Abstract
OBJECTIVES
Atrial fibrillation (AF) reduces survival and quality of life (QoL). It can be treated at the time of major cardiac surgery using ablation procedures ranging from simple pulmonary vein isolation to a full maze procedure. The aim of this study is to evaluate the impact of adjunct AF surgery as currently performed on sinus rhythm (SR) restoration, survival, QoL and cost-effectiveness.
METHODS
In a multicentre, Phase III, pragmatic, double-blinded, parallel-armed randomized controlled trial, 352 cardiac surgery patients with >3 months of documented AF were randomized to surgery with or without adjunct maze or similar AF ablation between 2009 and 2014. Primary outcomes were SR restoration at 1 year and quality-adjusted life years at 2 years. Secondary outcomes included SR at 2 years, overall and stroke-free survival, medication, QoL, cost-effectiveness and safety.
RESULTS
More ablation patients were in SR at 1 year [odds ratio (OR) 2.06, 95% confidence interval (CI) 1.20–3.54; P = 0.009]. At 2 years, the OR increased to 3.24 (95% CI 1.76–5.96). Quality-adjusted life years were similar at 2 years (ablation − control −0.025, P = 0.6319). Significantly fewer ablation patients were anticoagulated from 6 months postoperatively. Stroke rates were 5.7% (ablation) and 9.1% (control) (P = 0.3083). There was no significant difference in stroke-free survival [hazard ratio (HR) = 0.99, 95% CI 0.64–1.53; P = 0.949] nor in serious adverse events, operative or overall survival, cardioversion, pacemaker implantation, New York Heart Association, EQ-5D-3L and SF-36. The mean additional ablation cost per patient was £3533 (95% CI £1321–£5746). Cost-effectiveness was not demonstrated at 2 years.
CONCLUSIONS
Adjunct AF surgery is safe and increases SR restoration and costs but not survival or QoL up to 2 years. A continued follow-up will provide information on these outcomes in the longer term.
Study registration
ISRCTN82731440 (project number 07/01/34).
Keywords: Randomized trial, Atrial fibrillation, Ablation, Maze procedure, Cardiac surgery, Cost-effectiveness
INTRODUCTION
The prevalence of atrial fibrillation (AF) is 1–2% in the developed world, rising with age and comorbidity [1]. UK prevalence is 7.2% after age 65 and 10.3% after 75 [2] and will rise further with life expectancy. Symptoms include palpitations, chest pain, dizziness and breathlessness. Loss of atrial contractility increases the risk of thromboembolic stroke [3]. Anticoagulation reduces stroke but increases bleeding risk [4]. Atrial function loss may cause or exacerbate heart failure. AF has substantial impact on care and resources.
AF pathophysiology is now better understood: triggered most often by pulmonary vein foci, it is maintained through macro-re-entry circuits of 4–5 cm in diameter [5], leading to the development of the Cox-maze procedure in the 1980s [6]: through median sternotomy with cardiopulmonary bypass, the atria are cut and sutured to achieve pulmonary vein electrical isolation and interruption of macro-re-entry circuits. Despite success in restoring sinus rhythm (SR) [7], this challenging procedure is usually reserved for severely symptomatic patients, and the worldwide number of cut-and-sew Cox-maze procedures is extremely small in relation to AF prevalence.
Some or all the Cox-maze procedure blocks can be achieved by energy sources to ablate atrial tissue: easier, quicker and safer but expensive. Many cardiac surgery patients have AF. Whether they should routinely have adjunct AF surgery is unknown. Current practice varies widely between surgeons and hospitals. AF surgery increases SR restoration rate and decreases antiarrhythmic medication use [8–10]. However, the impact on patient-relevant outcomes, such as survival and health-related QoL (HRQoL), is uncertain. Cost-effectiveness analyses have mixed results [11, 12], are limited by the lack of HRQoL evidence in the short term and medium term (1–5 years) and economic models are not robust. The Amaze trial aimed to evaluate clinical and HRQoL outcomes and cost-effectiveness of this technology by comparing AF surgery as an adjunct to cardiac surgery with cardiac surgery alone.
METHODS
The Amaze trial was a Phase III, pragmatic, multicentre, double-blinded, parallel-armed, randomized controlled superiority trial (RCT) in 11 cardiac surgical centres. Thirty surgeons participated with at least 2 years of experience in AF surgery.
Patient recruitment
Consecutive cardiac surgery patients with a history of AF were screened. Inclusion criteria were age ≥18 years, elective or urgent cardiac surgery (coronary, valve, combined and other surgery requiring cardiopulmonary bypass), documented history (>3 months) of AF (non-paroxysmal or paroxysmal). Exclusion criteria were previous cardiac operations, emergency or salvage operations, off-pump surgery, unavailability for follow-up and inability to consent..
Randomization
Group allocation (1:1) was computer-generated by the trial statistician, using permuted block randomization (Sizes 6 and 8), stratified by surgeon and planned procedure. Randomization to planned cardiac surgery (control arm) or planned cardiac surgery with additional maze or similar ablation procedure (ablation arm) was performed on the operation day.
