Abstract
Introduction:
Patients in the intensive care unit (ICU) have significantly disrupted sleep. Sleep disruption is believed to contribute to ICU delirium, and ICU delirium is associated with increased mortality. Experts recommend sleep promotion as a means of preventing or shortening the duration of delirium. ICU Sleep promotion protocols are highly complex and difficult to implement. Our objective is to describe the development, pilot implementation, and revision of a medical ICU sleep promotion protocol.
Methods:
Naptime is a clustered-care intervention that provides a rest period between 00:00 and 04:00. We used literature review, medical chart review, and stakeholder interviews to identify sources of overnight patient disturbance. With stakeholder input, we developed an initial protocol that we piloted on a small scale. Then, using protocol monitoring and stakeholder feedback, we revised Naptime and adapted it for unitwide implementation.
Results:
We identified sound, patient care, and patient anxiety as important sources of overnight disturbance. The pilot protocol altered the timing of routine care with a focus on medications and laboratory draws. During the pilot, there were frequent protocol violations for laboratory draws and for urgent care. Stakeholder feedback supported revision of the protocol with a focus on providing 60- to 120-minute rest periods interrupted by brief clusters of care between 00:00 and 04:00.
Discussion:
Four-hour blocks of rest may not be possible for all medical ICU patients, but interruptions can be minimized to a significant degree. Involvement of all stakeholders and frequent protocol reevaluation are needed for successful adoption of an overnight rest period.
Keywords: delirium, intensive care unit, sleep, circadian rhythm, clustered care
Introduction
There is growing awareness of the importance of sleep in the intensive care unit (ICU) (1, 2). Critically ill patients experience sleep loss, poor sleep quality, and circadian disruption (3 –7). Sleep disruption may lead to delirium and persistent cognitive impairment after critical illness (8). Intensive care unit delirium is an independent predictor of mortality, increased length of stay, and postdischarge cognitive impairment (9 –13).
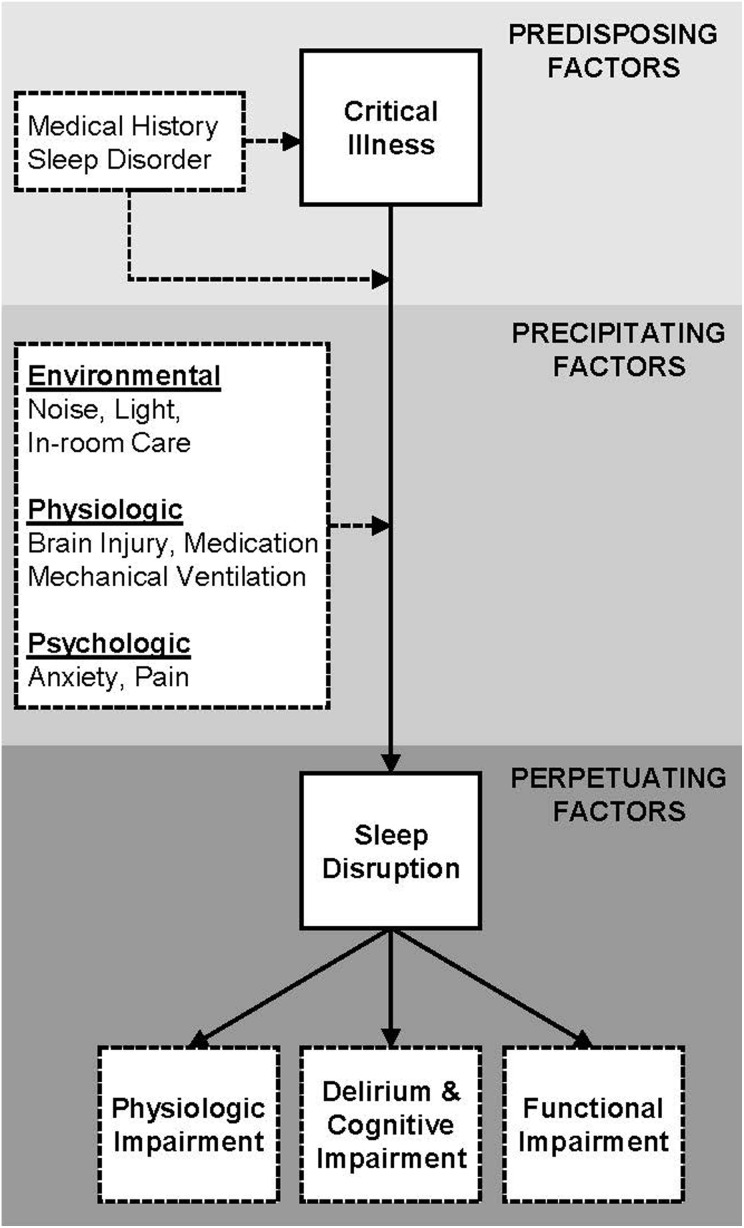
