Abstract
Background:
Microsampling techniques are alternative methods to venous sampling for obtaining blood for measurement of circulating biomarkers, offering the convenience of reduced sample volume and elimination of the need for phlebotomists. Dried blood spot (DBS) microsampling methods have been used for many years while more recently a volumetric absorptive microsampling device (VAMS™) has been introduced. In diabetes mellitus, circulating C-peptide is commonly used as an indicator of endogenous insulin secretion and clinical measurement can aid in diagnosis as well as informing on therapy. This pilot study investigated the effectiveness of microsampling collection of capillary blood for measurement of C-peptide.
Methods:
Capillary blood was collected into capillary tubes and centrifuged for plasma samples. Simultaneous samples were also collected using both microsampling methods (DBS and VAMS). Blood from both microsamplers was extracted prior to assaying for C-peptide alongside the corresponding plasma samples, using specific immunoassays and results obtained from microsampling compared to the reference plasma concentrations. Stability was determined by collecting duplicate DBS and VAMS and assaying both in a single assay after storing one at −20°C immediately and one at room temperature for 48 hours post-collection.
Results:
Good agreement was observed between C-peptide concentrations in plasma and equivalent DBS and VAMS samples (R2 = .929 and .9231, DBS and VAMS, respectively), with mean differences of 75.7 and 8.4 pmol/L observed for DBS and VAMS. Small decreases in C-peptide of 11.6% and 0.1% were observed after 48 hours storage for DBS and VAMS, respectively.
Conclusions:
C-peptide collected using DBS and VAMS showed good agreement with reference plasma concentrations, suggesting both would be an effective microsampling method for collection and measurement of C-peptide.
Keywords: C-peptide, microsampling, dried blood spot, volumetric absorptive microsampling
Use of microsampling techniques eliminates the need for large blood volume collection for the determination of circulating blood concentrations of biomarkers and drugs and in particular can be highly beneficial and convenient when monitoring young children and the elderly. Dried blood spot (DBS) collection has been an established method in blood analysis for many years, due to the elimination of the need for a phlebotomist and centrifugation step, lower blood volume and ease of transport (eg, postage). DBS collection, however, may be limited by factors including hematocrit, blood viscosity, inaccuracy of sample volume, sampling conditions, sampling quality and the nature/stability of analytes.1 A novel volumetric absorptive microsampling (VAMS™) device, consisting of an absorptive, fixed volume sampling pad attached to a plastic stem, has recently become available that reportedly is not affected by hematocrit levels2 or sampling conditions as it collects accurate, reproducible fixed sample volume.
In subjects with diabetes mellitus, plasma or serum C-peptide is commonly used as a measure of endogenous insulin secretion as both insulin and C-peptide are co-secreted in equimolar amounts.3 As approximately half of the insulin secreted by the pancreas is metabolized by the liver, measurement of peripheral C-peptide, which does not undergo hepatic extraction, rather than peripheral insulin allows a more accurate indication of insulin secretion. Unlike insulin, the rate of C-peptide clearance in the peripheral circulation is at a constant rate, giving more accurate and consistent measurements. Measurement of C-peptide also avoids potential issues of cross-reactivity between exogenous and endogenous insulin in insulin assays.4,5
As well as denoting endogenous insulin production, C-peptide has also been shown to change with the age of diagnosis, the type (type 1 or type 2) and duration of diabetes.6 Clinical measurement of C-peptide in diabetes can therefore aid in the diagnosis, prognosis and therapeutic response.4,7 It has previously been identified that measuring C-peptide collected using DBS can be used as a first line screening test monitoring beta cell function,1 with DBS correlating well with plasma levels.8
This study aimed to provide pilot data to inform the effectiveness of both DBS and a novel VAMS device for collection of capillary finger-prick samples for measuring C-peptide concentrations.
Methods
Sample Collection
Capillary blood samples were collected via finger-prick from adult staff volunteers at the Diabetes Research Unit Cymru, following informed consent. Fasting and postprandial samples were collected to ensure a wide C-peptide concentration range. All samples were disposed immediately following analysis.
Approximately 100 µl capillary blood, collected into EDTA capillary tubes (Sarstedt, UK), was centrifuged at 4000 rpm for 2 minutes and plasma removed. Plasma samples were frozen at −20°C until assayed.
