Abstract
It is unclear if ultrasound-assisted catheter-directed thrombolysis (UACDT) confers benefit over anticoagulation (AC) alone in the management of intermediate-risk (“submassive”) pulmonary embolism (PE), defined by evidence of right ventricular (RV) dysfunction in hemodynamically stable patients. This study sought to evaluate any lasting advantage of UACDT on mortality and resolution of RV dysfunction in intermediate-risk PE at a large academic medical center. Adults aged ≤ 86 years admitted with intermediate-risk PE from 2011 to 2016 were retrospectively identified. Patients were excluded if there was a history of cancer, pre-existing pulmonary hypertension, pregnancy or postpartum status, contraindication to AC, or treatment with systemic thrombolysis. Baseline Pulmonary Embolism Severity Index (PESI) scores were computed. Outcomes including length of stay (LOS), bleeding complications, resolution of RV dysfunction, and mortality were compared between patients who received UACDT and those managed with AC alone. A total of 104 patients met inclusion criteria, 65 of whom underwent UACDT. The cohorts had similar PESI scores (P = 0.45) and no clearly imbalanced confounding variables. There was no significant difference in LOS (P = 0.11). UACDT was associated with more bleeding complications (exact P = 0.04). Follow-up transthoracic echocardiograms performed in 54 UACDT and 24 AC patients demonstrated similar rates of resolution of RV dysfunction (61% in UACDT patients versus 75% in AC patients, P = 0.25). Overall one-year mortality was approximately 5% in both groups (exact P > 0.99). In this limited retrospective analysis of intermediate-risk PE patients, UACDT treatment was not associated with mortality benefit or increased resolution of RV dysfunction.
Keywords: mechanical thrombolysis, thrombolytic therapy, pulmonary thromboembolism
Introduction
Acute pulmonary embolism (PE) is a common cardiovascular disorder with a broad range of severity.1,2 Approximately 25% of patients presenting with acute PE are hemodynamically stable with clinical evidence of right ventricular (RV) dysfunction.3 The early approach to management in these intermediate-risk or “submassive” PEs remains uncertain. While they should all be promptly started on anticoagulation (AC), the role of reperfusion therapy is debated. Many clinicians believe that early thrombolytic treatment may minimize the risk of long-term right-sided heart failure by rapidly reducing thrombotic burden. Recent long-term data from a large international randomized controlled trial, however, found that systemic thrombolysis did not reduce residual dyspnea or RV dysfunction when compared to anticoagulation alone.4 Current evidenced-based guidelines recommend against peripherally administered thrombolysis because any acute hemodynamic improvements are offset by the increased risk of major hemorrhage.5–8
In May 2014, the U.S. Food and Drug Administration (FDA) approved an ultrasound-assisted catheter-directed low-dose fibrinolysis device (EkoSonic Endovascular System [EKOS], Bothell, WA, USA) for the management of intermediate-risk PEs based on a prospective, single-arm, multicenter trial.9,10 Preliminary data suggest this technique is safe and effective at rapidly decreasing RV strain, with potentially lower risk of bleeding complications than systemic thrombolysis.10–12 Although ultrasound-assisted catheter-directed thrombolysis (UACDT) has been gathering more popularity, it remains expensive and requires local expertise. Moreover, no study, to our knowledge, has assessed the long-term impact of this endovascular intervention.
We sought to evaluate whether UACDT affords any lasting benefit over AC alone in the management of intermediate-risk PEs. We retrospectively reviewed patients who presented to a large academic medical center with intermediate-risk PE and collected demographic information, clinical data, acute management details, and, when possible, outcome measures. We then assessed the impact of UACDT on length of stay (LOS), resolution of RV dysfunction, and one-year all-cause mortality.
Methods
Study design and data collection
This study was performed at the Brigham and Women’s Hospital in Boston, MA, USA. It was approved by the Partners Healthcare Institutional Review Board with a waiver of informed consent.