Impediments to sleep in the ICU are multifactorial. It is informative to consider ICU sleep disruption within the construct of the Spielman’s 3P model of insomnia or sleep disruption (Figure 1) (14). This model identifies predisposing, precipitating, and perpetuating factors. Of these factors, noise (eg, high sound), light, and in-room patient care activity are modifiable (15,16). Many studies demonstrate that average hospital and ICU sound levels are excessive and disruptive to patients (6,15,17 –20). In addition, abnormal light exposure, frequent use of continuous enteral feeding, and lack of physical activity predispose critically ill patients to circadian misalignment and amplitude loss which in turn contribute to further sleep disruption (21 –23).
Figure 1.
Model of intensive care unit sleep disruption based on Spielman’s 3P model of sleep disruption (insomnia). Many of the listed precipitating factors are modifiable in the intensive care unit setting.
Current expert guidelines suggest sleep promotion for delirium prevention (1). One sleep-promoting intervention with noise reduction and clustering of care significantly reduced the incidence of delirium (24), and a meta-analysis of environmental interventions demonstrated improvement in sleep quantity and decreased sleep fragmentation (25).
To address the problem of ICU sleep disruption, we developed a multifaceted, nonpharmacologic sleep promotion intervention, Naptime, to provide patients with a period of rest from 00:00 to 04:00. The purpose of this article is to describe the development, implementation, and iterative revision of our protocol.
Materials and Methods
The pilot implementation of Naptime was registered with ClinicalTrials.gov (NCT03119207) and approved by our institution’s Human Investigations Committee (HIC# 1112009428).
Setting
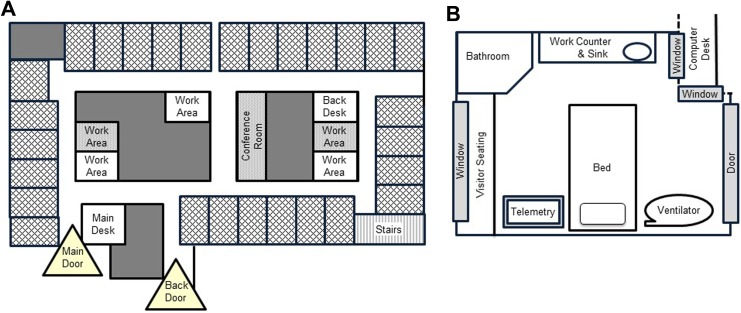
This project was conducted in the medical intensive care unit (MICU) of an academic tertiary medical center that admits over 3000 patients per year. Sepsis and acute respiratory failure are the most frequent reasons for admission. The patient–nurse ratio is 1:1 or 2:1. There is 24/7 attending physician staffing, with many admissions and discharges during the overnight period. The unit is rectangular with private, single-bed patient rooms on each of the four sides. In the center, there is a core of workstations, supply closets, and conference rooms (Figure 2). There is no central nursing station. Nurses work at standing-computer stations inside the patient room or at desks directly outside. No overhead paging is allowed on the unit. Our MICU Alarm Committee has modified alarm parameters and protocols to minimize nuisance alarms, limit signaling of alarms in other patient rooms, and promote rapid silencing of alarms. The MICU has no visiting hour restrictions, but visitors are not allowed to congregate in the unit hallways.