Blinding
The operating room staff could not be blinded to treatment allocation. After surgery, procedure details were kept in sealed envelopes in patient notes and only retrieved in a clinical emergency. Patients, cardiologists assessing electrocardiogram (ECG) results and researchers collecting HRQoL outcomes were unaware of treatment arm.
Clinical management
Local protocols were followed for operative and perioperative management and were identical in both arms. AF surgery in the intervention arm was conducted by an experienced surgeon. The Amaze trial was a pragmatic trial evaluating AF ablation as currently performed, so ablation methods and lesion sets were left to the surgeon: any device in clinical use was permitted, including bipolar and unipolar radiofrequency, ‘cut-and-sew’, cautery, cryotherapy, ultrasound, laser and microwave. Lesion sets and devices used were recorded.
Outcomes
SR restoration at 1 year after surgery and quality-adjusted life years (QALYs) over 2 years were joint primary outcomes. SR restoration required the absence of any AF on 4-day continuous ECG analysed by cardiologists unaware of patient identity or treatment arm. QALYs over 2 years were estimated from serial utility measurements from the UK population valuation of the EuroQoL EQ-5D-3l at randomization, discharge, 6 weeks, 6, 12 and 24 months postoperatively. Secondary outcomes were 2-year SR restoration, overall survival, stroke-free survival, hospital admission for haemorrhage, antiarrhythmic and anticoagulant drug usage, New York Heart Association (NYHA), HRQoL (SF-36), resource use and cost-effectiveness at 2 years. Prespecified subgroup analyses explored outcome differences using AF type, surgeons and cardiac procedure. Outcomes of lesion sets and method of ablation were compared within the trial arm.
Sample size
AF surgery was considered effective if there was a significant impact on either 1-year SR rates or 2-year quality-adjusted survival. The target (200 patients/arm) was based on detecting a 15% difference in the proportion of patients in SR at 1 year (45% vs 30%) or 1 additional month of quality-adjusted life [0.083 QALYs, standard deviation (SD) 0.3] over 2 years, with approximately 80% power, 2-sided significance of 5% and up to 15% death/loss to follow-up. Because of slower-than-expected accrual, recruitment stopped at 352 (88% target) patients, reducing the power to detect the proposed treatment effects to more than 70% for primary outcomes. To guard against overinterpretation of hypothesis tests due to multiple testing, we recommend that P-values between 0.025 and 0.05 are considered of borderline significance.
Statistical analysis
Primary outcome analysis was performed by intention to treat. SR restoration was analysed by logistic regression, including surgeon (random intercepts), baseline rhythm and planned procedure (fixed effects). For QALYs, linear regression, including surgeon (random intercepts), baseline utility and treatment arm (fixed effects), was fitted to utilities post-treatment. For survivors with missing EuroQoL measurements, multiple imputation was used, and QALY difference confidence interval (CI) was estimated using non-parametric bootstrapping. No primary outcome discounting was applied, and no adjustment was made for multiplicity. Sensitivity to assumptions surrounding missing data mechanisms were explored with no changes in results. For primary outcomes, prespecified subgroup effects were explored by including interaction terms, except for surgeon where a random effect was applied to the treatment coefficient. Lesion set effects were assessed in the ablation arm against a reference category. Adverse events by intervention were categorized by severity and relationship to procedure. Survival and stroke-free survival were analysed using the Kaplan–Meier and Cox regression. The SF-36 score analysis used linear regression, including time point, treatment arm, time-by-treatment arm interaction and baseline scores (fixed effects), with random intercepts for patients. Antiarrhythmic and anticoagulant use was tabulated by time and category and analysed by logistic regression.
Economic analysis
Resource use data from primary admission (time in theatre, intensive care and wards, hospital transfer, diagnostics and antiarrhythmic, antiplatelet, anticoagulant and cardiac drugs) were extracted from records, supplemented by patient-reported post-discharge health service use. Resources were valued using national estimates (https://www.evidence.nhs.uk/formulary/bnf/current; http://www.drugtariff.nhsbsa.nhs.uk/; http://www.pssru.ac.uk/project-pages/unit-costs/2015/; https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015/) literature (blood pressure monitoring and radiology) [13, 14] and data from the Papworth Hospital (operating room and device cost). High-intensity focussed ultrasound costed £3000, and other methods costed £1250. Type missingness was examined and replaced with mean or imputed values. Missing resource and utility data were imputed jointly using chained equations with predictive mean matching. Costs and QALYs were discounted at 3.5% in year 2. Incremental cost-effectiveness ratios relied on seemingly unrelated regression, controlling for baseline differences in age, gender, EQ-5D-3l, AF and (for QALYs) the primary surgery. Probabilistic sensitivity analysis used bootstrapping. Cost-effectiveness planes, acceptability curve and incremental net monetary benefit were estimated. Deterministic sensitivity analysis explored the impact of using SF-6D QALYs, complete case analysis, truncating costs and discharge QALYs, excluding outliers and alternative imputation strategies.