We retrospectively reviewed electronic medical records and identified all adults (aged 18–86 years) admitted from January 2011 to October 2016 with a primary diagnosis of intermediate-risk PE. Prior data were not included as they preceded the introduction of UACDT at our institution in 2011. Intermediate-risk PE was defined by hemodynamic stability as indicated by a systolic blood pressure (SBP) ≥ 90 mmHg and evidence of RV dysfunction. Computed tomography (CT) evidence alone of RV dysfunction did not qualify patients. We specifically included individuals with either dilated RV or reduced RV systolic function on transthoracic echocardiogram (TTE). These patients were identified by querying the Partners Healthcare Research Patient Data Registry, a clinical data registry derived from several sources including billing systems that contains comprehensive demographic and clinical information for patients seen in our hospital system. We requested lists of all patients with a primary diagnosis of PE who underwent TTE testing and/or catheter intervention during the index hospitalization and manually examined these records. We included only the first admission for acute PE in those patients with multiple admissions for intermediate-risk PE during the study period. Patients were excluded if there was a history of cancer, pre-existing pulmonary hypertension (PH), pregnancy or postpartum status, contraindication to AC (including goals of care), or treatment with systemic thrombolysis. Patients who presented after an episode of syncope were also excluded, as this was felt to represent transient hemodynamic instability suggestive of higher risk disease.13 Patients aged > 86 years were excluded, as this was the oldest age of someone who underwent UACDT at our institution.
Demographic variables comprising age, sex, race/ethnicity, and clinical characteristics including admission vital signs and co-morbidities were manually extracted along with whether individuals were admitted to an intensive care unit (ICU) and if UACDT was employed. Pulmonary Embolism Severity Index (PESI) scores were calculated.14 We reviewed available baseline and follow-up TTE final reports and specifically assessed RV size and systolic function. Reports that concluded normal RV size and normal RV function were categorized as negative. We subcategorized abnormalities of RV size and systolic function as subjectively described in reports: “borderline,” “mild,” “mild to moderate,” “moderate,” “moderate to severe,” “severe,” or “degree unknown.” Reports with no comment on RV size or function in the official report were categorized as “unknown.” Mortality records were obtained from the Social Security Master Death Index and Partners Healthcare Enterprise Master Patient Index. Given potentially incomplete death ascertainment from these indices, we also manually reviewed the electronic medical records to determine the date of last alive contact. These data were compiled in spreadsheet format (Microsoft Excel; Seattle, WA, USA).
Catheter-directed thrombolysis treatment
We limited our analysis to patients treated with the EkoSonic Endovascular System ([EKOS], Bothell, WA, USA), the only minimally invasive endovascular therapy approved by the FDA for the treatment of PE. Using this technology, one or two catheters were placed at the operator’s discretion based on the location and volume of PEs. A bolus dose of alteplase 0–5 mg/strand was usually administered followed by an infusion rate of 0.5–1 mg/h/strand. Infusion rates were adjusted such that target dose of 20 mg would be reached during standard working hours to facilitate prompt device removal. Patients remained on intravenous unfractionated heparin with a goal partial thromboplastin time of 40–60 during the alteplase infusion. All patients were admitted to an ICU for monitoring for the duration of alteplase infusion.
Study outcomes
Outcomes including LOS, ICU admission status, bleeding complications, resolution of RV dysfunction, and mortality were compared between patients who received UACDT and those managed with AC alone. LOS was measured from date of admission at our institution to date of discharge. Patients were said to require an ICU if this level of care was utilized at any point during the index hospitalization. Bleeding events were classified by the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries bleeding criteria.15 Hemorrhage was deemed severe it if was intracerebral or resulted in substantial hemodynamic compromise requiring treatment. Moderate bleeding was defined by the need for transfusion and minor bleeding referred to all other events (e.g. hematoma at catheter insertion site). Resolution of RV dysfunction was established by normal size and function on any follow-up TTE. Survival was defined as the time from admission to reported death from any cause and was assessed at hospital discharge and through one year.