Figure 2.
A, Medical intensive care unit floor plan. Cross-hatched rectangles around the perimeter are patient rooms. Unlabeled gray areas are offices or closed supply closets. Open (white) and closed (gray) work areas, a closed conference room, stairs, and unit doors are located as indicated. B, Patient room floor plan. Standard furniture and common clinical equipment are located as indicated. The door is sliding glass. Interior windows allow monitoring from hallway computer desk. There is a large exterior window in every patient room.
There is a hospital-wide quiet time protocol from 23:00 to 06:00 which includes dimming hallway lights and limiting overhead pages to cardiac arrest or fire announcements. On admission, patients receive a quiet pack including headphones, earplugs, and eye masks, and a brief explanation card which emphasizes the importance of sleep and requests that patients keep sound levels low overnight. The use of earplugs and eyes masks varies based on preference but can be effective for those who are comfortable using these devices (26). The institution-wide protocol includes changes in workflow by pharmacy, laboratory, information technology, and facility services. For example, under the hospital-wide protocol, in-room stocking of supplies, floor maintenance, and trash collection occur before 23:00 or after 05:00.
Stakeholder Inclusion
We recruited a diverse set of MICU stakeholders for all stages of protocol development. Stakeholders from hospital and unit leadership facilitated communication between our group and hospital-wide services (eg, pharmacy, laboratory, information technology, and facilities services). We recruited MICU clinical staff (physicians, nurses, respiratory therapists, pharmacists, and patient care assistants) to facilitate changes in direct care. Patient and visitor stakeholders provided insight into their needs. We also collaborated with special interest groups, including the ICU alarm, ICU infection control, and ICU skin care committees.
Identification of Sources of Disturbance
During the first stage, we conducted a literature review via PubMed to generate a list of factors that may contribute to sleep disruption. We used the search terms “intensive care unit” and “critical care” combined with “sleep,” “rest,” “disruption,” “disturbance,” “sound,” “noise,” “light, “lux,” “nocturnal care interactions,” and “nocturnal nursing interactions.” We identified a set of relevant articles in English. We discussed the resulting list of factors with stakeholders and addressed these factors in our protocol per stakeholder recommendation.
We abstracted overnight care activities from the electronic medical record (EMR) to understand workflow. We conducted interviews with physicians, nurses, patient care assistants, and respiratory therapists who worked nights and with patients regarding sources of sleep disturbance (27). We also met with the MICU pharmacists to discuss medications that require frequent dosing or long run times to determine ways to avoid disturbance due to medication administration.
Protocol Development and Pilot Implementation
We used the data obtained in the first phase to develop a protocol. We selected 00:00 to 04:00 as the Naptime period to include most patients’ sleep midpoint (28). With the support of our stakeholders, we delineated 4 categories of care activities: (1) untimed, (2) timed outside of Naptime, (3) time critical within Naptime, and (4) urgent or emergent (Table 1). We also developed care protocols to mitigate the disturbances that we identified (Table 2). The bedside nurse served as the gatekeeper to minimize in-room disturbance levels between 00:00 and 04:00. Emergency interventions were not limited or altered.
Table 1.
Categorization of Bedside Care Activities.
| Activity Type | Common Activity Examples |
|---|---|
| Restricted 00:00 to 04:00 | |
| Not timed |
|
| Timed and can be rescheduled |
|
| Permitted 00:00 to 04:00 | |
| Time sensitive or frequent (cluster as possible) |
|
| Emergency care |
|
Abbreviations: CAM-ICU, confusion assessment method for the intensive care unit; GCS, Glasgow Coma Scale; RASS, Richmond Agitation and Sedation Scale.