RESULTS
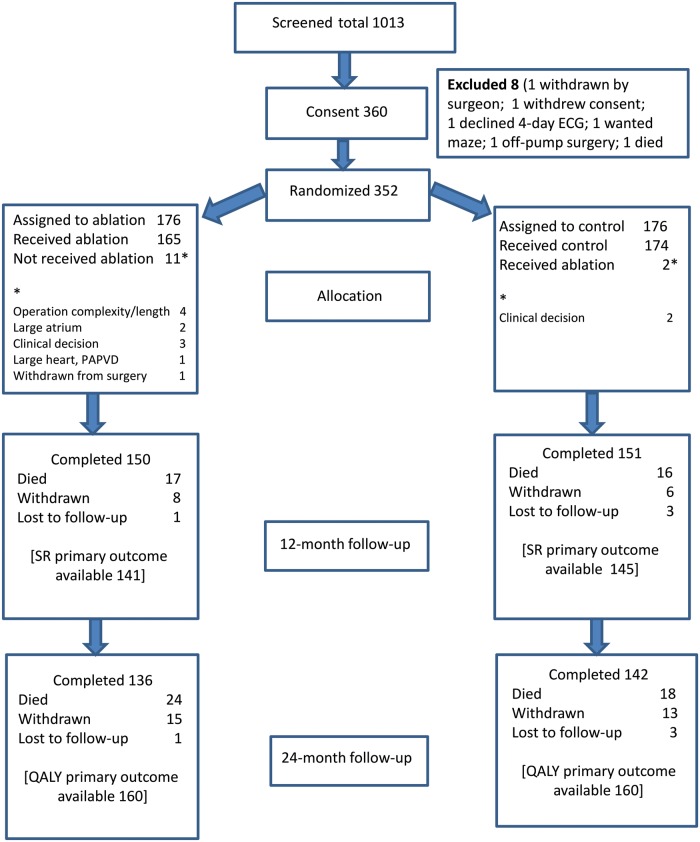
Between February 2009 and March 2014, 1013 patients were screened by 30 surgeons in 11 centres: 352 were randomized (176 each) to control or ablation arms. Exclusions applied to 366 patients (Supplementary Material, Table S1). Thirteen (3.7%) patients did not receive allocated treatment: 11 (6.3%) patients from the ablation arm due to technical issues and 2 (1.1%) from the control arm due to surgeon-perceived benefit after randomization (Fig. 1). One-year SR status was available for 141 (80%) ablation and 145 (82%) control patients, and 2-year QALYs were known for 160 patients in each arm (91%). Loss-to-follow-up reasons were similar for the 2 groups (Fig. 1), which were also similar in demographics, symptomatic status, cardiovascular profile and operations performed (Tables 1 and 2). The left atrial (LA) appendage was resected or excluded in 97 (55.1%) ablation arm patients and in 53 (30.1%) control patients.
Figure 1:
Patient flow through the Amaze trial. ECG: electrocardiogram; PAPVD: partial anomalous pulmonary venous drainage; QALY: quality-adjusted life year; SR: sinus rhythm.
Table 1:
Baseline characteristics of patients randomized in the Amaze trial
| Ablation | Control | Total | |
|---|---|---|---|
| (n = 176) | (n = 176) | (n = 352) | |
| Age (years) | |||
| Mean (SD) | 72.3 (7.53) | 71.4 (7.81) | 71.9 (7.67) |
| Range | 50.0–86.0 | 48.0–89.0 | 48.0–89.0 |
| Sex | |||
| Male, n (%) | 112 (63.6) | 120 (68.2) | 232 (65.9) |
| Female, n (%) | 64 (36.4) | 56 (31.8) | 120 (34.1) |
| Body mass index (kg/m2) | |||
| Mean (SD) | 28.1 (5.27) | 27.6 (4.62) | 27.9 (4.96) |
| Range | 17.4–46.0 | 17.9–42.8 | 17.4–46.0 |
| Logistic EuroSCORE (%) | |||
| Mean (SD) | 6.94 (5.489) | 6.64 (4.869) | 6.79 (5.184) |
| Range | 0.88–30.41 | 1.40–23.85 | 0.88–30.41 |
| CCS class, n (%) | |||
| 0 | 125 (71.0) | 133 (75.6) | 258 (73.3) |
| 1 | 13 (7.4) | 17 (9.7) | 30 (8.5) |
| 2 | 21 (11.9) | 16 (9.1) | 37 (10.5) |
| 3 | 10 (5.7) | 8 (4.5) | 18 (5.1) |
| 4 | 1 (0.6) | 1 (0.6) | 2 (0.6) |
| Missing/not known | 6 (3.4) | 1 (0.6) | 7 (2.0) |
| NYHA classification, n (%) | |||
| I | 31 (17.6) | 30 (17.0) | 61 (17.3) |
| II | 74 (42.0) | 68 (38.6) | 142 (40.3) |
| III | 59 (33.5) | 71 (40.3) | 130 (36.9) |
| IV | 10 (5.7) | 6 (3.4) | 16 (4.5) |
| Missing/not known | 2 (1.1) | 1 (0.6) | 3 (0.9) |
CCS: Canadian Cardiac Society; NYHA: New York Heart Association; SD: standard deviation.
