Abstract
Ortner’s syndrome or cardiovocal syndrome is a rare condition and refers to the association between cardiovascular conditions, usually cardiac enlargement from mitral stenosis, and recurrent laryngeal nerve palsy. We reported an interesting case of a patient with end-stage renal disease on regular dialysis who developed both Ortner’s syndrome and dysphagia aortica as a result of an aortic arch aneurysm. The aneurysm underwent a rapid increase in size, likely as a result of Staphylococcus aureus infection (mycotic aneurysm) from an internal jugular dialysis catheter. This case highlighted the importance of cardiovascular conditions as rarer causes of dysphonia and dysphagia, particularly with the existence of an extrinsic infective source.
Keywords: Ortner’s syndrome, dysphagia aortica, mycotic thoracic aortic aneurysm
Introduction
Dysphonia (inability to produce normal voice sounds through the vocal organs) secondary to left recurrent laryngeal nerve paralysis was first described in 1897 by Ortner, a Viennese physician, in a patient with mitral stenosis and left atrial enlargement.1 The condition is also now referred to as cardiovocal syndrome because the left recurrent laryngeal nerve palsy can be caused by any enlarged cardiovascular structures. Many cardiac diseases that cause left atrial enlargements (i.e. myxoma, pulmonary hypertension and congenital cardiac disease) can cause this condition. In addition, numerous associations with aortic and pulmonary artery aneurysms have been reported in the literature2,3
Dsyphagia secondary to external oesophageal compression by the thoracic aorta is termed as dysphagia aortica. This can be caused by compression at the upper oesophagus level by disorders of the thoracic aorta such as aortic arch or descending thoracic aortic aneurysm or the lower oesophagus by an aneurysmal or atherosclerotic aorta. It is associated with old age, hypertension and kyphoscoliosis.4 We present a case report of a haemodialysis patient with simultaneous presentation of both Ortner’s syndrome and dysphagia aortica.
Case
A 64-year-old lady with end-stage renal disease on haemodialysis through an internal jugular tunnelled dialysis catheter was referred with intermittent dysphagia. She had multiple co-morbidities including type 2 diabetes mellitus, hypertension, dyslipidemia, hypothyroidism and hyperuricemia. Her dysphagia became more pronounced over several months with accompanying reduced appetite and weight loss (5 kg within 2 months). At around the same time, she also began suffering from dysphonia and frequent dizzy spells. An oesophagoduodenoscopy (OGD) was performed a month later, and this revealed left vocal cord palsy and gastritis, but no other oesophageal pathology to account for the dysphagia. Left vocal card paralysis was reconfirmed by an indirect laryngioscopy. A chest X-ray showed soft tissue opacity in left upper zone with displaced aortic calcification. A subsequent computed tomography (CT) scan confirmed an aortic aneurysm with 4.4 cm of aneurysmal sac arising from the arch of the aorta containing significant atheroma. The aneurysm commenced proximal to the origin of the left subclavian artery and was associated with cardiomegaly. The patient was recommended for surgical intervention but she declined. She was admitted 4 months later with worsening dysphagia and bradycardia. Her dysphagia had deteriorated to the point that she could not swallow food without coughing. In addition, she was increasingly troubled by dizzy episodes which were attributed to junctional bradycardia. Her heart rate dropped to 38 beats/min with significant pauses in the rhythm. Despite the bradycardia, she continued to suffer from high blood pressure (up to 180/100 mmHg) necessitating adjustments to her numerous antihypertensive medications. During the course of her admission, she suffered from septicaemia from Staphylococcus aureus, presumably from her internal jugular dialysis catheter. She was treated with a prolonged course of flucloxacillin which was later changed to vancomycin because of poor clinical response. Her tendency to defaulting to accept treatment had resulted in late creation and usage of arterio venous fistula for dialysis, necessitating a prolonged usage of temporary catheter for dialysis. A repeat CT thorax showed two aneurysms. An enlarging aortic arch aneurysm of 5.3 cm × 5.1 cm proximal to left subclavian artery and pericardial effusion of 1.3 cm. There was a second aneurysm with mural thrombus arises from the proximal descending aorta, distal to the origin of left subclavian artery (Figures 1 and 2). A subsequent CT angiogram with contrast showed a 4.6 cm × 3.5 cm saccular aneurysm in the arch of the aorta causing mild compression of the origin of left carotid and left subclavian arteries with mild displacement of trachea and oesophagus (Figure 3). Another OGD was done in view of worsening dysphagia. This again confirmed left vocal cord palsy with an inward bulge (extrinsic compression) at 19–20 cm resulting from an extraluminal mass compressing on the oesophagus. Finally, she was transferred to the cardiothoracic centre; however, she was deemed a high anaesthetic and surgical risk. Therefore, she did not proceed with any surgical intervention and passed away from progressive septicaemia and cardiovascular shut down within 1 week.