Table 2.
Summary of Naptime Protocol Elements.
| Institution level |
|
| Unit level |
|
| Bedside |
|
| Direct care |
|
| Challenging cases |
|
Abbreviation: ICU, intensive care unit.
We conducted a pilot test of the protocol from August 2013 until June 2014. We implemented the protocol for individual patients, and research staff provided one-on-one coaching to the bedside nurses. We did not alter clinical staffing for the protocol. Patients underwent the Naptime protocol every night of their MICU stay. During the day, medications, diagnostic imaging, laboratory draws, and care orders were reviewed and retimed by the principal investigator (M.P.K.) in conjunction with the primary team. The overnight nurse and physician discussed and retimed any newly ordered medications, imaging, or laboratory draws. The nurse prevented other nonurgent room visits on an as-needed basis.
To assist with pilot protocol implementation and standardization, we developed a checklist of care activities that should be completed before Naptime (Supplement 1). We also monitored adverse events (eg, unintended device removal, fall, or unexpected death) during Naptime.
Adaptation for Unitwide Implementation
We elicited challenges or violations to the protocol by staff. The bedside nurse completed a confidential survey (Supplement 2) to identify the frequency and reason for Naptime interruptions. We reviewed the medical record for the occurrence of medication administration, diagnostic imaging, or laboratory draws. We repeated meetings with stakeholders. To facilitate future monitoring, we developed automated reporting of overnight blood draws, medication administration, and radiologic studies in the EMR. Based on this feedback, we adapted the Naptime protocol for unitwide implementation and launched the protocol in March of 2017. The details of the protocol are reported below.
Results
Sources of Disturbance
Major themes from the literature review included disturbance from the following: staff and visitor voices, general sound, sound from alarms, sound from carts or moving equipment, room maintenance, skin care and bathing, laboratory draws, medication administration, frequent overnight care, pain, and anxiety or sadness (selected articles 15,16,19,29,30). Review of the medical record revealed that laboratory draws, diagnostic imaging, medication administration, transfusions, skin and wound care, dressing changes, and procedures related to intravenous access frequently occurred overnight. Baseline analysis of unitwide practice revealed that 10.5% of blood transfusions, 8.7% of diagnostic imaging, 27.0% of laboratory draws, and 9.2% of medications occurred between 00:00 and 04:00. Stakeholder interviews are reported in detail elsewhere (27). Briefly, both staff and patients identified environmental factors in sleep disruption; however, patients also identified emotions and a need for reassurance as major factors in sleep disruption.
Naptime Pilot Implementation
We piloted Naptime with 26 patients. The mean patient age was 62.3 years (standard deviation [SD] = 16.3). Acute Physiology and Chronic Health Evaluation II mean was 18.9 (SD 6.3); 25% of patients had sepsis, 28% had acute respiratory failure, 34% were intubated, and 74% were on vasopressors. Mean length of stay was 4.1 (SD 2.9) days. The primary team and bedside nurse agreed with Naptime enrollment in all cases. Daytime medical record review most frequently resulted in an adjustment in the timing of routine medications (42% of patients, not including piperacillin–tazobactam or insulin), piperacillin–tazobactam (31%), insulin sliding scale (38%), and ventilator checks (34%). Laboratory draws frequently occurred during the Naptime protocol; the median number of laboratory draws for Naptime patients was 1 with an interquartile range of 0 to 3.
We collected 24 post-Naptime surveys from bedside nurses. Survey responses indicated the following estimates of the number room entries: “None” 8%, “1-2 times” 21%, “3-4 times” 38%, and “5 or more times” 33%. Urgent or time critical care was the most frequently cited reason for room entrance (58%). Care activities that required room entrance included monitoring of continuous hemodialysis, titration of vasopressors, position changes, and agitated delirium. Only 8% of respondents indicated that there was not enough time outside of Naptime to conduct routine care. Discussion with stakeholders revealed persistent beliefs that routine daily laboratory draws are needed at 02:00 to allow time for electrolyte repletion before the change of shift. There was also concern that day shift caregivers would be frustrated if certain tasks (eg, bathing) did not occur overnight. There were no adverse events related to the Naptime protocol.
