Abstract
Background:
Emerging therapies such as closed-loop (CL) glucose control, also known as artificial pancreas (AP) systems, have shown significant improvement in type 1 diabetes mellitus (T1DM) management. However, demanding patient intervention is still required, particularly at meal times. To reduce treatment burden, the automatic regulation of glucose (ARG) algorithm mitigates postprandial glucose excursions without feedforward insulin boluses. This work assesses feasibility of this new strategy in a clinical trial.
Methods:
A 36-hour pilot study was performed on five T1DM subjects to validate the ARG algorithm. Subjects wore a subcutaneous continuous glucose monitor (CGM) and an insulin pump. Insulin delivery was solely commanded by the ARG algorithm, without premeal insulin boluses. This was the first clinical trial in Latin America to validate an AP controller.
Results:
For the total 36-hour period, results were as follows: average time of CGM readings in range 70-250 mg/dl: 88.6%, in range 70-180 mg/dl: 74.7%, <70 mg/dl: 5.8%, and <50 mg/dl: 0.8%. Results improved analyzing the final 15-hour period of this trial. In that case, the time spent in range was 70-250 mg/dl: 94.7%, in range 70-180 mg/dl: 82.6%, <70 mg/dl: 4.1%, and <50 mg/dl: 0.2%. During the last night the time spent in range was 70-250 mg/dl: 95%, in range 70-180 mg/dl: 87.7%, <70 mg/dl: 5.0%, and <50 mg/dl: 0.0%. No severe hypoglycemia occurred. No serious adverse events were reported.
Conclusions:
The ARG algorithm was successfully validated in a pilot clinical trial, encouraging further tests with a larger number of patients and in outpatient settings.
Keywords: artificial pancreas, carbohydrate counting, clinical trial, sliding mode control, switched control
Type 1 diabetes mellitus (T1DM) is a chronic disease characterized by the destruction of the pancreatic β-cells, resulting in insulin deficiency. Typical treatments require patient interaction with glucose sensors and insulin pumps, and as consequence, are burdensome and subject to human errors.1 Ideally, a closed-loop (CL) approach provides the patient with a more autonomous, and therefore, improved quality of life. This is the foundation for the development of an artificial pancreas (AP).
The AP project is a worldwide very active development.2-6 It consists of a glucose sensor and an insulin pump connected through a control algorithm. The control algorithm commands the pump to deliver the corresponding amount of insulin to take the patient into normoglycemia. Although AP systems have shown improved glycemic control in T1DM, glucose regulation during postprandial and exercise periods remains a challenge.7-9 It has been essential for gaining momentum in designing and testing glucose controllers, the development of elaborated simulators, such as the Universities of Virginia/Padova metabolic simulator that was accepted by the US Food and Drug Administration (FDA) in lieu of animal trials.10-12
Various infusion and sensing routes are possible,13,14 however, most AP systems are based on subcutaneous (SC) glucose sensing and insulin infusion. That yields to a minimally invasive AP scheme, but affects significantly the controller performance, because large lag-times in glucose measurement and insulin action are introduced.15,16
Several single- and dual-hormone control strategies have been designed for glucose control in T1DM. Most of them are based on proportional-integral-derivative (PID), model predictive control (MPC), and fuzzy logic controllers. Generally, they are arranged into a semiautomatic framework, where feedforward insulin boluses are applied at meal times. The current objective is the validation of these algorithms in patients during normal life conditions. To that end, multiple clinical trials were performed in several countries of the EU, United States, Israel, and Australia.17-36 Robust and linear parameter-varying (LPV) controllers have also been developed, but tested only in silico.37-42 To date, the only commercially available system that automates insulin delivery is the hybrid CL Medtronic MiniMed 670G system (Medtronic, Minneapolis, MN), which is based on a PID controller.26 Clinical trials without meal announcements have also been performed.43-51 There, the main challenge is to mitigate postprandial hyper-and hypoglycemia, because no proactive insulin adjustments are commanded as with the hybrid strategy. In a fully feedback scheme, the control signal is only reactive, and consequently there is a higher risk of initial hyperglycemia and late hypoglycemia during the postprandial period.
Increase in incidence and prevalence of T1DM in Latin America is similar to the worldwide trend.52-54 In this work, we present the first clinical trial in this region to validate a glucose controller, the so-called automatic regulation of glucose (ARG) algorithm. The main characteristic of the ARG algorithm is that it automatically adjusts the glucose concentration without requiring premeal insulin boluses. First, the algorithm was coded in Matlab and rigorously tested in silico on the complete adult cohort of the UVA/Padova simulator.55 Then, it was implemented in Java and migrated to the Diabetes Assistant (DiAs) system developed by UVA.56 The modular architecture of the DiAs facilitated the implementation process that also involved a redesign of the meal user-interface. Finally, the controller was validated in a pilot CL clinical study on five T1DM subjects.
Materials and Methods
Description of the CL System’s Components
The AP scheme employed in this study is depicted in Figure 1. Glucose concentration was measured by a Dexcom G4 sensor (Dexcom, San Diego, CA). Every 5 minutes, a new reading was transmitted using Bluetooth low energy (BLE) to a Google Nexus 5 smartphone (LG Electronics, Seoul, South Korea) within the UVA DiAs platform. Then, a new insulin dose was computed by the ARG algorithm, and transmitted to a Roche Accu-Chek Spirit Combo insulin pump (Roche, Diagnostics, Mannheim, Germany) using classic Bluetooth. Finally, encrypted AP data were sent in real time to a remote server via Wi-Fi using the DiAs Web Monitoring (DWM) system.57
Figure 1.