Statistical analysis
All data were exported from Microsoft Excel for statistical analysis in StataMP 15.0 (StataCorp, College Station, TX, USA). Clinical data including vital signs at presentation were assumed to be normal when data were missing, an approach used in the derivation of the PESI.14 Continuous variable means were compared using Wilcoxon rank-sum test and categorical variables were evaluated using Fisher’s exact test. We used a multivariable logistic regression model to appraise the impact of demographic and clinical variables on the propensity to undergo UACDT. Additionally, we performed a multivariable logistic regression analysis controlling for PESI score to assess the effect of UACDT on resolution of RV dysfunction. To measure differences in overall mortality we compared reported death rates between treatment groups at one year using Fisher’s exact test. Unadjusted Kaplan–Meier curves were calculated for each treatment group censoring for the date when individuals were last reported alive and compared statistically using the log-rank test. A P value < 0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
A total of 104 patients were included in our analysis, 65 of whom received UACDT. The analytic cohort comprised 103 patients (99.0%) diagnosed by CT pulmonary angiography and one patient (1.0%) diagnosed by ventilation perfusion scan.
There were no significant differences between the groups with respect to demographic data, co-morbidities, or smoking status as described in Table 1. Mean PESI scores were 85.4 (range = 33–171, standard deviation [SD] = 29.7) in the patients who underwent UACDT and 90.8 (range = 23–183, SD = 33.0) in those treated with AC alone with no significant difference between the groups (P = 0.39). The two cohorts had similar baseline assessments of RV size (exact P = 0.12) and systolic function (exact P = 0.43) as summarized in Table 2. Sensitivity analyses including only those patients with follow-up TTEs showed no differential loss to follow-up.
Table 1.
Patient characteristics.
| Clinical values | AC (n = 39) | UACDT (n = 65) | P value |
|---|---|---|---|
| Age (years) | 58.4 ± 18.6 | 53.9 ± 16.9 | 0.22 |
| Female sex | 24 (61.5) | 34 (52.3) | 0.42* |
| Race | 0.11* | ||
| White | 25 (64.1) | 53 (81.5) | |
| Black | 8 (20.5) | 9 (13.9) | |
| Hispanic | 5 (12.8) | 2 (3.1) | |
| Other | 1 (2.6) | 1 (1.5) | |
| History of obesity | 23 (59.0) | 39 (60.0) | >0.99* |
| History of DVT/PE | 11 (28.2) | 15 (23.1) | 0.64* |
| Hormonal use including oral contraceptives | 7 (18.0) | 8 (12.3) | 0.57* |
| Recent hospitalization, surgery or reduced mobility† | 15 (38.5) | 35 (53.9) | 0.16* |
| History of heart failure | 1 (2.6) | 1 (1.5) | >0.99* |
| History of chronic lung disease | 11 (28.2) | 18 (27.7) | >0.99* |
| History of hypertension | 22 (56.4) | 30 (46.2) | 0.42* |
| History of neurologic disease | 9 (23.1) | 8 (12.3) | 0.18* |
| History of chronic kidney disease | 2 (5.1) | 5 (7.7) | 0.71* |
| History of diabetes | 4 (10.3) | 13 (20.0) | 0.28* |
| History of chronic infection | 3 (7.7) | 6 (9.2) | >0.99* |
| Ever smoker | 11 (28.2) | 21 (32.3) | 0.83* |
| Heart rate ≥ 110 | 19 (48.7) | 29 (44.6) | 0.69* |
| Systolic blood pressure <100 mmHg | 3 (7.7) | 5 (7.7) | >0.99* |
| Respiratory rate ≥ 30 | 2 (5.1) | 6 (9.2) | 0.71* |
| Temperature < 36℃/96.8°F | 6 (15.4) | 10 (15.4) | >0.99* |
| O2 saturation < 90% | 10 (25.6) | 20 (30.8) | 0.66* |
| Altered mental status‡ | 3 (7.7) | 2 (3.1) | 0.36* |
| PESI score | 90.8 ± 33.0 | 85.4 ± 29.7 | 0.45 |
Data are presented as n (%) or mean ± SD.
Exact P value.
In the prior three months.