Table 2:
Cardiovascular status at the baseline of patients randomized in the Amaze trial
| Ablation | Control | Total | |||
|---|---|---|---|---|---|
| (n = 176) | (n = 176) | (n = 352) | |||
| Left ventricular function, n (%) | |||||
| Poor (LVEF <30%) | 4 (2.3) | 8 (4.5) | 12 (3.4) | ||
| Moderate (LVEF 30–50%) | 50 (28.4) | 56 (31.8) | 106 (30.1) | ||
| Good (LVEF >50%) | 122 (69.3) | 112 (63.6) | 234 (66.5) | ||
| Previous PCI, n (%) | 16 (9.1) | 14 (8.0) | 30 (8.5) | ||
| Congestive cardiac failure, n (%) | 5 (2.8) | 1 (0.6) | 6 (1.7) | ||
| Diabetes, n (%) | |||||
| Insulin dependent | 5 (2.8) | 7 (4.0) | 12 (3.4) | ||
| Non-insulin dependent | 27 (15.3) | 17 (9.7) | 44 (12.5) | ||
| Hyperlipidaemia, n (%) | 70 (39.8) | 63 (35.8) | 133 (37.8) | ||
| Atrial fibrillation class, n (%) | |||||
| Paroxysmal | 44 (25.0) | 48 (27.3) | 92 (26.1) | ||
| Persistent | 30 (17.0) | 19 (10.8) | 49 (13.9) | ||
| Permanent | 102 (58.0) | 109 (61.9) | 211 (59.9) | ||
| Atrial fibrillation history (months), n (%) | |||||
| 0–3 | 4 (2.3) | 2 (1.1) | 6 (1.7) | ||
| 3–6 | 25 (14.2) | 25 (14.2) | 50 (14.2) | ||
| 6–12 | 31 (17.6) | 23 (13.1) | 54 (15.3) | ||
| >12 | 115 (65.3) | 126 (71.6) | 241 (68.5) | ||
| Not known | 1 (0.6) | 1 (0.3) | |||
| Permanent pacemaker, n (%) | 7 (4.0) | 8 (4.5) | 15 (4.3) | ||
| Previous cardioversion, n (%) | 24 (13.6) | 23 (13.1) | 47 (13.4) | ||
| Previous ablation, n (%) | 3 (1.7) | 1 (0.6) | 4 (1.1) | ||
| Other arrhythmias, n (%) | 2 (1.1) | 2 (1.1) | 4 (1.1) | ||
| Anticoagulants, n (%) | 137 (77.8) | 137 (77.3) | 274 (77.6) | ||
| Antiarrhythmics, n (%) | 145 (82.4) | 148 (84.1) | 293 (83.2) | ||
| Actual procedure category, n (%) | |||||
| MVR | 39 (22.2) | 48 (27.3) | 87 (24.7) | ||
| CABG | 35 (19.9) | 34 (19.3) | 69 (19.6) | ||
| AVR | 32 (18.2) | 23 (13.1) | 55 (15.6) | ||
| CABG + AVR | 16 (9.1) | 21 (11.9) | 37 (10.5) | ||
| CABG + MVR | 14 (8.0) | 13 (7.4) | 27 (7.7) | ||
| All others | 40 (22.7) | 37 (21.0) | 77 (21.9) | ||
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; LVEF: left ventricular ejection fraction; MVR: mitral valve repair or replacement; PCI: percutaneous coronary intervention.
Primary outcome: sinus rhythm at 1 year
Among cases with complete ECG data, 87 of 141 (61.7%) ablation patients were in SR at 1 year vs 68 of 145 (46.9%) controls (Fig. 2). In the intention-to-treat analysis, the odds ratio (OR) (95% CI) for 1-year SR restoration in the ablation arm was 2.06 (1.20–3.54), P = 0.0091. This increased from 1.6 (0.6–4.0) for the first 120 randomized patients to 2.9 (0.9–9.6) for the final 71 patients randomized in the last 18 months. This suggests a learning curve, but this was not proved by the probability analysis (Supplementary Material, Fig. S1).
Figure 2:
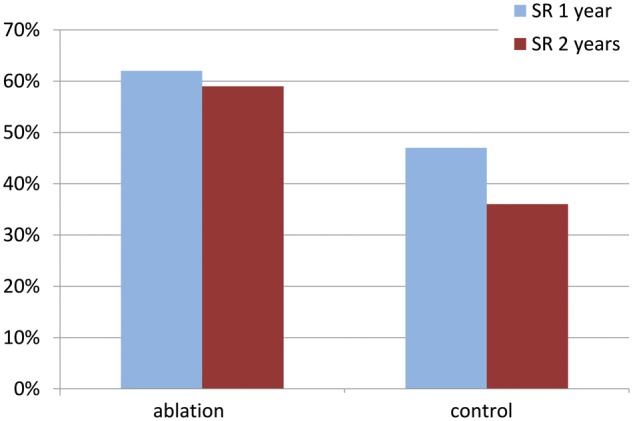
Percentage of patients in SR and free from atrial fibrillation at 1 year and 2 years after randomization. SR: sinus rhythm.
Primary outcome: quality-adjusted life years
The unadjusted, undiscounted mean (95% CI) QALY over 2 years was 1.489 (1.416–1.558) for the ablation arm and 1.485 (1.403–1.559) in the control arm. In the intention-to-treat analysis, the adjusted mean difference (95% CI) in QALYs at 2 years (ablation − control) was −0.025 (−0.129 to 0.078, P = 0.6319).