Schematic of the AP system.
ARG Algorithm
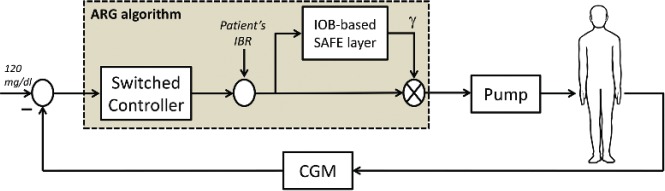
The ARG control algorithm consists of a switched linear quadratic Gaussian (LQG) controller combined with a sliding-mode safety layer to include insulin on board (IOB) constraints. This control structure not only aims a good glucose control, but also a reduction of patient intervention by commanding the insulin infusion without the need of feedforward insulin boluses. The block diagram is depicted in Figure 2.
Figure 2.
Block diagram of the ARG algorithm tested on the clinical study.
The switched LQG controller is based on the Youla parametrization approach.58 Here, two LQG controllers are switched to have different responses to fasting and prandial periods. One LQG controller is conservative and performs slight changes on the patient’s insulin basal rate (IBR). The other one is aggressive and is selected when higher insulin doses are needed, for example, at meal times. The safety auxiliary feedback element (SAFE) block quickly adapts (reduces) the insulin infusion when a constraint on the active insulin is reached. The pharmacokinetic IOB model developed by Willinska et al59 has been used in this work to estimate the IOB value, because it is an easy-to-tune model. However, any other IOB model could be employed with the SAFE mechanism, including those describing insulin pharmacodynamics. In addition, it is worth remarking that this version of the SAFE mechanism restricts only the maximum IOB value, but other strategies that provide a time-varying IOB constraint have already been explored by some of the authors.60
To make the ARG algorithm more robust against factors affecting the glucose level, two auxiliary modules based on the SAFE block have been included. One lowers the IOB limit when low glucose values are detected or predicted (hypo-related module), and the other generates a correction bolus when a persistent hyperglycemia cannot be mitigated by the conservative controller (hyper-related module).
The SAFE block, apart from safety issues, allows generating an insulin spike in a CL manner. In this way, the response of the controller can be shaped to obtain both slight and sharp changes in the insulin infusion rate. Although the controller will tend to operate at its safety limits when it is in the aggressive mode to generate the insulin spike, this does not imply that the postprandial glucose response only depends on the value of IOB constraint. The latter is simply an upper bound to the amount of insulin to be injected. In a previous work,55 it was shown that the ARG algorithm presents robustness against errors in setting the IOB constraint.
To switch between the conservative and aggressive modes, any meal detection algorithm can be used. However, a cautious approach was followed during this pilot trial. Here, the patient had to announce the meal time and select the meal size from three possible sizes (small, medium or large) by pushing a button. Thus, the DiAs system was alerted that a meal could be ingested. That announcement did not generate any meal-related insulin bolus. It only triggered the control algorithm to a listening mode. During that mode, the continuous glucose monitoring (CGM) trend was analyzed for 90 minutes at most, and the aggressive mode was selected only if rising glucose values were detected.
The purpose of the listening mode is to avoid false positives when the patient delays his/her meal or even forgets to eat. In this first trial, the tolerance to switch from the listening to the aggressive mode was defined as a minimum increase of 2 mg/dl of the glucose level per sample during the last 4 samples. This low tolerance helped to rapidly generate an insulin spike, facilitating the postprandial control. However, through analyzing the results, it was detected that there was margin to increase this tolerance without affecting the controller response. This suggests that the commutation condition can be relaxed, making the control algorithm be more robust to real life situations. Even if the glucose concentration was high due to a late notification of the meal, the controller is likely to commute to the aggressive mode. This notification has to occur late enough (hours approximately) not to detect an increase in the glucose level. Moreover, if there is a late notification and the conservative mode cannot correctly mitigate the glucose excursion, the hyper-related module will generate a correction bolus to reduce the postprandial glucose excursion.
When the aggressive mode was triggered, the controller became more sensitive to changes in glucose values, and the IOB limit was adjusted according to the selected meal size. The aggressive mode commanded the insulin infusion during an hour, and afterward, the ARG algorithm was automatically triggered to the conservative mode again. Note that in Figure 2, a 120 mg/dl target is defined for all subjects. However, it is not intended to be an exact target as in a tracking problem. In this case, the controller has no integral action to avoid insulin stacking, and as a consequence, there is a certain tolerance with respect to the desired target. In addition, it is worth mentioning that such tolerance is also in part regulated by the two auxiliary hypo- and hyper-related modules of the ARG algorithm that assist in reducing the insulin infusion or generating correction boluses when necessary.
To cover the interpatient uncertainty, the ARG algorithm is tuned using a priori clinical information that can easily be obtained: the patient’s total daily insulin (TDI) (U) of the previous week, carbohydrate/insulin ratio (CR) (gCHO/U), body weight (kg), and correction factor (CF) (mg/dl/U). Further details and formalisms of this control strategy can be found in Colmegna et al.55
Study Design and Participants
Main eligibility criteria included clinical diagnosis of T1DM ≥ 2 years; use of insulin pump and CGM sensor for ≥6 months; age 18-65 years; and HbA1c < 10%. Main exclusion criteria included diabetic ketoacidosis (DKA) within last 12 months; severe hypoglycemia with loss of consciousness within last 12 months; and cardiac condition.