Defined by disorientation, lethargy, stupor, or coma.
AC, anticoagulation; UACDT, ultrasound-assisted catheter-directed thrombolysis; PE, pulmonary embolism; DVT, deep vein thrombosis; PESI, Pulmonary Embolism Severity Index; SD, standard deviation.
Table 2.
Baseline right ventricle assessment.
| Baseline TTE results | AC (n = 39) | UACDT (n = 65) | P value |
|---|---|---|---|
| RV size | 0.12* | ||
| Normal | 0 (0.0) | 0 (0.0) | |
| Borderline enlarged | 6 (15.4) | 2 (3.1) | |
| Mildly enlarged | 7 (18.0) | 11 (16.9) | |
| Mild to moderately enlarged | 1 (2.6) | 1 (1.5) | |
| Moderately enlarged | 17 (43.6) | 27 (41.5) | |
| Moderate to severely enlarged | 0 (0.0) | 3 (4.6) | |
| Severely enlarged | 6 (15.4) | 8 (12.3) | |
| Enlarged, degree unknown | 2 (5.1) | 12 (18.5) | |
| Unknown | 0 (0.0) | 1 (1.5) | |
| RV systolic function | 0.42* | ||
| Normal | 7 (18.0) | 7 (10.8) | |
| Borderline reduced | 0 (0.0) | 0 (0.0) | |
| Mildly reduced | 10 (25.6) | 10 (15.4) | |
| Mild to moderately reduced | 3 (7.7) | 2 (3.1) | |
| Moderately reduced | 13 (33.3) | 24 (36.9) | |
| Moderate to severely reduced | 1 (2.6) | 3 (4.6) | |
| Severely reduced | 3 (7.7) | 7 (10.8) | |
| Reduced, degree unknown | 2 (5.1) | 9 (13.9) | |
| Unknown | 0 (0.0) | 3 (4.6) |
Data are presented as n (%).
Exact P value.
TTE, transthoracic echocardiogram; AC, anticoagulation; UACDT, ultrasound-assisted catheter-directed thrombolysis; RV, right ventricle; SD, standard deviation.
Assessment of outcomes
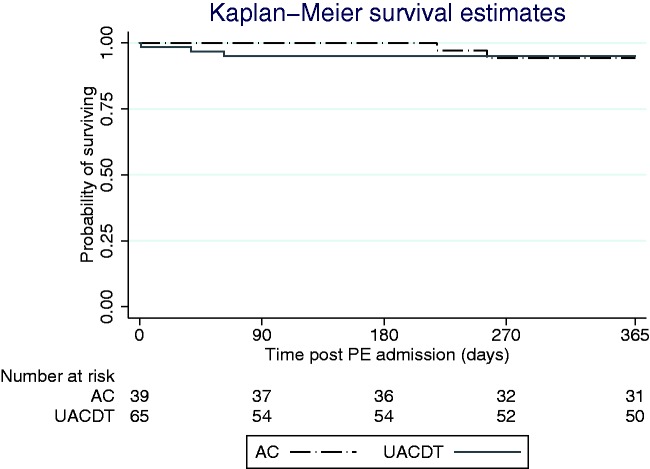
Outcomes are summarized in Table 3. The mean length of stay was 5.1 days (range = 1–23, SD = 3.5) in patients managed with UACDT and 3.9 days (range = 1–9, SD = 1.7) for those treated with AC alone (P = 0.11). All 65 patients treated with UACDT were admitted to an ICU per protocol whereas eight (20.5%) patients managed with AC were admitted to an ICU (exact P < 0.01). Overall, there were more bleeding events in the UACDT group compared with the AC cohort (exact P = 0.04). There were two (3.1%) severe bleeding events in patients treated with UACDT: one death before discharge in the UACDT cohort due to massive hemothorax confirmed on autopsy and one intracerebral hemorrhage during the index hospitalization. UACDT was associated with no moderate bleeding and five (7.7%) minor bleeding complications including two hematomas, prolonged bleeding from the catheter site, gross hematuria, and a stable gastrointestinal bleed that did not require transfusion. There were no deaths during the index hospitalization in the AC group and no minor, moderate, or severe bleeding complications in this group. The incidence of confirmed mortality at one year was similar with three (4.6%) and two (5.1%) deaths in the UACDT and AC groups, respectively (exact P > 0.99). The vital status could not be confirmed in 12 (18.5%) and six (15.4%) patients in the UACDT and AC groups, respectively, at one year (exact P = 0.79). Unadjusted Kaplan–Meier survival curves accounting for censorship are depicted in Fig. 1 (log-rank P = 0.97).