Unitwide Adaptation
The number of protocol violations during the Naptime pilot suggests that an uninterrupted 4-hour period is not achievable for a significant proportion of patients. We adapted the protocol to emphasize alternate strategies to cluster care and minimize disturbance so that patients have at least 60 to 120 minutes at a time for rest. For the unitwide protocol, the nurse remains the gatekeeper for preventing in-room activity during Naptime. For unitwide implementation, we identified nurse champions, created educational materials, and held in-service meetings to publicize protocol relaunch. Caregiver education focused on laboratory draw practices and acceptance of delaying nonurgent care activities to times outside of Naptime.
Unitwide Protocol
Clinical staff complete routine nighttime care before midnight. The care that must occur during Naptime is clustered to decrease the number of room entrances; caregivers use flashlights, nightlights, and low voices to minimize the light and sound associated with care that occurs during Naptime. At the start of Naptime, the nurse dims or turns off lights and closes the window curtain, the door curtain, and the room door. We suggest a small opening in the door curtain to allow viewing of the head of the bed, the ventilator, and the telemetry monitor. We encourage patients to turn televisions off or use headphones. We have a policy against turning the television on for unresponsive patients or in empty rooms. Our protocol also includes keeping window shades up during the day to promote wakefulness. Although heart rate and oximetry are continuous on our unit, we turn telemetry alarms to a minimum volume inside the patient room. We complete temperature checks before Naptime. Blood pressure monitoring occurs no more frequently than every 60 minutes if clinically allowable.
We also ask the bedside nurse to provide a verbal “signal to sleep.” This signal lets the patient know that they have time to sleep for a few hours and explains the ongoing remote monitoring. This explanation reassures patients that they are safe and that we are continuing to take care of them. Nurses ask the patients if they have toileting, pain, or other needs and assure that the call bell is within reach. Nurses provide a similar signal for unresponsive or sedated patients that is adapted to their abilities.
Pharmacy protocols schedule routine medications outside of Naptime. Medications that require administration every 6 hours are given at 05:00, 11:00, 17:00, and 23:00. With the support and input of our pharmacy department, the EMR prevents inadvertent overnight medication scheduling via system defaults which offer a “now” dose and then automatically schedules medications during appropriate daytime periods. If needed, the bedside nurse can also change medication timing without physician approval. Inhaled medications that are “as needed” or “standing” are administered under an institutional protocol that prevents waking the patient. Exceptions are allowed for patients requiring continuous or frequent dosing of inhaled medications.
Infused medications are a major source of disruption due to intravenous pump beeping. Beeping from continuous infusions can be minimized with the strategic use of bag sizes and concentrations that ensure sufficient supply for the entire or at least one-half of the rest period. Routine tubing changes needed for continuous infusions occur outside of Naptime. Difficult medications with frequent dosing or long run times (eg, piperacillin–tazobactam) cause disruption. We minimize disruption by starting infusions at midnight and maximizing run time; in many cases, a 4-hour run time can be used to avoid starts or stops during 00:00 to 04:00.
Routine laboratory draws occur after 04:00. This avoids disturbance related to phlebotomy and disturbance for nonurgent electrolyte repletion or transfusion. Patients requiring frequent laboratory monitoring every 4 to 6 hours have their presleep laboratory draws ordered “stat” at 22:00; adjustments or changes can be made before midnight. For every 6-hour fingersticks, our protocol suggests a 05:00, 11:00, 17:00, and 23:00 schedule.
Ventilated patients must have a ventilator check at least every 8 hours by a respiratory therapist; we complete this check before Naptime for stable patients. Also, ventilator alarm settings can be optimized to prevent nuisance alarms, and ventilator screens can be positioned to be visible through the glass wall for out-of-room monitoring. For most patients, suctioning can be timed just before and after Naptime. Patients who require suctioning more frequently are suctioned a minimum number of times during Naptime, and we cluster the suctioning with other necessary care.