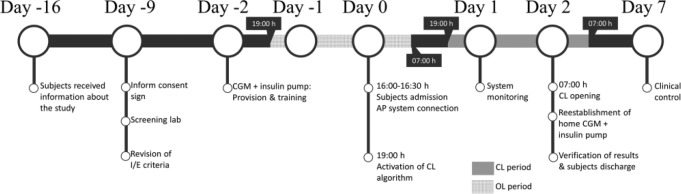
The timeline of the protocol is presented in Figure 3. Two days before the inpatient trial, each subject was fitted with a Dexcom CGM sensor and a Roche insulin pump to become acquainted with the study devices. They continued with their usual care at home until the day of the CL study. During that time their diabetes data was gathered at baseline. On admission day, patients met the study team at the Hospital Italiano de Buenos Aires (HIBA) at 16:00-16:30 h without previous restrictions on diet. Screening tests were performed, selection criteria was reevaluated, and the DiAs system was paired with the glucose sensor and insulin pump. After 19:00 h, the CL system was activated. Five standardized meals were served during the study: one breakfast, one lunch, one afternoon snack, and two dinners. Breakfast and afternoon snack were a cup of tea or coffee, two slices of whole-meal bread or five crackers, diet jam, and spreadable cheese (≈28 gCHO). Dinners consisted of whole pasta, lean meat, and fresh fruit (≈55 gCHO). Lunch was the same as dinners, but with mashed potatoes instead of whole pasta (≈55 gCHO). Before each meal, subjects had to inform the meal size to the DiAs system as small (<35 gCHO), medium (35-65 gCHO), or large (>65 gCHO). During the study, each patient had a capillary glucose measurement at least 7 times/day, and CGM calibrations were performed according to the manufacturer recommendation in situations of stable glycemia. Patients were remotely monitored by the team, and treated with a 15 g of rescue carbohydrates when their blood glucose levels dropped below 70 mg/dl. After 36 hours, the loop was opened (at 07:00 h), concluding the trial. Then, participants were fitted with their home devices, and invited to have breakfast. After verifying the results and performing a clinical evaluation, subjects were discharged.
Figure 3.
Timeline of the protocol.
The protocol was approved by the ethics committee of the HIBA and the Argentine Health Agency (ANMAT).
Outcomes
The purpose of this supervised clinical study was to obtain clinical validation of the ARG algorithm by testing its ability to maintain the patient’s glucose concentration in a safe range for 36 hours without feedforward insulin boluses. The a priori condition for the ARG controller to be verified was that the system works properly in terms of hardware communication and glucose control at least 80% of the total connection time. Secondary outcomes were percentage of total time within the desirable glycemia range (70-180 mg/dl); percentage of total time within the acceptable glycemia range (70-250 mg/dl); percentage of total time in hypoglycemia (<70 mg/dl); percentage of total time in hyperglycemia (>180 mg/dl); number of mild (50-69 mg/dl) and severe (<50 mg/dl) hypoglycemic episodes; and comparison of the glycemic registries obtained during the trial with the registries before hospitalization.
Results
Five T1DM patients (three women and two men) were enrolled for this study according to the inclusion and exclusion criteria previously defined. The mean ± SD age was 43 ± 6 years, TDI was 38.1 ± 13.5 U, HbA1c was 7.4 ± 0.7 %, weight was 65.3 ± 15.8 kg, and duration of diabetes was 19 ± 5 years (see Table 1 for a summary of the demographic data).
Table 1.
Patient Demographics.
| Patient # | Sex | Age (years) | TDI (u) | HbA1c (%) | Weight (kg) | DD (years) |
|---|---|---|---|---|---|---|
| 54112 | F | 48 | 23.5 | 8.1 | 48.0 | 18 |
| 54113 | M | 44 | 58.7 | 8.1 | 91.0 | 26 |
| 54114 | F | 32 | 30.3 | 6.5 | 60.5 | 14 |
| 54115 | M | 45 | 34.8 | 7.3 | 61.5 | 16 |
| 54116 | F | 45 | 43.0 | 6.8 | 65.5 | 23 |
| Mean ± SD | 43 ± 6 | 38.1 ± 13.5 | 7.4 ± 0.7 | 65.3 ± 15.8 | 19 ± 5 | |
Because the main objective of this pilot clinical trial was to validate the ARG control algorithm, the protocol defined for the CL period was not strictly followed during the 36 hours of usual care (OL, open loop). While patients were instructed to follow similar food choices between the OL and CL period, we were unable to track patients’ activities at home. Therefore, this study lacks the rigor of a comparison of identical settings for both periods. However, to illustrate how the usual glucose control of the enrolled T1DM patients is, data registered prior to the CL test are also presented in this section. Patients were not subject to physical activity during this CL study, but they were not bedridden, but instead allowed to walk around freely when desired.