Secondary outcomes
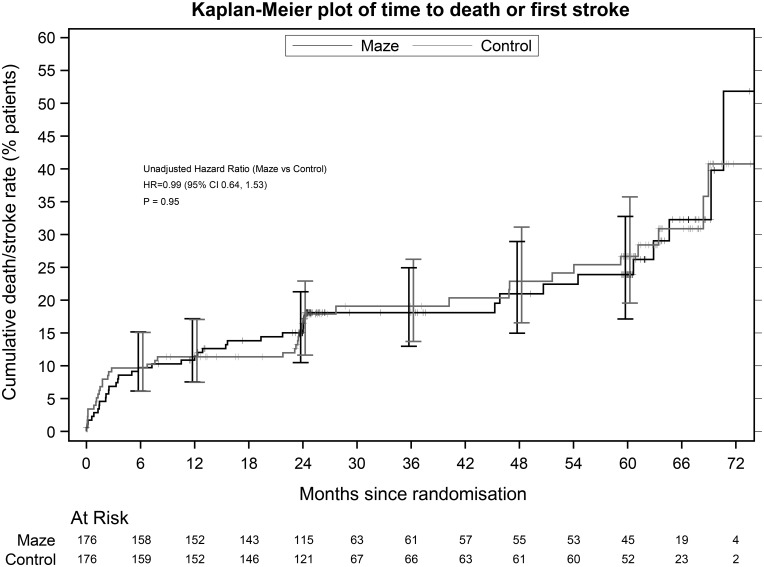
At 2 years, 69 of 118 (58.5%) ablation completers were in SR when compared with 47 of 129 (36.4%) controls (Fig. 2). The adjusted OR for SR at 2 years was 3.24 (95% CI 1.76–5.96). Significantly fewer ablation patients received anticoagulants from 6 months (Supplementary Material, Table S2) without a higher stroke rate: 13 strokes in 10 (5.7%) ablation patients and 19 in 16 (9.1%) control patients; the difference of −3.4% (95% CI −14.1–7.3%) was not significant (the Fisher’s exact test P = 0.3083) nor was the difference in stroke events between the 2 groups (log-linear model relative rate 0.68 (95% CI 0.34–1.39, P = 0.292). Stroke-free survival was similar in the 2 arms (HR = 0.99, 95% CI 0.64–1.53, P = 0.949, Fig. 4). Fifteen (7 ablation and 8 control) patients already had permanent pacemakers at surgery. Ablation did not increase the need for permanent pacemaker implantation after surgery (ablation 15 and control 17). Sixty (34.1%) ablation patients required 65 cardioversions and 67 (38.1%) control patients required 72 cardioversions. Immediate cardioversion success rates were similar [48/65 (73.8%) ablation and 54/72 (75.0%) control]. There was no significant difference in antiarrhythmic drug use throughout follow-up (Supplementary Material, Table S2). There were no significant differences between the 2 arms in any of the following outcomes at any time point: NYHA, EQ-5D-3l and SF-36.
Figure 4:
Six-year cumulative mortality rate or stroke rate after patient randomization in the Amaze trial. ‘Maze’ refers to all atrial fibrillation ablation surgeries. CI: confidence interval; HR: hazard ratio.
Safety
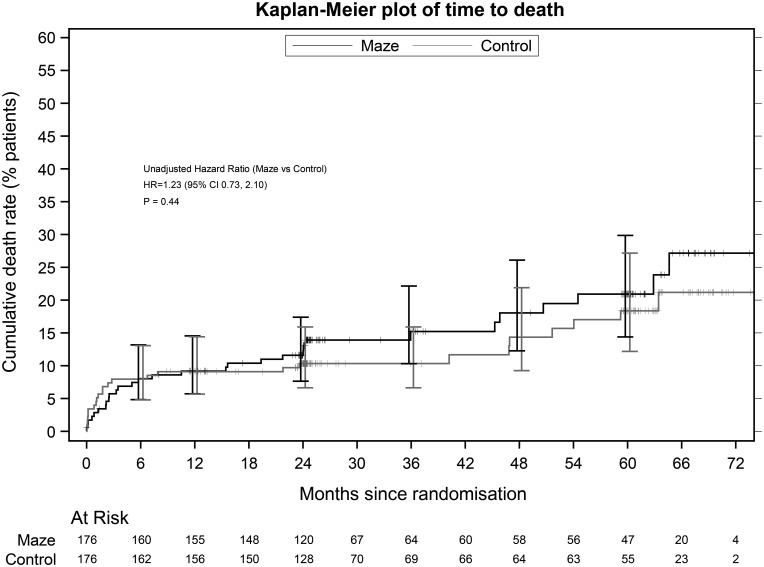
The mean (SD) cross-clamp time was longer by 5.1 min in the ablation group [82.2 (37.2) vs 77.2 (48.6)], and bypass time was longer by 18.9 min [118.1 (43.4) vs 99.3 (41.8)]. There were 5 (2.8%) operative deaths in the ablation group and 9 (5.1%) among controls (P = 0.414). Over the trial course, there were 30 ablation and 25 control deaths (HR 1.23, 95% CI 0.73–2.10; P = 0.437), so that adding AF surgery did not significantly affect early or late mortality (Fig. 3).