Position changes are required every 2 hours for immobile patients. We position patients on their side with pillows for support at 00:00. At 02:00, the nurse pulls the pillows out from under the patient which allows the patient to roll onto their back and change position. We believe that this “pillow pull” position change is the least disturbing during the rest period. We cluster other care needed during Naptime with this position change. We complete routine wound care, dressing changes, and monitoring of central lines outside of Naptime.
Challenging Cases
Naptime does not change the timing of urgent or emergent patient care. During emergencies, we mitigate disruption to adjacent patient rooms by closing doors and preventing staff and family from congregating outside the patient’s room. As noted above, some patients have frequent or time-intensive care needs that prevent a continuous 4-hour rest period. Examples include patients in the prone position, patients supported by continuous renal replacement, and patients who have unstable ventilator or vasopressor needs. Our protocol describes strategies for these patients to cluster interventions that could provide shorter blocks of rest (eg, minutes) between 00:00 and 04:00. As mentioned above, the 02:00 position change is often used as a time point to deliver a cluster of care to patients.
Finally, overnight admissions are frequent, disruptive to the patient, and disruptive to patients in adjacent rooms. Our protocol suggests a coordinated examination by all members of the medical team, immediate laboratory draws, rapid completion of any diagnostic work or procedures, and clustering of in-room care. We delay any care per Naptime protocol if it is safe for the patient. Overnight discharges are also very disturbing to patients. We continue to work with our hospital-wide bed management teams to avoid transfers between 00:00 and 04:00.
Discussion
Implementing Naptime presented challenges related to the complexity of care in the MICU. Nonurgent laboratory draws and urgent or time critical care were the most frequent reasons for protocol violation. Furthermore, pilot implementation suggested that an uninterrupted 4-hour period is not achievable for a significant proportion of patients. Based on our pilot, we adapted the protocol to emphasize alternate strategies to cluster care and minimize disturbance so that most patients have at least 60 to 120 minutes at a time for rest. The protocol must adapt to the individual patient and individual ICU needs. Although we have identified and addressed many sources of overnight disturbance, ICU sleep promotion requires vigilance for new sources of disturbance and frequent protocol adjustment.
There is a gap between the perceived importance of sleep and the implementation of sleep promotion protocols (2,31). This protocol conflicts with ICU culture and practice on many levels. Because of this, we have involved stakeholders, recruited nurse champions, and garnered the support of hospital and ICU leadership. We are leveraging successful ICU tools such as checklists to facilitate workflow. Future directions include consulting with implementation experts to facilitate long-term maintenance and expansion of the protocol beyond our MICU.
Ultimately, changes to ICU staffing models may be needed to allow a greater amount of work to be done outside of the Naptime. Also, there is little focus on changing unit or building design or altering building materials to minimize patient disturbance. To justify modifications to the physical structure of the ICU, we need to demonstrate patient benefit. We are currently monitoring environmental disturbance levels, patient sleep, delirium incidence, and patient and staff satisfaction to document the benefit of Naptime.
Conclusions
There are many obstacles to sleep in the ICU. As the pressure to shorten inpatient admissions has increased, workflow and system defaults have evolved with little regard for patient sleep. Each hospital environment may have unique challenges, but many of the outlined solutions will have relevance to other ICUs. Notably, the involvement of broad stakeholders to identify sources of disturbance in an individual ICU will improve the design and implementation of a rest period such as Naptime. Finally, sleep improvement protocols require monitoring, a mechanism for stakeholder feedback, and iteration to maintain effectiveness over time. We believe that the wide dissemination of sleep promotion interventions will positively impact outcomes in critically ill patients.