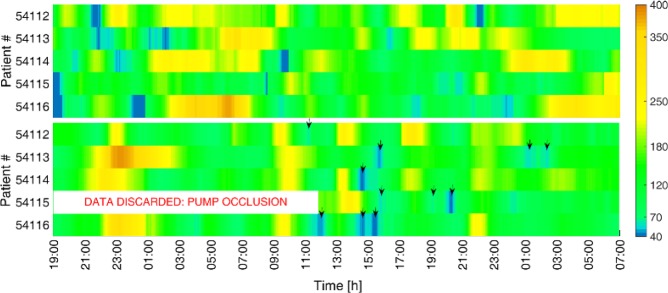
The CGM measurements for all five patients during the OL and CL periods are presented in Figure 4. There, it is shown that most of the carb rescues during the CL period occurred after having lunch (approximately at 13:00 h). After the first dinner, a tendency toward hyperglycemia was detected using the standard IOB limit. Therefore, it was increased for the following breakfast and lunch as a way to prevent high postprandial glucose excursions. However, this two significant and consecutive increases in the IOB constraint lead to insulin stacking and as a consequence, to hypoglycemic episodes around lunch. We consider that if the standard IOB limit were maintained, these hypoglycemic events would not have occurred. Indeed, the standard IOB limit was not changed for the other meals, and adequate postprandial glucose control was achieved. In addition, note that readings for patient 54115 in CL were discarded during the first night and the following morning due to pump occlusion. Despite that inconvenience, the ARG algorithm commanded the insulin infusion during the 90.3% of time in CL, and 99.6% if the time interval associated with the pump occlusion is not considered.
Figure 4.
CGM readings for all the five patients during 36 hours in OL following a standard basal-bolus therapy (above) and in CL using the ARG algorithm (below). Down arrows indicate the carb treatments.
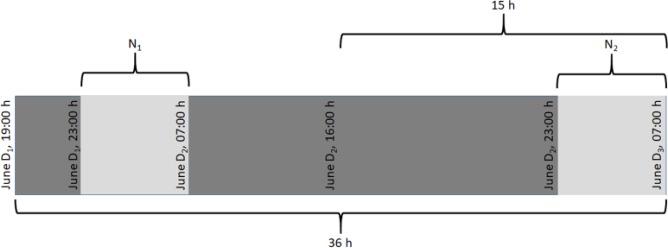
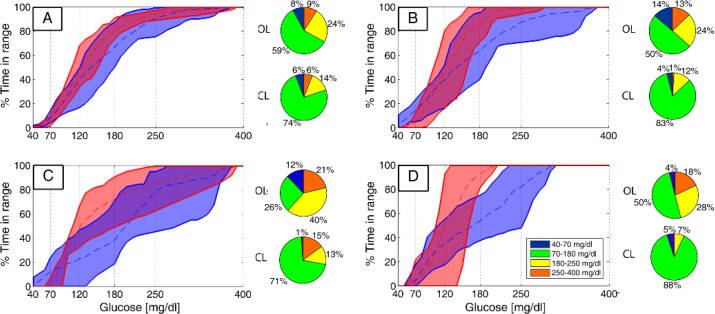
The average results are presented in Table 2, considering the time periods defined in Figure 5. As shown in Table 2, a mean low blood glucose index (LBGI) ≤ 2.5 (low risk of hypoglycemia)61 was achieved with the ARG algorithm. In addition, the overall percentage time in range < 70 mg/dl were 5.8% and 4.1% for the 36-hour and 15-hour periods, respectively, and 1.0% and 5.0% for the nights N1 and N2, respectively. On the other hand, the control strategy yielded a mean high blood glucose index (HBGI) ≤ 4.5 (low risk of hyperglycemia)61 for the 15-hour period and night N2, and a mean HBGI ≤ 9 (moderate risk)61 for the 36-hour period and night N1. Furthermore, the mean percentage time in range > 180 mg/dl was 19.5% for the 36-hour period, 13.3% for the 15-hour period, and 27.9% and 7.3% for the nights N1 and N2, respectively. In terms of the proportion of time in the desired and acceptable glycemia ranges during the whole 36-hour period, CL insulin delivery yielded a mean percentage time in range 70-250 mg/dl of 88.6%, and in range 70-180 mg/dl of 74.7%. Higher increases in the mean percentage time in ranges 70-250 mg/dl (94.7%) and 70-180 mg/dl (82.6%) were obtained during the 15-hour period. Note that there was a trend toward less hyperglycemia over the course of the CL study, while low risk of hypoglycemia was achieved at all time intervals.
Table 2.
Average Clinical Results in OL and in CL.