Table 3.
Outcomes.
| Outcomes | AC (n = 39) | UACDT (n = 65) | P value |
|---|---|---|---|
| Length of stay (days) | 3.9 ± 1.9 | 5.1 ± 3.5 | 0.11 |
| Admitted to an ICU | 8 (20.5) | 65 (100.0) | <0.01* |
| Follow-up TTE available | 24 (61.5) | 54 (83.1) | |
| Resolution of RV dysfunction | 18 (75.0) | 33 (61.1) | 0.25 |
| Mortality at 1 year | 2 (5.1) | 3 (4.6) | >0.99* |
| Total bleeding complications | 0 (0.0) | 7 (10.8) | 0.04* |
| Mild | 0 (0.0) | 5 (7.7) | |
| Moderate | 0 (0.0) | 0 (0.0) | |
| Severe | 0 (0.0) | 2 (3.1) |
Data are presented as n (%) or mean ± SD.
Exact P value.
AC, anticoagulation; UACDT, ultrasound-assisted catheter-directed thrombolysis; ICU, intensive care unit; TTE, transthoracic echocardiogram; RV, right ventricle; SD, standard deviation.
Fig. 1.
Kaplan–Meier survival estimates following admission for intermediate-risk PE in those treated with UACDT and AC alone (log-rank P = 0.97). PE, pulmonary embolism; AC, anticoagulation; UACDT, ultrasound-assisted catheter-directed thrombolysis.
Follow-up TTEs were performed in 54 (83.1%) patients managed with UACDT compared with 24 (61.5%) in those treated with AC alone. Resolution of RV dysfunction was observed in 33 (61.1%) patients managed with UACDT and 18 (75.0%) patients in the AC cohort with no significant difference between the groups (P = 0.25 by logistic regression adjusted for PESI score). Even though PESI score is in part determined by patient age, we performed a sensitivity analysis controlling the logistic regression model for PESI score and age with no change in our results. The interval time between PE diagnosis and follow-up TTE was in the range of 1 day to 4.4 years in the UACDT group (median 50 days) and 3 days to 5.0 years in the AC group (median 126 days). Table 3 summarizes these outcome data. Sensitivity analyses including only those patients with at least moderate abnormalities in either RV size or function at the time of PE diagnosis were consistent with the primary analysis.
Discussion
The role of catheter-directed therapies in the management of intermediate risk PE remains contentious. In this single-center retrospective study, UACDT using the only FDA-approved device for management of intermediate-risk PE was not associated with benefit over AC alone with respect to LOS, long-term resolution of RV dysfunction, and all-cause mortality at one year. Patients treated with UACDT had a higher rate of bleeding complications, though the overall event rate was low. Power was limited for most endpoints.
The two cohorts appear to be well matched with similar baseline PESI scores that correspond to intermediate-risk disease.16 There were no significant differences in co-morbidities identified, though some imbalances may not have been detected given the small sample size. Importantly, we excluded patients with a history of malignancy to avoid potential confounders. Patients with cancer-associated thromboembolism have a higher risk of recurrent disease and hemorrhagic complications that are significantly impacted by the type of cancer and treatment history.17
We observed a similar average LOS in both groups: approximately four days in those managed with AC and 5 days in those treated with UACDT. While one small single-center study of 45 patients with acute intermediate- or high-risk PE suggested a decreased LOS associated with UACDT from approximately seven days to three days, this finding has not been replicated.18 Indeed, multiple studies of patients with intermediate-risk PEs have suggested average hospitalization lengths of approximately five days regardless of whether UACDT was employed.10,19 It is worth noting that at our institution all patients who undergo UACDT are observed in an ICU for the duration of thrombolytic infusion. Only 21% of patients managed with AC alone required ICU-level care. It is therefore reasonable to assume that with similar LOS, the hospital cost for those treated with UACDT was higher given expenses associated with the intervention along with any time spent in the ICU that may not have otherwise been required.