Figure 3:
Six-year cumulative mortality rate after patient randomization in the Amaze trial. ‘Maze’ refers to all atrial fibrillation ablation surgeries. CI: confidence interval; HR: hazard ratio.
There were 330 adverse events in 100 AF surgery patients and 333 in 111 controls (each 60%). Of them, 71 (42.5%) ablation and 84 (45.5%) control patients had at least 1 moderately severe event, and 31 (18.6%) ablation and 38 (20.5%) control patients had a severe event. Few events were ‘possibly related’ to treatment: 23 in 17 (10.2%) ablation patients and 28 in 19 (10.3%) control patients; 1 patient admitted to hospital for atrial flutter (classed as ‘definitely related’ to treatment) was subsequently found to be in the control group.
Subgroup analysis
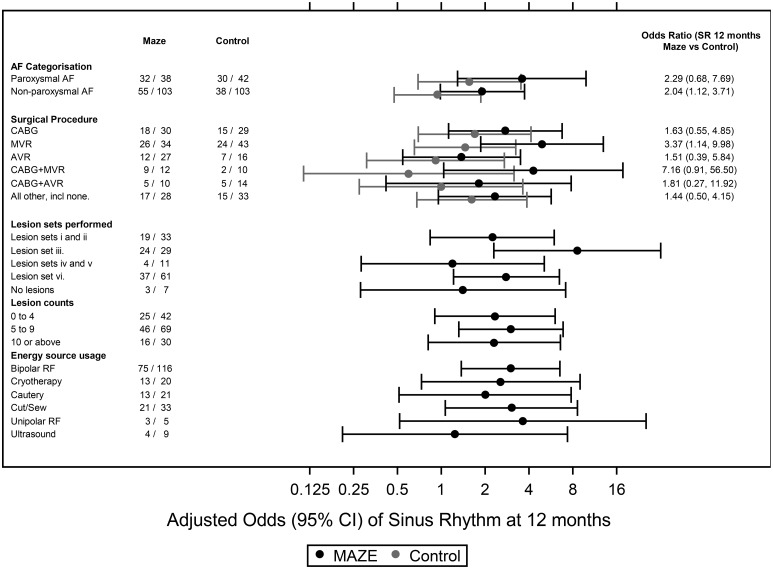
Preplanned subgroup analysis showed no significant interaction between 1-year SR restoration and type of AF (paroxysmal or non-paroxysmal) or planned cardiac procedure (Fig. 5). The random intercepts analysis showed that SR restoration rates varied by surgeon across both arms, with an intraclass correlation coefficient of 0.089. In the ablation arm, the highest odds for 1-year SR restoration occurred with a comprehensive LA lesion set including the mitral isthmus lesion. Adding right atrial (RA) lesions conveyed no further increase in SR restoration odds (to be interpreted cautiously because of confounding associations between lesion sets and surgeons). Post hoc analysis of the LA appendage excision showed a significant interaction and was increasingly used as the trial progressed for the ablation group but not for controls.
Figure 5:
Forest plot showing the odds ratio of SR restoration at 1 year after randomization for predefined subgroups in the Amaze trial. Lesion set groupings are provided below [the left atrium, right atrium and pulmonary vein (PV)]. ‘Maze’ refers to all AF ablation surgeries. (i) Minimal left atrial (LA) lesion set: PV isolation only ± the LA appendage line, (ii) more extensive LA-only lesion set excluding the mitral annulus, (iii) more extensive LA-only lesion set including the mitral annulus, (iv) minimal LA lesion set and the right atrial (RA) lesion set, (v) more extensive LA lesion set excluding the mitral annulus + the RA lesion set and (vi) more extensive LA lesion set including the mitral annulus + the RA lesion set. AF: atrial fibrillation; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; CI: confidence interval; MVR: mitral valve repair or replacement; RF: radiofrequency; SR: sinus rhythm.
Cost-effectiveness
Higher ablation costs resulted from the ablation device, length of stay in critical care and readmissions (Table 3). The adjusted mean incremental maze cost was £3533 (95% CI £1321–£5746), significantly higher than control (P < 0.01). The adjusted mean QALY difference was not significant (−0022, 95% CI −01231 to 00791; P = 0.67, Supplementary Material, Table S3). No analyses suggested that ablation was cost-effective at 2 years at £30 000 per QALY. The smallest incremental cost-effectiveness ratio was £53 538/QALY from an unplanned analysis limited to patients randomized in the 2nd half of the trial (Supplementary Material, Figs S2–S5).
Table 3:
Mean (SD) of per-patient costs of resource use with imputation
| Ablation (n = 176) |
Control (n = 176) |
Difference (ablation − control) | |||
|---|---|---|---|---|---|
| Mean cost/patient | SD | Mean cost/patient | SD | ||
| Health service use | |||||
| Primary admission | |||||
| Theatre use | £5225 | £1594 | £4949 | £1863 | £276 |
| Ablation device | £1212 | £408 | £14 | £133 | £1197 |
| Adult critical care | £4029 | £7600 | £3065 | £5586 | £964 |
| Cardiac ward | £3397 | £4661 | £3064 | £2014 | £333 |
| Rehabilitation | £48 | £325 | £148 | £1082 | −£100 |
| Acute trust | £937 | £6105 | £165 | £1409 | £772 |
| Subtotal | £14 847 | £12 474 | £11 404 | £7194 | £3 443 |
| Medication (whole trial period) | £618 | £1584 | £681 | £2765 | −£63 |
| Follow-up | |||||
| Readmissions | £1650 | £4192 | £1220 | £2994 | £430 |
| Tests | £388 | £376 | £344 | £283 | £44 |
| Healthcare visits | £1179 | £1061 | £1193 | £1052 | −£14 |
| Subtotal | £3217 | £5629 | £2757 | £4329 | £460 |
| Grand total | £18 681 | £13 340 | £14 842 | £8295 | £3839 |
SD: standard deviation.