| 36 hoursa | OL |
CL |
|||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | P valueb | |
| Glucose (mg/dl) | 153 | [132, 175] | 138 | [119, 156] | 0.120 |
| % time in (70, 250) mg/dl | 82.9 | [67.3, 98.6] | 88.6 | [82.4, 94.7] | 0.315 |
| % time in (70, 180) mg/dl | 59.1 | [41.9, 76.2] | 74.7 | [68.1. 81.4] | 0.036 |
| % time <70 mg/dl | 7.6 | [2.9, 12.4] | 5.8 | [1.6, 10.0] | 0.290 |
| % time >180 mg/dl | 33.3 | [16.6, 50.0] | 19.5 | [10.6, 28.4] | 0.027 |
| % time <50 mg/dl | 1.7 | [0.3, 3.1] | 0.8 | [0.2, 3.5] | 0.190 |
| LBGIc | 2.8 | [1.8, 3.7] | 2.3 | [1.4, 3.1] | 0.214 |
| HBGI | 7.2 | [3.4, 11.0] | 4.9 | [2.9, 6.9] | 0.182 |
| 15 hoursa | |||||
| Glucose (mg/dl) | 156 | [125, 188] | 129 | [102, 157] | 0.057 |
| % time in (70, 250) mg/dl | 73.5 | [49.8, 97.2] | 94.7 | [83.8, 98.4] | 0.083 |
| % time in (70, 180) mg/dl | 49.8 | [24.5, 75.1] | 82.6 | [69.9, 95.2] | 0.014 |
| % time <70 mg/dl | 13.6 | [4.4, 22.7] | 4.1 | [0.8, 18.0] | 0.049 |
| % time >180 mg/dl | 36.6 | [11.5, 61.7] | 13.3 | [3.6, 38.9] | 0.012 |
| % time <50 mg/dl | 5.4 | [1.6, 16.4] | 0.2 | [0.0, 3.5] | 0.083 |
| LBGIc | 4.2 | [2.1, 6.2] | 1.8 | [0.3, 3.3] | 0.038 |
| HBGI | 8.7 | [2.9, 14.5] | 2.8 | [0.1, 5.5] | 0.047 |
| N1a | |||||
| Glucose (mg/dl) | 196 | [133, 258] | 155 | [90, 220] | 0.336 |
| % time in (70, 250) mg/dl | 66.8 | [23.6, 92.9] | 84.4 | [37.2, 98.0] | 0.176 |
| % time in (70, 180) mg/dl | 26.4 | [2.3, 50.5] | 71.1 | [36.9, 91.2] | 0.072 |
| % time <70 mg/dl | 12.0 | [3.2, 35.9] | 1.0 | [0.0, 20.8] | 0.104 |
| % time >180 mg/dl | 61.6 | [25.0, 98.3] | 27.9 | [8.4, 61.8] | 0.198 |
| % time <50 mg/dl | 3.4 | [0.4, 23.6] | 0.0 | [0.0, 0.0] | 0.226 |
| LBGIc | 3.1 | [0.8, 7.1] | 1.6 | [0.5, 2.7] | 0.337 |
| HBGI | 14.8 | [2.5, 27.0] | 8.1 | [0.0, 10.0] | 0.346 |
| N2a | |||||
| Glucose (mg/dl) | 169 | [128, 210] | 125 | [94, 156] | 0.033 |
| % time in (70, 250) mg/dl | 78.1 | [29.1, 96.9] | 95 | [66.9, 99.4] | 0.341 |
| % time in (70, 180) mg/dl | 50.3 | [23.2, 77.4] | 87.7 | [76.5, 99.0] | 0.035 |
| % time <70 mg/dl | 3.6 | [0.3, 29.5] | 5.0 | [0.6, 33.1] | 0.821 |
| % time >180 mg/dl | 46.1 | [23.7, 68.4] | 7.3 | [1.2, 33.0] | 0.004 |
| % time <50 mg/dl | 0.0 | [0.0, 0.0] | 0.0 | [0.0, 0.0] | – |
| LBGIc | 2.0 | [0.6, 3.4] | 1.5 | [0.4, 4.1] | 0.471 |
| HBGI | 9.8 | [2.8, 16.8] | 1.9 | [0.4, 5.7] | 0.031 |
The 36-hour and 15-hour periods and the nights (N1 and N2) are analyzed separately.
Statistically significant at P < .05.
LBGI adapted to the characteristics of Dexcom G4 CGM system.62
Figure 5.
Definition of the time periods used for result analysis (36 hours is the overall time interval). D1 = 21, D2 = 22, and D3 = 23 for the OL period; D1 = 23, D2 = 24, and D3 = 25 for the CL period.
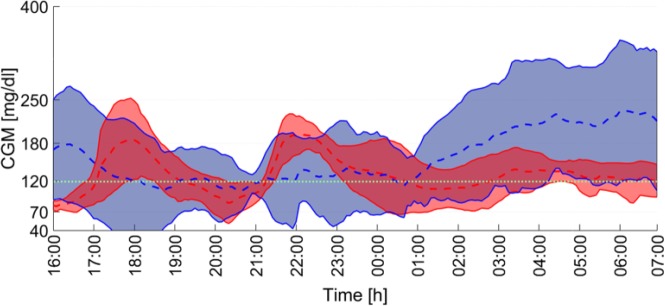
Regarding the overnight control, where a comparison between the CL and OL strategies may be performed, the increase in the mean percentage time in range 70-250 mg/dl during the night N1 was 17.6% (P = .176) and in range 70-180 mg/dl was 44.7% (P = .072). Similar results are obtained when the night N2 is analyzed. In that case, the increase in the mean percentage time in range 70-250 mg/dl was 16.9% (P = .341) and in range 70-180 mg/dl was 37.4% (P = .035). To illustrate this analysis, the average, the minimum and the maximum cumulative time in range are presented in Figure 6, and the average time responses in OL and CL for the 15-hour period are depicted in Figure 7. It is worth noting that the average glucose concentration at 07:00 h of day 2 (when the CL was opened) was 120 mg/dl, which coincides exactly with the CL setpoint (see Figure 2).
Figure 6.