A further 25 µl blood was collected onto a 226 Spot Saver RUO Card (PerkinElmer, UK). Samples were left to dry for at least 1 hour at room temperature, then 6 mm spots were punched from the card and placed in sealable plastic bags with desiccant.
Either 10 µl or 20 µl of blood was also collected onto Mitra® VAMS devices (Neoteryx, Torrance, CA, USA), by placing the sampling tip to the blood sample and allowing the tip to fully turn red. Following this, the device was transferred to a plastic “clamshell” storage package and left to dry at room temperature overnight.
Blood Extraction
DBS
The 6 mm spots punched from the DBS card were added directly to the assay microplate wells. No extraction was carried out beforehand.
VAMS Device
Prior to assay, the VAMS tips were removed from the device and placed into a mini spin column (EZ-10, Biobasic, Canada) at room temperature in either 140 µl antibody conjugate (chemiluminescence assay) or 110 µl assay buffer (ELISA). Assay method details are given below. Spin columns were incubated at room temperature while shaking (900 rpm) for 1 hour and then centrifuged at 13 000 rpm for 3 minutes. The supernatant was transferred into 1.5 ml microtubes until assayed.
Assay Protocols
The reference method of determining C-peptide was a sensitive chemiluminescence assay (IV2-004, Invitron, UK). To establish if the extraction method was transferable to alternative assay formats, an ELISA (10-1136-01, Mercodia, Sweden) was also performed on a smaller number of samples.
Chemiluminescence Assay
Manufacturer’s protocol was followed for measuring from both plasma and DBS. Briefly, 100 µl labelled antibody (a soluble antibody labelled with a chemiluminescent acridinium ester) was added to each well followed by either 25 µl standard, quality control, plasma sample, or a single DBS. For measuring from the VAMS device, a slight adaptation to this protocol was made; 125 µl extracted blood in antibody conjugate (extraction described above) was added directly to the assay well.
The plate was then incubated at 37°C for 2 hours, washed and read immediately on a plate luminometer (Berthold Centro LB960).
ELISA
Manufacturer’s protocol was followed for measuring plasma and DBS; 25 µl standard, quality control, plasma sample, or a single DBS was added to each well followed by 50 µl assay buffer. For measuring from the VAMS device, 75 µl extracted blood in assay buffer (as described above) was added. The plate was then incubated at room temperature on a shaker for an hour and washed. Antibody conjugate (100 µl) was then added to all assay wells, incubated for a further hour at room temperature on a shaker before being washed. TMB (200 µl) was added to the assay wells and incubated for 15 minutes at room temperature before adding 50 µl stop solution. The plate was read immediately on a Multiskan™ GO Microplate Spectrophotometer (Thermo, UK) at 450 nm.
MikroWin 2000 curve fitting software (MikroWin ver. 4.29, Mikrotek Laborsysteme GmbH) was used to obtain C-peptide concentrations from luminometer or spectrophotometer outputs.
Plasma Equivalent Correction Factors
A correction factor (CF) for extracted samples was employed to give plasma equivalent values to account for the use of whole blood and for differences in blood volumes used in the assays.
DBS
Due to the nature of DBS, a variable volume of blood may be collected so a consistent theoretical equation could not be calculated. Analysis via regression equation comparing plasma and DBS gave a plasma equivalent CF of 4.3.
VAMS
The following factors were taken into consideration when calculating the CF for VAMS: Use of whole blood (*0.5 for plasma); VAMS sample volume versus 25 µl plasma assay volume (*0.4 [10 µl VAMS] or *0.8 [20 µl VAMS]); Volume of total extracted sample added to assay well (*0.89 [125 ul of 140 ul in chemiluminescence assay] or *0.68 [75 µl of 110 µl in ELISA]). This generated a theoretical plasma equivalent CF of 5.6 and 2.8 for the chemiluminescence assay (10 µl and 20 µl VAMS, respectively) and 7.35 and 3.67 for the ELISA (10 µl and 20 µl VAMS, respectively). Theoretical CFs were confirmed using linear regression equation comparing VAMS and plasma results.
Statistical Analysis
Linear regression analysis was used to evaluate the relationship between plasma and DBS or VAMS C-peptide levels. Comparison of blood collection methods against the plasma reference, assayed using the chemiluminescence assay was displayed using Bland-Altman plots and analyzed using Passing-Bablok analysis to identify any constant or proportional bias.