The proportion of patients with residual RV dysfunction in our cohorts (39% of patients managed with UACDT and 25% of patients treated with AC alone) agrees with previously published rates. The Pulmonary Embolism Thromboembolism (PEITHO) trial demonstrated similar rates of residual RV dysfunction on TTE in patients treated with systemic thrombolysis and AC only.5 In another prospective study of 180 patients with intermediate-risk PE, Kline et al. found that at least 20% of individuals demonstrated sonographic evidence of either persistently dilated RV or depressed RV systolic function at six-month follow-up, including in a small subset treated with systemic thrombolysis.20 In our analysis, we assumed that patients with at least borderline increase in RV size or decline in RV systolic function had significant baseline RV dysfunction associated with their PE; patients with pre-existing PH were excluded. We only assessed for normalization of RV size and function as subjective assessments of RV size and function were not standardized making it difficult to compare, for example, improvement from moderate to mild dysfunction on serial exams. We did not include estimated mean pulmonary arterial pressure in our assessment of RV dysfunction as this non-invasive approach has several limitations, especially when image acquisition and data interpretation are not standardized.21 We also excluded patients without an initial TTE as these individuals would complicate RV follow-up assessment; most patients at our institution diagnosed with intermediate-risk PE have this study performed at the time of diagnosis.
All-cause mortality was comparably low in both cohorts, and power to detect a difference between groups was limited. Our study, by design, was not representative of the intermediate-risk PE patient population. We excluded older patients and individuals with poor prognosticating co-morbidities such as malignancy and pre-existing PH to eliminate potential confounders and better evaluate the impact of UACDT on outcomes. It is therefore not surprising that our rates are significantly lower than those observed in other trials of intermediate-risk PE4,20,22 and those expected based on PESI scores alone.16 It is also worth noting that the Social Security Master Death and Partners Healthcare Enterprise Master Patient Indices are not comprehensive records of death. We confirmed vital status in majority of individuals, but some unaccounted deaths could further explain our overall low mortality rates. While UACDT does not appear to be associated with major changes in one-year mortality, the sample size was too small to confidently measure minor differences.
The results of this study must be interpreted in the context of these and other limitations. The retrospective design restricts the data available for review. For example, not all patients had follow-up TTEs and the timing of these studies was inconsistent. More patients in the UACDT group underwent follow-up studies, but there was no significant differential loss to follow-up. The cohorts were not randomly assigned. At our institution, management of intermediate-risk PE is decided by the primary physician team, often with guidance from either a cardiology or pulmonary consultant which may have resulted in biased samples. To mitigate this risk, we limited our analysis to those patients without malignancy and pre-existing PH as these were felt to be potentially confounding variables. After these exclusions, the cohorts had similar assessments of RV dysfunction and PESI scores suggesting similar risk stratification. Finally, our single-center study conclusions may not be generalizable to all practice settings, which may vary in terms of spectrum of pathology and diagnostic study ordering patterns.