DISCUSSION
In this pragmatic, multicentre trial, 1-year and 2-year SR restoration rates were significantly higher for ablation patients than controls and slightly higher than reported in a recent RCT meta-analysis [15]. SR restoration rate in the control group was higher than any previously reported through cardiac surgery alone [16, 17]. Control patients received the same postoperative care as trial patients including postoperative cardioversion suggesting that, with a determined effort, cardiac surgery alone can restore SR in one-third of patients at 2 years, an outcome worth pursuing in the absence of adjunct AF surgery.
The optimal lesion set remains controversial. The full Cox-maze lesion set is established [18], and if there is a ‘dose–response’ relationship, SR restoration rates should be better with a more complete lesion. One RCT of AF surgery in mitral patients found no significant difference in SR restoration between the complete lesion set and pulmonary vein isolation alone [16], although it was probably insufficiently powered to detect such a difference. Many surgeons carry out only parts of the full Cox-maze, and there is a wide range of lesion sets used. Terminology is unhelpful with such procedures variously described as maze, mini-maze, the LA maze or simply AF ablation. The Amaze trial showed higher SR restoration rates with a complete LA lesion set including the mitral annulus or ‘isthmus’ lesion but did demonstrate the benefit of adding RA lesions, although the power to detect these differences was low and adding such lesions has little impact on operative time or complexity above a full LA lesion set.
We found no impact in QoL at 2 years, but this is relatively short follow-up, and cardiac surgery alone achieves such an increase in QoL [19] that it may be difficult to discern additional benefits from AF surgery at this stage. Two factors may modify this conclusion in future: there was significantly less anticoagulation in ablation patients postoperatively with no increase in stroke rate, and the HESTER study [20] showed LA contractile recovery in most but not all patients when ablation restores SR. These results lend support to anticoagulation withdrawal when SR is restored after AF surgery, but the varying extent of the LA contractile recovery suggests that the LA function should be measured before contemplating withdrawal. A continued follow-up of the Amaze patients will establish whether QoL and survival advantages accrue over time.
The per-patient cost over 2 years was higher in the ablation arm with no significant impact on discounted QALYs. Deterministic and probabilistic sensitivity analyses confirmed this and the probability that AF surgery would be cost-effective at 2 years was less than 5%, and alternative assumptions do not alter this conclusion.
Strengths and limitations
The Amaze trial is the largest randomized trial to date to evaluate adjunct AF surgery. It is unique in including all cardiac (not only mitral) procedures, in having both patients and outcome assessors blinded to treatment arm and in incorporating survival, stroke-free survival and QoL as outcome measures. The pragmatic design evaluated AF surgery as currently performed in clinical practice, rather than what may be achievable in specialist centres. The number of participating units and surgeons, the variety of ablation devices and lesion sets and the interaction between these variables have improved result generalizability but reduced the power to draw firm conclusions about the optimal device and lesion set.
Recruitment is a widespread RCT problem. Logistic delays, activity overestimation and rising awareness of AF surgery among patients and clinicians affected recruitment rate. This led to early stoppage of the trial at 88% recruitment when no further funding was available, but the lower-than-expected loss to follow-up reduced any impact on the trial power. Infrequent follow-up (6, 12 and 24 months) is associated with under-reporting of frequent events, illness severity and intensive service use, but there is no recommended interval between follow-ups [21, 22]. In the Amaze trial, 95% of the difference in follow-up costs is related to readmissions (infrequent major events), making cost underestimation unlikely. The cost-effectiveness analysis was limited to 2 years and may not reflect long-term benefits.
CONCLUSION
Adjunct AF surgery can be practised safely in a routine cardiac surgical setting and increases SR restoration up to 2 years after surgery. This electrophysiological success did not translate into better 2-year survival or QoL, and the procedure is, therefore, not proved to be cost-effective at 2 years. A longer follow-up will determine whether AF surgery has an impact on these outcomes.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the work of Fay Cafferty, Mark Schulpher and Derek Todd who formed the data monitoring committee chaired by James Roxburgh. We thank Brian Elliott and Paul Kinnersley who participated in the Trial Steering Committee chaired by Dai Rowlands. We thank surgeons Haitham Abunasra, Simon Allen, Benjamin Bridgewater, Pedro Catarino, Stephen Clarke, John Dark, John Dunning, Leonidas Hadjinikolao, Jonathan Hyde, David Jenkins, Anthony de Souza, Stephen Large, Michael Lewis, Neil Moat, Narain Moorjani Suku Nair, Choo Ng, Rajesh Patel, Jacek Szostek, Augustine Tang, Graham Venn, John Wallwork and Nizar Yonan who also recruited to the trial. The Papworth Trials Unit staff supported the design, recruitment, co-ordination, conduct, data management, analysis and quality assurance. We also thank Hester Goddard for the trial set-up and grant application, and Donna Alexander for continuous support throughout.