Results
Plasma Comparison
The relationship between plasma C-peptide concentrations and plasma equivalent DBS and VAMS concentrations, measured using the chemiluminescence assay is shown in Figure 1. Good overall agreement was observed between plasma and plasma equivalent C-peptide concentrations collected using either DBS (R2 = .929, P < .0001; y = 1.1468x – 133.55) or VAMS (R2 = .9231, P = .0001; y = 1.0024x – 10.198). No constant or proportional bias was observed.
Figure 1.
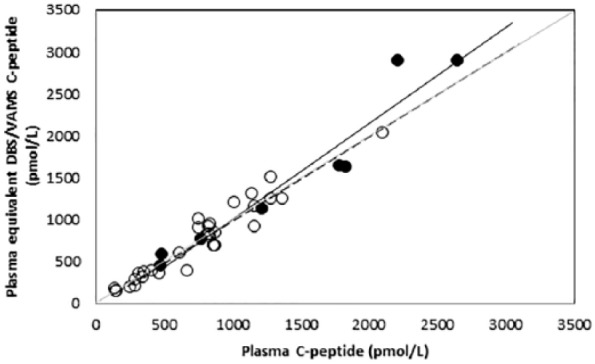
Relationship between plasma C-peptide and DBS (closed circles, solid regression line) or VAMS (open circles, dashed regression line) plasma equivalent C-peptide concentrations measured in the chemiluminescence assay.
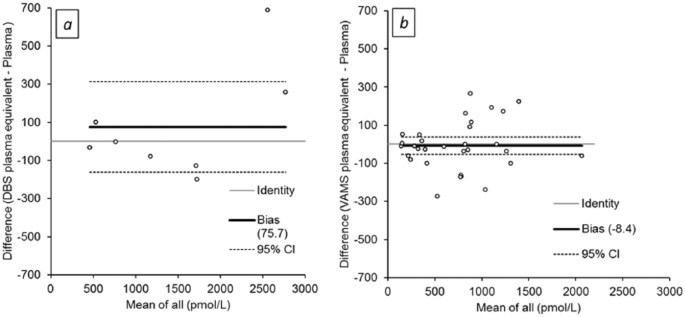
Difference (Bland-Altman) plots for plasma and plasma equivalent C-peptide concentrations are shown in Figure 2. Mean differences of 75.7 pmol/L (2.8%) and −8.4 pmol/L (–3.0%) were observed for DBS and VAMS, respectively.
Figure 2.
Difference plots for plasma C-peptide versus plasma equivalent (a) DBS and (b) VAMS C-peptide concentrations assayed using chemiluminescence assay.
Stability
Stability at room temperature was determined by collecting two DBS and two VAMS samples per subject. One sample of each collection method was stored at −20°C immediately (baseline), while a second sample was stored at room temperature for 48 hours, and then stored at −20°C until assay. After allowing samples to reach room temperature, all were extracted and analyzed in the same assay. Mean (SD) baseline C-peptide concentrations of 992 (747) pmol/L and 1280 (1459) pmol/L were observed for DBS and VAMS respectively. Decreases of 11.6% and 0.1% were observed after 48 hours storage at room temperature for DBS and VAMS respectively.
Reproducibility
DBS and VAMS finger prick blood samples (n = 2, collected in triplicate) were collected and dried overnight, before extraction and analysis in a single assay. Mean coefficients of variation of 26.4% and 4.3% were observed for DBS and VAMS, respectively.
Discussion
The objective of this pilot study was to evaluate whether capillary blood samples collected using either dried blood spots or a novel volumetric absorptive microsampling device can provide an accurate measurement of C-peptide compared to a reference plasma sample. Both microsampling methods offer greater convenience over venous samples; samples can be transported without dry ice or biohazard needs, do not require immediate sample processing upon collection and use a direct sampling approach via finger prick, eliminating the need for a nurse or phlebotomist. Previous bio-analytical studies measuring alternative analytes show VAMS (Mitra devices) perform better than conventional DBS sampling in terms of stability and reproducibility.9,10
We identified that both DBS and VAMS showed good agreement in the measurement of C-peptide when compared with their corresponding plasma results with little or no bias; supporting work by Willemsen et al, who have previously shown a good correlation (R = .95) between plasma C-peptide and levels of C-peptide from samples collected by DBS.8
Microsampling has the advantage of being able to be collected at home and transported to a lab through the postal system; therefore stability of the analyte of interest at ambient temperature is critical to its usefulness. Numerous studies have shown C-peptide to be stable when collected using DBS for up to 7 days when stored at room temperature, with Johansson et al identifying that C-peptide was stable for 6 months on DBS in these conditions.1,11,12 No work has yet been published on C-peptide stability on VAMS devices specifically, however, in a small number of samples we identified that C-peptide collected using VAMS devices proved to be more stable than samples collected using DBS, with C-peptide levels remaining constant in VAMS after 48 hours storage at room temperature compared to a drop of 11% for DBS. This difference could potentially be in part be due to the greater variation observed with DBS.