In conclusion, this limited retrospective study of intermediate-risk PE found that UACDT treatment was not associated with any benefit over AC alone as assessed by LOS, resolution of RV dysfunction, and one-year mortality. Based on these findings, we believe future prospective randomized controlled trials are urgently needed to evaluate if UACDT confers any benefit in this population before it is incorporated into routine clinical practice.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was supported by the National Institutes of Health (grant numbers NHLBI U01 HL125215, R42 HL132742, and R01 HL131910) and the Harvard Catalyst | The Harvard Clinical and Translational Science Center (grant numbers NCRR & NCATS UL1 TR001102). The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
References
- 1.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012; 379(9828): 1835–1846. [DOI] [PubMed] [Google Scholar]
- 2.Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010; 363(3): 266–274. [DOI] [PubMed] [Google Scholar]
- 3.Piazza G. Submassive pulmonary embolism. JAMA 2013; 309(2): 171–180. [DOI] [PubMed] [Google Scholar]
- 4.Konstantinides SV, Vicaut E, Danays T, et al. Impact of thrombolytic therapy on the long-term outcome of intermediate-risk pulmonary embolism. J Am Coll Cardiol 2017; 69(12): 1536–1544. [DOI] [PubMed] [Google Scholar]
- 5.Meyer G, Vicaut E, Danays T, et al. PEITHO Investigators. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014; 370(15): 1402–1411. [DOI] [PubMed] [Google Scholar]
- 6.Jaff MR, McMurtry MS, Archer SL, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123(16): 1788–1830. [DOI] [PubMed] [Google Scholar]
- 7.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest 2016; 149(2): 315–352. [DOI] [PubMed] [Google Scholar]
- 8.Konstantinides SV, Barco S, Lankeit M, et al. Management of pulmonary embolism: an update. J Am Coll Cardiol 2016; 67(8): 976–990. [DOI] [PubMed] [Google Scholar]
- 9.Owings K. FDA clears EKOS EkoSonic Endovascular System. Available at: www.fdanews.com/articles/164759-fda-clears-ekos-ekosonic-endovascular-system (accessed 15 November 2017).
- 10.Piazza G, Hohlfelder B, Jaff MR, et al. SEATTLE II Investigators. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: The SEATTLE II Study. JACC Cardiovasc Interv 2015; 8(10): 1382–1392. [DOI] [PubMed] [Google Scholar]
- 11.Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 2014; 129(4): 479–486. [DOI] [PubMed] [Google Scholar]
- 12.Kuo WT, Banerjee A, Kim PS, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest 2015; 148(3): 667–673. [DOI] [PubMed] [Google Scholar]
- 13.Altınsoy B, Erboy F, Tanrıverdi H, et al. Syncope as a presentation of acute pulmonary embolism. Ther Clin Risk Manag 2016; 12: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donzé J, Le Gal G, Fine MJ, et al. Prospective validation of the Pulmonary Embolism Severity Index. A clinical prognostic model for pulmonary embolism. Thromb Haemost 2008; 100(5): 943–948. [DOI] [PubMed] [Google Scholar]
- 15.GUSTO investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329(10): 673–682. [DOI] [PubMed] [Google Scholar]
- 16.Dentali F, Riva N, Turato S, et al. Pulmonary embolism severity index accurately predicts long-term mortality rate in patients hospitalized for acute pulmonary embolism. J Thromb Haemost 2013; 11(12): 2103–2110. [DOI] [PubMed] [Google Scholar]
- 17.Khorana AA, Carrier M, Garcia DA, et al. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis 2016; 41(1): 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nykamp M, VandenHull A, Remund T, et al. Safety and efficacy of ultrasound-accelerated catheter-directed lytic therapy in acute pulmonary embolism with and without haemodynamic instability. J Vasc Surg Venous Lymphat Disord 2015; 3(3): 251–257. [DOI] [PubMed] [Google Scholar]
- 19.Bagla S, Smirniotopoulos JB, van Breda A, et al. Ultrasound-accelerated catheter-directed thrombolysis for acute submassive pulmonary embolism. J Vasc Interv Radiol 2015; 26(7): 1001–1006. [DOI] [PubMed] [Google Scholar]
- 20.Kline JA, Steuerwald MT, Marchick MR, et al. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136(5): 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rich JD, Shah SJ, Swamy RS, et al. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. Chest 2011; 139(5): 988. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro A, Lindmarker P, Johnsson H, et al. Pulmonary embolism: one-year follow-up with echocardiography doppler and five-year survival analysis. Circulation 1999; 99(10): 1325–1330. [DOI] [PubMed] [Google Scholar]