Figure 1.

Coronal contrast-enhanced CT image shows aortic arch saccular aneurysm (A) slightly compressing the origin of the left common carotid artery (arrow).
Ao: ascending aorta.
*Brachiocephalic trunk.
Figure 2.

Coronal contrast-enhanced CT image posterior to Figure 1 shows two saccular aneurysms (A and B). Aneurysm A arises from the aortic arch proximal to left subclavian artery, compressing the proximal part of the left subclavian artery (arrow). Aneurysm B with mural thrombus (#) arises from the proximal descending aorta, distal to the origin of left subclavian artery (arrow).
Figure 3.
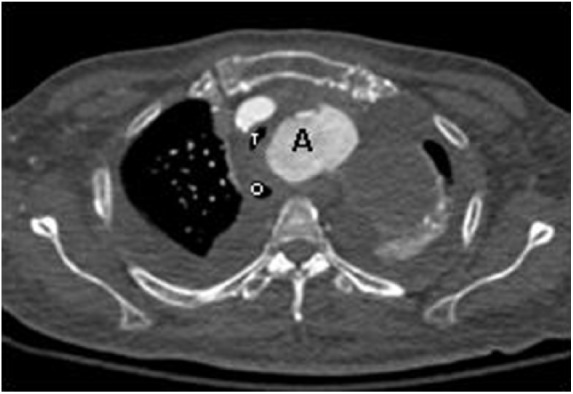
Axial contrast-enhanced CT image shows that the saccular aortic arch aneurysm (A) displaces the trachea (T) and oesophagus (O) to the right. The tracheal is partly compressed by the aneurysm.
Discussion
Ortner’s syndrome, or cardiovocal syndrome, is a clinical condition associated with left recurrent laryngeal nerve palsy due to cardiovascular disease. The palsy arises from compression of the recurrent laryngeal nerve as it passes between the arch of the aorta and the pulmonary artery. The left vagus nerve gives rise to the left recurrent laryngeal nerve at the level of the aortic arch. The nerve then curves around the aorta on the outer side of the ligamentum arteriosum and ascends in the groove between the oesophagus and the trachea. The nerve then continues along the tracheo-oesophageal groove to supply all the muscles of the left vocal cord except the cricothyroid muscle. This muscle is innervated by yet another branch of the vagus nerve, namely, the superior laryngeal nerve. The tortuous course of the left recurrent laryngeal nerve makes it vulnerable to injury due to pathological conditions in surrounding structures.5 Most authors believe that pressure in the pulmonary artery causes the nerve compression, although the dilated left atrium found in mitral stenosis or patent ductus arteriosus may also cause anatomical compression of the vagus nerve.6 The symptoms may be determined by any compression of the vagus nerve, at its origin in the lateral sulcus posterior to the bulb, on its course in the bulbo-cerebellar cistern, at the point where it emerges from the skull through the jugular foramen, in the carotid sheath or adjacent to the sites of recurrent laryngeal nerves deflection in the above-mentioned vessel.7
The oesophagus usually begins its descent into the mediastinum to the right of the aorta. It crosses the aorta at the aorta-oesophageal decussation site in the lower third of the posterior mediastinum before penetrating the diaphragm through the diaphragmatic hiatus.5 Atherosclerotic and degenerative changes in the aorta cause loss of elasticity resulting in a dilated and distorted aorta.8 This can result in the oesophagus being pushed and compressed by the aorta against the cardiac chambers resulting in dyspahagiaaortica.9
The occurrence of both dysphagia and dysphonia is rare in patients with thoracic aortic aneurysm. PubMed literature review showed only four cases with a similar presentation. Zangirolami et al.10 reported a case with thickness of voice and dysphagia in a 42-year-old man with a fusiform aneurysm in the aortic arch leading to recurrent compression of the left laryngeal nerve. The patient had a good outcome through endovascular repair of the aneurysm. Three other cases of concurrent dysphagia and dysphonia were reported; two in cases with enlarged left atrium from mitral valve pathology11,12 and with aortic dissection.6 The CT images showed the location of the thoracic aortic aneurysm and its close proximity to the surrounding cardiac and oesophageal structures (Figures 1–3).