Cumulative time in range for all the five patients in OL (blue) and in CL (red) for the 36-hour (A) and 15-hour periods (B), and the nights N1 (C) and N2 (D). The dashed lines are the mean values, and the continuous lines are the envelopes.
Figure 7.
Average CGM readings for all the five patients in OL (blue) and in CL (red) during the 15-hour period. The filled areas represent ±1 SD. The dotted green line indicates the glycemic control target.
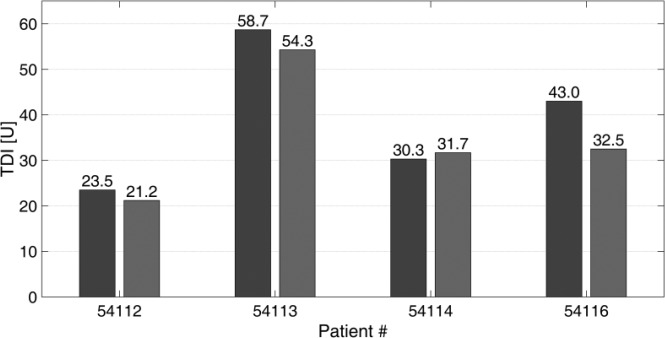
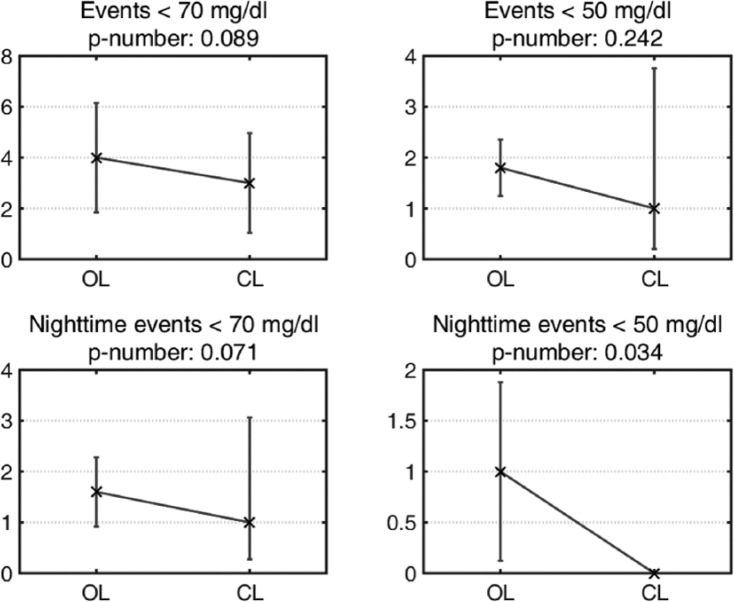
Data for the patients’ TDI amounts are illustrated in Figure 8, where OL TDI correspond to an average of the values obtained during the trial’s previous week. There was no significant difference in the mean TDI in CL compared with the one obtained in OL (P = .1227). An analysis of the hypoglycemic events detected using CGM measurements is presented in Figure 9. There were fewer hypoglycemic events using the ARG compared with the usual OL treatment. During the CL period, none of the CGM readings were below 50 mg/dl, and 80% of the CGM readings below 70 mg/dl were confirmed with a reference glucose measurement. No severe episodes of hypoglycemia or serious adverse events were reported.
Figure 8.
Patients’ TDI obtained from biometric data before the CL clinical study (dark gray bars), and patients’ TDI obtained considering the last 24 hours of the CL clinical study (light gray bars). Patient 54115 is not included here because he or she did not complete 24 hours in CL.
Figure 9.
Hypoglycemic events registered by the CGM sensor during the 36-hour time interval. The mean values are represented by crosses, and the 95% confidence intervals are represented by vertical bars. Statistically significant at P < .05.
It is worth remarking that the comparison between CL and OL results presented here was only for illustrative purposes. Further research with a larger sample size and randomized crossover conditions are needed for conclusions.
Discussion
The first clinical trials using an AP system in Latin America were defined in two stages. The first stage was carried out in November 2016. There, the UVA hybrid CL system63 was used for training purposes.64 Here, the second stage to validate the ARG algorithm was presented.
There are numerous studies and reviews on hybrid AP, but few of them are based on algorithms without premeal insulin boluses. Postprandial glucose control is a major challenge using a fully CL AP system. Therefore, in this first-ever test of the ARG controller the meal time and a classification of the meal size (small, medium, or large) were informed to the controller. The use of a meal size classification can be found in other works as well.32,65,66 Here, it was used to adjust the IOB limit via the subject’s CR, but not to generate a meal-related insulin bolus. The higher the IOB limit is set, the less restrictive the safety layer becomes. If a single standard meal size were used to define the IOB limit for every meal, this limit might be too high for small meals or too low for large meals. Thus, this three-meal-size classification scheme added an extra degree of freedom to the ARG algorithm, allowing to reduce the uncertainty in the IOB constraint. In a next stage, this constraint could be automatically adapted based on a carbohydrate estimation algorithm.67
It is worth noting that the ARG algorithm was personalized based solely on a priori clinical information that was easily obtainable, that is, participants were not involved in any previous tuning or identification process. In addition, the computational burden was low. The synthesis and tuning of the controller was performed offline. Then, the personalized controller was migrated to the Nexus 5 smartphone (2.26 GHz quad-core processor) within the DiAs system. Every 5 minutes the main tasks were to update the 13-state switched LQG controller and the two-state IOB model. The state space realization of both the conservative and aggressive LQG controllers were the same, except for a submatrix of the C-matrix that was the only element that changed at switching times. Consequently, the controller’s state was never reset, being always consistent with the estimation of the T1DM model’s state. Regarding the IOB model, it was implemented in the SAFE block, which was based on sliding mode conditioning concepts. Therefore, a smaller sampling time of 0.1 minutes was defined for that block. The 0.1-minute sampling time did not represent a problem during the implementation, because the SAFE algorithm was completely software-based. Here, every 5 minutes the two-state IOB model was updated 50 times to obtain the current state. In terms of computation-times, that was computed in a fraction of a second.
