been reported to be a radiologic subtype present in substantial fraction of patients with IPF.23 Third, the HRCT was not centrally read, which may introduce heterogeneity in the patient population. Fourth, while the quantitative HRCT findings did not show a statistically significant treatment effect, the imaging techniques are susceptible to potential artifacts from inspiratory effort and the expected change over a short trial period is small, which can limit interpretability. Fifth, although the distance walked in 6 minutes has prognostic value and potentially indicated real-world functional status, these data align with earlier studies that suggest this test has weak correlation to other quantifiable parameters. This signal in early data for the small trial is an opportunity for further refinement in the 6-minute walk testing of future trials and determination of correlation with other indicators of disease severity and functional status.
Conclusions
In this preliminary study, infusions of recombinant human pentraxin 2 vs placebo resulted in a slower decline in lung function over 28 weeks for patients with idiopathic pulmonary fibrosis. Further research should more fully assess efficacy and tolerability.
Trial Protocol and Statistical Analysis Plan
eAppendix. Methods
eFigure 1. Box Plots of Observed Change in Forced Vital Capacity Percentage of Predicted Value From Baseline toWeek 28
eFigure 2. Secondary End Point: Least-Squares Mean (Secondary End Point) and Box Plot of Observed Change From Baseline to Week 28 in Total Lung Volume, Percentage of Normal Lung Volume, and Percentage of Interstitial Lung Abnormalities by Background Therapy Status
eFigure 3. Least-Squares Mean (Secondary End Point) and Observed Mean Change From Baseline to Week 28 in Dlco by Background Therapy Status
eFigure 4. Box Plots of Observed Mean Change in the 6-Minute Walk Test From Baseline to Week 28
eTable 1. Categorical Changes in FVC Percentage of Predicted Value
eTable 2. Adverse Events
References
- 1.Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med. 2007;176(3):277-284. [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795-806. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Chen SY, Hou Q, Yeh WS, Collard HR. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48(1):179-186. [DOI] [PubMed] [Google Scholar]
- 4.King TE Jr, Bradford WZ, Castro-Bernardini S, et al. ; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083-2092. [DOI] [PubMed] [Google Scholar]
- 5.Richeldi L, du Bois RM, Raghu G, et al. ; INPULSIS Trial Investigators . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071-2082. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941-1952. [DOI] [PubMed] [Google Scholar]
- 7.De Biasi S, Cerri S, Bianchini E, et al. Levels of circulating endothelial cells are low in idiopathic pulmonary fibrosis and are further reduced by anti-fibrotic treatments. BMC Med. 2015;13:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naik-Mathuria B, Pilling D, Crawford JR, et al. Serum amyloid P inhibits dermal wound healing. Wound Repair Regen. 2008;16(2):266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171(10):5537-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreira AP, Cavassani KA, Hullinger R, et al. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease. J Allergy Clin Immunol. 2010;126(4):712-721. [DOI] [PubMed] [Google Scholar]
- 11.Murray LA, Chen Q, Kramer MS, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int J Biochem Cell Biol. 2011;43(1):154-162. [DOI] [PubMed] [Google Scholar]
- 12.Murray LA, Rosada R, Moreira AP, et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages. PLoS One. 2010;5(3):e9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pilling D, Roife D, Wang M, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179(6):4035-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dillingh MR, van den Blink B, Moerland M, et al. Recombinant human serum amyloid P in healthy volunteers and patients with pulmonary fibrosis. Pulm Pharmacol Ther. 2013;26(6):672-676. [DOI] [PubMed] [Google Scholar]
- 15.van den Blink B, Dillingh MR, Ginns LC, et al. Recombinant human pentraxin-2 therapy in patients with idiopathic pulmonary fibrosis: safety, pharmacokinetics and exploratory efficacy. Eur Respir J. 2016;47(3):889-897. [DOI] [PubMed] [Google Scholar]
- 16.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117. [DOI] [PubMed] [Google Scholar]
- 18.du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011;184(12):1382-1389. [DOI] [PubMed] [Google Scholar]
- 19.du Bois RM, Weycker D, Albera C, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183(9):1231-1237. [DOI] [PubMed] [Google Scholar]
- 20.Ayele BT, Lipkovich I, Molenberghs G, Mallinckrodt CH. A multiple-imputation-based approach to sensitivity analyses and effectiveness assessments in longitudinal clinical trials. J Biopharm Stat. 2014;24(2):211-228. [DOI] [PubMed] [Google Scholar]
- 21.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365(12):1079-1087. [DOI] [PubMed] [Google Scholar]
- 22.du Bois RM, Albera C, Bradford WZ, et al. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2014;43(5):1421-1429. [DOI] [PubMed] [Google Scholar]
- 23.Chung JH, Lynch DA. The value of a multidisciplinary approach to the diagnosis of usual interstitial pneumonitis and idiopathic pulmonary fibrosis: radiology, pathology, and clinical correlation. AJR Am J Roentgenol. 2016;206(3):463-471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Methods
eFigure 1. Box Plots of Observed Change in Forced Vital Capacity Percentage of Predicted Value From Baseline toWeek 28
eFigure 2. Secondary End Point: Least-Squares Mean (Secondary End Point) and Box Plot of Observed Change From Baseline to Week 28 in Total Lung Volume, Percentage of Normal Lung Volume, and Percentage of Interstitial Lung Abnormalities by Background Therapy Status
eFigure 3. Least-Squares Mean (Secondary End Point) and Observed Mean Change From Baseline to Week 28 in Dlco by Background Therapy Status
eFigure 4. Box Plots of Observed Mean Change in the 6-Minute Walk Test From Baseline to Week 28
eTable 1. Categorical Changes in FVC Percentage of Predicted Value
eTable 2. Adverse Events