Supplemental Material
Supplemental Material, JPX-17-0053_Knauert_et_al_Naptime_Supplement_1 for Creating Naptime: An Overnight, Nonpharmacologic Intensive Care Unit Sleep Promotion Protocol by Melissa P Knauert, Nancy S Redeker, Henry K Yaggi, Michael Bennick, and Margaret A Pisani in Journal of Patient Experience
Supplemental Material
Supplemental Material, JPX-17-0053_Knauert_et_al_Naptime_Supplement_2 for Creating Naptime: An Overnight, Nonpharmacologic Intensive Care Unit Sleep Promotion Protocol by Melissa P Knauert, Nancy S Redeker, Henry K Yaggi, Michael Bennick, and Margaret A Pisani in Journal of Patient Experience
Acknowledgment
The authors acknowledge the care providers at Yale-New Haven Hospital and especially the overnight MICU teams who have been open to change, creative, and tireless in providing patients the best care possible.
Author Biographies
Melissa P Knauert is an assistant professor of Medicine at the Yale School of Medicine. She serves as the associate program director for the Sleep Medicine Fellowship at the Yale School of Medicine. Her research is focused on the impact of sleep and circadian disruption on critical illness outcomes.
Nancy S Redeker is the Beatrice Renfield Term professor of Nursing. Her program of research focuses on the contributions of sleep and sleep disorders in acute and chronic conditions and the role of self-management and environmental interventions focused on sleep among patients with these conditions.
Henry K Yaggi is an associate professor of Medicine at the Yale School of Medicine. He serves as the director of the Yale Centers for Sleep Medicine. His research investigates the cardiovascular consequences of sleep disordered breathing and examines the impact of interventions targeting sleep disorders that may help to improve health outcomes.
Michael Bennick is an associate clinical professor of Medicine at the Yale School of Medicine. He is the medical director of the Patient Experience and Chairman of the Patient Experience Council at Yale-New Haven Hospital.
Margaret A Pisani is an associate professor of Medicine at the Yale School of Medicine and the Fellowship Program director for the Pulmonary and Critical Care Fellowships at the Yale School of Medicine. Dr. Pisani’s research focuses on improving the care and outcomes of older critically ill patients. She is particularly interested in the prevention and treatment of intensive care unit delirium.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by project funds to M. P. Knauert (P20 NR014126, Redeker and Yaggi mPI) and project funds to M. A. Pisani from The Patrick and Catherine Weldon Donaghue Medical Research Foundation. M. P. Knauert also received a career development support from the National Center for Advancing Translational Science, a component of the National Institutes of Health (KL2 TR000140, Sherwin PI).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306. [DOI] [PubMed] [Google Scholar]
- 2. Kamdar BB, Knauert MP, Jones SF, Parsons EC, Parthasarathy S, Pisani MA, et al. Perceptions and practices regarding sleep in the intensive care unit. A survey of 1,223 critical care providers. Ann Am Thorac Soc. 2016;13:1370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knauert MP, Haspel JA, Pisani MA. Sleep loss and circadian rhythm disruption in the intensive care unit. Clin Chest Med. 2015;36:419–29. [DOI] [PubMed] [Google Scholar]
- 4. Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117:809–18. [DOI] [PubMed] [Google Scholar]
- 6. Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–7. [DOI] [PubMed] [Google Scholar]
- 7. Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weinhouse GL, Schwab RJ, Watson PL, Patil N, Vaccaro B, Pandharipande P, et al. Bench-to-bedside review: delirium in ICU patients – importance of sleep deprivation. Crit Care. 2009;13:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. [DOI] [PubMed] [Google Scholar]
- 10. Milbrandt EB, Deppen S, Harrison PL, Shintani AK, Speroff T, Stiles RA, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–62. [DOI] [PubMed] [Google Scholar]
- 11. Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. [DOI] [PubMed] [Google Scholar]