In a hybrid CL system when a meal is announced, an insulin bolus is infused to cover that meal depending on the carbohydrate patient estimation. This could be a problem if the subject misestimates meal size or if he/she finally decides not to eat, because a large amount of active insulin will still be in his/her body. On the other hand, despite reducing patient intervention, there is still a strong compromise between the aggressiveness of the controller and the postprandial glucose excursion in fully closed-loop AP systems.48-51 In Turksoy et al51 this problem is tackled using an additional module different from the main controller which infuses an insulin bolus when a meal is detected. Here, both the patient burden and the performance trade-off are first addressed via a switched controller structure (combining both aggressive and conservative controller) together with the nonlinear action introduced by an IOB-based sliding mode safety layer. In this way, no premeal boluses are infused with the ARG controller, and when a meal is announced (only required in this first clinical validation for IOB limit definition), the controller will be triggered to the listening mode. Being in that mode, it will be triggered to the aggressive mode only when rising glucose values are detected. Even in the worst case scenario where rising glucose values were detected only because of CGM long drift, the amount of insulin that would be infused by the reactive controller will be significantly lower than a meal-related insulin bolus.
Carbohydrate counting is a difficult task, even for experienced subjects.68 The fact of not having to count carbohydrates would be expected to strengthen users perceived ease of use of AP systems.69 As a result, higher user acceptance of emerging technologies for the management of T1DM may be achieved with a CL strategy like the ARG algorithm.
Limitations and Future Work
This study had many limitations. The main one was probably the lack of an OL arm, which made a fair comparison of OL and CL periods not possible. In particular, we were unable to track the quantity and quality of food intake during the home-care period. Also, the food intakes were neither realistic nor normalized to each patient. Prior information about average carbohydrates consumed per meal by each patient would have greatly strengthened the validation of the ARG algorithm. Finally, regarding the algorithm, the initial calibration of IOB limit was not always respected, probably leading to worse results than with the a-priori defined bounds.
Regarding how the control algorithm could be expanded, it should be remarked that the controller design was based on a linear time-invariant (LTI) version of a LPV control-oriented model.70 Nevertheless, the final ARG controller is not LTI, but a switched-LTI controller with a sliding-mode outer controller (SAFE), for example, a nonlinear controller. However, more general LPV or switched LPV controllers could be synthetized. Furthermore, more complex scenarios could be potentially addressed by redesigning the switching policy and/or the IOB constraints. In that sense, a third controller, either LTI as in this case or LPV, could be included to take into account other perturbations like physical activity.71 IOB limits could take into account both the IOB value and its tendency previous to the meal intake.
In a future clinical test, any meal detection strategy could be employed67,72-74 to completely avoid meal announcement because the ARG controller is independent on how meals are detected. Indeed, in silico CL trials using a meal detector algorithm had been carried out previously with good results.55
Conclusion
This was the first AP clinical trial without premeal insulin boluses in Latin America. Here, the ARG algorithm was validated in a pilot study on five T1DM subjects. During the CL period, the controller was able to safely regulate the glucose level, minimizing risks of hypo- and hyperglycemia. Although promising results were obtained, new larger and longer trials that include usual living conditions are necessary.
Acknowledgments
The essential pump donation by Roche and the collaboration of Dexcom are truly appreciated by all authors.
Footnotes
Abbreviations: AP, artificial pancreas; ARG, automatic regulation of glucose; BLW, Bluetooth low energy; CF, correction factor; CGM, continuous glucose monitoring; CI, confidence interval; CL, closed loop; CR, carbohydrate ratio; CSII, continuous subcutaneous insulin infusion; DD, duration of diabetes; DiAs, Diabetes Assistant; DKA, diabetic ketoacidosis; DWM, DiAs Web Monitoring; FDA, Food and Drug Administration; gCHO, grams of carbohydrates; HBGI, high blood glucose index; HIBA, Hospital Italiano de Buenos Aires; IBR, insulin basal rate; IOB, insulin on board; LBGI, low blood glucose index; LPV, linear parameter-varying; LQG, linear quadratic Gaussian; LTI, linear time invariant; MPC, model predictive control; OL, usual care/open loop; PID, proportional-integral-derivative; SAFE, safety auxiliary feedback element; SC, subcutaneous; SD, standard deviation; T1DM, type 1 diabetes mellitus; TDI, total daily insulin; UVA, University of Virginia.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MB is the cofounder of TypeZero Technologies, Inc, receives research support from Dexcom, Tandem, Ascensia, Senseonics and Sanofi, and consults for Dexcom, Roche, Ascensia, and Safoni. DC is TypeZero’s part-time CMO. All other authors declare they have no conflicts of interest
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Nuria/Cellex Foundations (Argentina/Spain), CONICET, UNLP, UNQ, ITBA (Argentina). During this study, ECN was part of the Center for Diabetes Technology, UVA (USA), funded by JDRF grant 2-SRA-2016-291-Q-R.