This patient had end-stage renal disease secondary to diabetes mellitus and was on dialysis for 18 months before her demise. We suspect that she developed progressive enlargement of the thoracic aortic aneurysm through progression of macrovascular complications from diabetes mellitus and hypertension. There was a rapid progression in aneurysm size from 4.4 cm from the arch of the aorta to 6.2 cm in the space of 4 months. The thoracic aorta usually grows very slowly at approximately 0.1 cm per year.13 Rapid growth of the thoracic aorta is usually reflective of measurement errors across the oblique portion of the aorta, especially the aortic arch.14 In this case, the most likely factor for the rapid increase in size was the probable development of mycotic aneurysm from S. aureus infection. Infected aneurysms can develop from haematogenous spread of infectious microemboli into the vasovasorum of a preexisting aneurysm or infection of a preexisting intimal defect by a circulating infectious agent. An infectious arteritis causes destruction of the arterial wall with subsequent contained rupture and formation of a pseudoaneurysm or saccular aneurysm, which can rapidly develop and enlarge.15 We suspect that other factors like uncontrolled hypertension and grossly dilated vascular structures secondary to chronic fluid overload (from non-compliance with fluids) may have also contributed to the enlargement. Her clinical progress through dialysis had not been smooth and was marred by recurrent admissions for fluid overload and infections, likely secondary to macrovascular and microvascular complications of her diabetes. She was also troubled by dizzy spells attributed to bradycardia. In retrospect, we speculate that she may have had carotid sinus hypersensitivity secondary to direct intrusion of the aneurysm onto her left carotid artery. Alternatively, impingement of her right and left vagus nerve may have caused bradycardia through involvement of the sinoatrial and atrioventricular nodes, respectively. Her clinical management was compromised by her reluctance to consistently accept medical treatment and subsequent delay in surgical intervention of her thoracic aneurysm.
Conclusion
We conclude that thoracic aortic aneurysm can present with both Ortner’s syndrome and dysphagia aortica through the compressive effects of the aneurysm on its surrounding structures. Both these entities are rare in their own right and even rarer when occurring together. The progression of the disease may be accelerated by infection through the development of mycotic aneurysm. Clinicians should remain vigilant and cognizant on cardiovascular causes of dysphonia and dysphagia.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Informed consent: The patient described herein had given written informed consent to the use of de-identified patient data for use in research and education. Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
References
- 1. Ortner N. Recurrenssl ähmungbei mitral stenose. Wien Klein Wochenschr 1897; 10: 753–755. [Google Scholar]
- 2. Kamp O, van Rossum AC, Torenbeek R. Transesophageal echocardiography and magnetic resonance imaging for the assessment of saccular aneurysm of the transverse thoracic aorta. Int J Cardiol 1991; 33: 330–333. [DOI] [PubMed] [Google Scholar]
- 3. Harano M, Tanemoto K, Kuinose M, et al. A case of chronic traumatic dissecting aneurysm of the thoracic aorta. Nihon Kyobugekagakkai Zasshi 1994; 47: 1023–1025. [PubMed] [Google Scholar]
- 4. McMillian IKR, Hyde I. Compression of the oesophagus by the aorta. Thorax 24: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Snell RS. Clinicalanatomy for medical student. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2000, 113 pp. [Google Scholar]
- 6. Lee S. Dysphagia and hoarseness associated with painless aortic dissection: a rare case of cardio vocal syndrome. Dysphagia 2006; 21(2): 129–132. [DOI] [PubMed] [Google Scholar]
- 7. Paquette CM, Manos DC, Psooy BJ. Unilateral vocal cord paralysis: a review of CT findings, mediastinal causes, and the course of the recurrent laryngeal nerves. Radiographics 2012; 32: 721–740. [DOI] [PubMed] [Google Scholar]
- 8. Keates PG, Magidson O. Dysphagia associated with sclerosis of the aorta. Brit J Radiol 1955; 28: 184–190. [DOI] [PubMed] [Google Scholar]
- 9. Mucklow EH, Smith OE. Dysphagia and the unusual radiographic appearances associated with the variable relationships of the aorta and lower oesophagus. J Fac Radiol 1954; 6: 88–95. [DOI] [PubMed] [Google Scholar]
- 10. Zangirolami AC, Oliveira FV, Tepedino MS. Ortner’s syndrome: secondary laryngeal paralysis caused by a great thoracic aorta aneurysm. Int Arch Otorhinolaryngol 2015; 2: 180–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kishan CV. Ortner’s syndrome in association with mitral valve prolapsed. Clin Cardiol 2000; 23: 295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arifputera A, Loo G, Chang P, et al. An unusual case of dysphonia and dysphagia. Singapore Med J 2014; 55(2): e31–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002; 73: 17–28. [DOI] [PubMed] [Google Scholar]
- 14. Elefteriades JA, Emily A. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol 2010; 55(9): 841–857. [DOI] [PubMed] [Google Scholar]
- 15. Macedo TA, Stanson AW, Oderich GS, et al. Infected aortic aneurysms: imaging findings. Radiology 2004; 23(1): 250–257. [DOI] [PubMed] [Google Scholar]