- 12. Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9:R375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spielman A, Glovinsky P. In: Hauri P, ed. Case Studies in Insomnia. New York, NY: Plenum Medical Book; 1991. [Google Scholar]
- 15. Tamburri LM, DiBrienza R, Zozula R, Redeker NS. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13:102–13. [PubMed] [Google Scholar]
- 16. Le A, Friese RS, Hsu CH, Wynne JL, Rhee P, O’Keeffe T. Sleep disruptions and nocturnal nursing interactions in the intensive care unit. J Surg Res. 2012;177:310–4. [DOI] [PubMed] [Google Scholar]
- 17. Knauert M, Jeon S, Murphy TE, Yaggi HK, Pisani MA, Redeker NS. Comparing average levels and peak occurrence of overnight sound in the medical intensive care unit on A-weighted and C-weighted decibel scales. J Crit Care. 2016;36:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Darbyshire JL, Young JD. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit Care. 2013;17:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167:708–15. [DOI] [PubMed] [Google Scholar]
- 20. Tembo AC, Parker V, Higgins I. The experience of sleep deprivation in intensive care patients: findings from a larger hermeneutic phenomenological study. Intensive Crit Care Nurs. 2013;29:310–6. [DOI] [PubMed] [Google Scholar]
- 21. Duffy JF, Czeisler CA. Effect of light on human circadian physiology. Sleep Med Clin. 2009;4:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edwards BJ, Reilly T, Waterhouse J. Zeitgeber-effects of exercise on human circadian rhythms: what are alternative approaches to investigating the existence of a phase-response curve to exercise? Biol Rhythm Res. 2009;40:53–69. [Google Scholar]
- 23. Jiang P, Turek FW. Timing of meals: when is as critical as what and how much. Am J Physiol Endocrinol Metab. 2017;312:E369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, et al. The effect of a quality improvement intervention on perceived sleep quality and cognition in a medical ICU. Crit Care Med. 2013;41:800–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poongkunran C, John SG, Kannan AS, Shetty S, Bime C, Parthasarathy S. A meta-analysis of sleep-promoting interventions during critical illness. Am J Med. 2015;128:1126–37.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamdar BB, Yang J, King LM, Neufeld KJ, Bienvenu OJ, Rowden AM, et al. Developing, implementing, and evaluating a multifaceted quality improvement intervention to promote sleep in an ICU. Am J Med Qual. 2014;29:546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding Q, Redeker NS, Pisani MA, Yaggi HK, Knauert MP. Factors influencing patients’ sleep in the intensive care unit: perceptions of patients and clinical staff. Am J Crit Care. 2017;26:278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kryger MH, Roth T, Dement WC. Principles and practice of sleep medicine. 6th ed Philadelphia, PA: Elsevier; 2017:1730. [Google Scholar]
- 29. Xie H, Kang J, Mills GH. Clinical review: the impact of noise on patients’ sleep and the effectiveness of noise reduction strategies in intensive care units. Crit Care. 2009;13:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Little A, Ethier C, Ayas N, Thanachayanont T, Jiang D, Mehta S. A patient survey of sleep quality in the Intensive Care Unit. Minerva Anestesiol. 2012;78:406–14. [PubMed] [Google Scholar]
- 31. Knauert MP, Kamdar BB, Sleep in the ICUTF. Reply: sleep in the intensive care unit is a priority. Ann Am Thorac Soc. 2016;13:1868–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, JPX-17-0053_Knauert_et_al_Naptime_Supplement_1 for Creating Naptime: An Overnight, Nonpharmacologic Intensive Care Unit Sleep Promotion Protocol by Melissa P Knauert, Nancy S Redeker, Henry K Yaggi, Michael Bennick, and Margaret A Pisani in Journal of Patient Experience
Supplemental Material, JPX-17-0053_Knauert_et_al_Naptime_Supplement_2 for Creating Naptime: An Overnight, Nonpharmacologic Intensive Care Unit Sleep Promotion Protocol by Melissa P Knauert, Nancy S Redeker, Henry K Yaggi, Michael Bennick, and Margaret A Pisani in Journal of Patient Experience