Funding
This work was supported by the NIHR Health Technology Assessment programme.
Conflict of interest: Amaze operations used a multitude of devices, including Atricure products (although this is not specified in the manuscript and no specific products are mentioned anywhere). SAMN and YAO have received expenses and lecture fees from Atricure for participating in educational courses on atrial fibrillation.
REFERENCES
- 1. Kannel WB, Wolf PA, Benjamin EJ, Levy D.. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2N–9N. [DOI] [PubMed] [Google Scholar]
- 2. Hobbs FD, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S. et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9:iii–v, ix–x, 1–74. [DOI] [PubMed] [Google Scholar]
- 3. Wolf PA, Abbott RD, Kannel WB.. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 4. Friberg L, Rosenqvist M, Lip GY.. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500–10. [DOI] [PubMed] [Google Scholar]
- 5. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G. et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 6. Cox JL, Schuessler RB, Cain ME, Corr PB, Stone CM, D'Agostino HJ. et al. Surgery for atrial fibrillation. Semin Thorac Cardiovasc Surg 1989;1:67–73. [PubMed] [Google Scholar]
- 7. Cox JL, Boineau JP, Schuessler RB, Kater KM, Lappas DG.. Five-year experience with the maze procedure for atrial fibrillation. Ann Thorac Surg 1993;56:814–23; discussion 23–4. [DOI] [PubMed] [Google Scholar]
- 8. Damiano RJ, Schwartz FH, Bailey MS, Maniar HS, Munfakh NA, Moon MR. et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg 2011;141:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weimar T, Schena S, Bailey MS, Maniar HS, Schuessler RB, Cox JL. et al. The Cox-maze IV procedure for lone atrial fibrillation: a single center experience in 100 consecutive patients. J Interv Card Electrophysiol 2012;5:8–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ware JE. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Quality Metric Inc, 2000. [Google Scholar]
- 11. Van Breugel HN, Nieman FH, Accord RE, Van Mastrigt GA, Nijs JF, Severens JL. et al. A prospective randomized multicenter comparison on health-related quality of life: the value of add-on arrhythmia surgery in patients with paroxysmal, permanent or persistent atrial fibrillation undergoing valvular and/or coronary bypass surgery. J Cardiovasc Electrophysiol 2010;21:511–20. [DOI] [PubMed] [Google Scholar]
- 12. Gillinov AM, Argenziano M, Blackstone EH, Iribarne A, DeRose JJ, Ailawadi G. et al. Designing comparative effectiveness trials of surgical ablation for atrial fibrillation: experience of the Cardiothoracic Surgical Trials Network. J Thorac Cardiovasc Surg 2011;142:257–64.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD. et al. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet 2011;378:1219–30. [DOI] [PubMed] [Google Scholar]
- 14. Auguste P, Barton P, Hyde C, Roberts TE.. An economic evaluation of positron emission tomography (PET) and positron emission tomography/computed tomography (PET/CT) for the diagnosis of breast cancer recurrence. Health Technol Assess 2011;15:iii–v, 1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huffman MD, Karmali KN, Berendsen MA, Andrei AC, Kruse J, McCarthy PM. et al. Concomitant atrial fibrillation surgery for people undergoing cardiac surgery. Cochrane Database Syst Rev 2016;8:CD011814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillinov AM, Gelijns AC, Parides MK, DeRose JJ, Moskowitz AJ, Voisine P. et al. CTSN Investigators. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med 2015;372:1399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoo JS, Kim JB, Ro SK, Jung Y, Jung SH, Choo SJ. et al. Impact of concomitant surgical atrial fibrillation ablation in patients undergoing aortic valve replacement. Circ J 2014;78:1364–71. [DOI] [PubMed] [Google Scholar]
- 18. Badhwar V, Rankin JS, Damiano RJ, Gillinov AM, Bakaeen FG, Edgerton JR. et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg 2017;103:329–41. [DOI] [PubMed] [Google Scholar]
- 19. Grady KL, Lee R, Subačius H, Malaisrie SC, McGee EC, Kruse J. et al. Improvements in health-related quality of life before and after isolated cardiac operations. Ann Thorac Surg 2011;91:777–83. [DOI] [PubMed] [Google Scholar]
- 20. Abu-Omar Y, Thorpe BS, Freeman C, Mills C, Stoneman VEA, Gopalan D. et al. Recovery of left atrial contractile function after maze surgery in persistent longstanding atrial fibrillation. J Am Coll Cardiol 2017;70:2309–11. [DOI] [PubMed] [Google Scholar]
- 21. Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.. Assessing the costs of healthcare technologies in clinical trials. Health Technol Assess 1999;3:1–76. [PubMed] [Google Scholar]
- 22. Ridyard CH, Hughes DA.. Methods for the collection of resource use data within clinical trials: a systematic review of studies funded by the UK Health Technology Assessment program. Value Health 2010;13:867–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.