We found that the VAMS device gave more reproducible C-peptide results than DBS, with a mean CV% of 4.3% compared to 26.4%, when tested in triplicate on the same samples. This could be due to the more accurate absorption of the VAMS device, independent of hematocrit levels, compared to the potential variable volume collection of DBS. The ease of use of the Mitra could also play a role in the accuracy of collection and reduce the need for repeat sampling.
The main limitation of this study is the relatively small sample size. Although the sample numbers analyzed were less than would be recommended by guidelines, the encouraging results would justify performing a full method comparison study.
Conclusions
In this small pilot study, measurement of C-peptide in capillary blood collected onto DBS and a novel VAMS device, showed good agreement with a reference plasma C-peptide concentration. The VAMS device showed less bias, albeit in a larger number of samples, was more reproducible and displayed better stability than corresponding DBS collected samples. Both DBS and VAMS show promise as an effective method for the convenient collection of samples in the measurement of C-peptide, and the findings warrant further analysis in a larger, more formal method comparison study.
Footnotes
Abbreviations: CF, correction factor; CV, coefficient of variation; DBS, dried blood spot; ELISA, enzyme linked immunosorbent assay; VAMS, volumetric absorptive microsampling.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Johansson J, Becker C, Persson N, Fex M, Torn C. C-peptide in dried blood spots. Scand J Clin Lab Invest. 2010;70(6):404-409. [DOI] [PubMed] [Google Scholar]
- 2. Denniff P, Spooner N. Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Anal Chem. 2014;86(16):8489-8495. [DOI] [PubMed] [Google Scholar]
- 3. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. 2013;30(7):803-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leighton E, Sainsbury CAR, Jones GC. A practical review of C-peptide testing in diabetes. Diabetes Ther. 2017;8(3):475-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steiner DF, Cunnignham D, Spigelman L, Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967;157(3789):697-700. [DOI] [PubMed] [Google Scholar]
- 6. Thunander M, Petersson C, Jonzon K, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82(2):247-255. [DOI] [PubMed] [Google Scholar]
- 7. Gjessing HJ, Matzen LE, Faber OK, Froland A. Fasting plasma C-peptide, glucagon stimulated plasma C-peptide, and urinary C-peptide in relation to clinical type of diabetes. Diabetologia. 1989;32(5);305-311. [DOI] [PubMed] [Google Scholar]
- 8. Willemsen R, Burling K, Ackland F, et al. Evaluation of a novel method to detect residual β-cell function by dried blood spots in children and adolescent with a recent diagnosis of type 1 diabetes. ESPE Abstracts. 2016;86:FC95. [Google Scholar]
- 9. Luo Y, Korfmacher W, Ho S, et al. Evaluation of two blood microsampling approaches for drug discovery PK studies in rats. Bioanalysis. 2015;7(18): 2345-2359. [DOI] [PubMed] [Google Scholar]
- 10. Kip A, Kiers KC, Rosing H, Schellems JHM, Beijnen JH, Dorlo TPC. Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J Pharm Biomed Anal. 2017;135:160-166. [DOI] [PubMed] [Google Scholar]
- 11. Lehmann S, Delaby C, Vialaret J, Ducos J, Hirtz C. Current and future use of “dried blood spot” analyses in clinical chemistry. Clin Chem Lab Med. 2013;51(10):1897-1909. [DOI] [PubMed] [Google Scholar]
- 12. Prentice P, Turner C, Wong MCY, Dalton RN. Stability of metabolites in dried blood spots stored at different temperatures over a 2-year period. Bioanalysis. 2013;5(12):1507-1514. [DOI] [PubMed] [Google Scholar]