ORCID iDs: Ricardo Sánchez-Peña  https://orcid.org/0000-0001-9190-2576
https://orcid.org/0000-0001-9190-2576
Marcela Moscoso-Vásquez  https://orcid.org/0000-0003-4691-0096
https://orcid.org/0000-0003-4691-0096
References
- 1. Brazeau AS, Mircescu H, Desjardins K, et al. Carbohydrate counting accuracy and blood glucose variability in adults with type 1 diabetes. Diabetes Res Clin Pr. 2013;99(1):19-23. [DOI] [PubMed] [Google Scholar]
- 2. Cobelli C, Renard E, Kovatchev BP. Artificial pancreas: past, present, future. Diabetes. 2011;60(11):2672-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atlas E, Thorne A, Lu K, Phillip M, Dassau E. Closing the loop. Diabetes Technol Ther. 2014;16(suppl 1):s23-s33. [DOI] [PubMed] [Google Scholar]
- 4. Doyle FJ, III, Huyett L, Lee J, Zisser H, Dassau E. Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care. 2014;37(5):1191-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haidar A. The artificial pancreas: how closed-loop control is revolutionizing diabetes. IEEE Control Syst Mag. 2016;36(5):28-47. [Google Scholar]
- 6. Turksoy K, Frantz N, Quinn L, et al. Automated insulin delivery—the light at the end of the tunnel. J Pediatr. 2017;186:17-28. [DOI] [PubMed] [Google Scholar]
- 7. Riddell MC, Zaharieva DP, Yavelberg L, Cinar A, Jamnik VK. Exercise and the development of the artificial pancreas. J Diabetes Sci Technol. 2015;9:1217-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Breton MD, Cherñavvsky D, Forlenza GP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40(12):1644-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes Metab. 2018;20(2):245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovatchev BP, Breton M, Dalla Man C, Cobelli C. Food and Drug Administration Master File; MAF 1521, 2008. In silico model and computer simulation environment approximating the human glucose/insulin utilization. 2008. [Google Scholar]
- 11. Kovatchev BP, Breton M, Dalla Man C, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3(1):44-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalla Man C, Micheletto F, Lv D, Breton M, Kovatchev BP, Cobelli C. The UVA/PADOVA type 1 diabetes simulator: new features. J Diabetes Sci Technol. 2014;8(1):26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burnett DR, Huyett LM, Zisser HC, Doyle FJ, III, Mensh BD. Glucose sensing in the peritoneal space offers faster kinetics than sensing in the subcutaneous space. Diabetes. 2014;63(7):2498-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Renard E. Insulin delivery route for the artificial pancreas: Subcutaneous, intraperitoneal, or intravenous? Pros and cons. J Diabetes Sci Technol. 2008;2(4):735-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bequette BW. Challenges and recent progress in the development of a closed-loop artificial pancreas. Annu Rev Control. 2012;36(2):255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steil GM, Panteleon AE, Rebrin K. Closed-loop insulin delivery-the path to physiological glucose control. Adv Drug Deliv Rev. 2004;56(2):125-144. [DOI] [PubMed] [Google Scholar]
- 17. Castle JR, Engle JM, El Youssef J, et al. Novel use of glucagon in a closed-loop system for preventing of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33(6):1282-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steil GM, Palerm CC, Kurtz N, et al. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96(5):1402-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phillip M, Battelino T, Atlas E, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824-833. [DOI] [PubMed] [Google Scholar]
- 20. Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37(11):3025-3032. [DOI] [PubMed] [Google Scholar]
- 21. Kovatchev BP, Renard E, Cobelli C, et al. Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care. 2014;37(7):1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thabit H, Lubina-Solomon A, Stadler M, et al. Home use of closed-loop insulin delivery for overnight glucose control in adults with type 1 diabetes: a 4-week, multicentre, randomised crossover study. Lancet 2014;2(9):701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harvey RA, Dassau E, Bevier WC, et al. Clinical evaluation of an automated artificial pancreas using zone-model predictive control and health monitoring system. Diabetes Technol Ther. 2014;16(6):348-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hovorka R, Elleri D, Thabit H, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37(5):1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Bock M, Roy A, Cooper M, et al. Feasibility of outpatient 24-hour closed-loop insulin delivery. Diabetes Care. 2015;38(11):e186-e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38(7):1205-1211. [DOI] [PubMed] [Google Scholar]
- 27. Del Favero S, Boscari F, Messori M, et al. Randomized summer camp crossover trial in 5- to 9-year-old children: outpatient wearable artificial pancreas is feasible and safe. Diabetes Care. 2016;39(7):1180-1185. [DOI] [PubMed] [Google Scholar]
- 28. Gondhalekar R, Dassau E, Doyle FJ., III Periodic zone-MPC with asymmetric costs for outpatient-ready safety of an artificial pancreas to treat type 1 diabetes. Automatica. 2016;71(9):237-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ly TT, Keenan DB, Roy A, et al. Automated overnight closed-loop control using a proportional-integral-derivative algorithm with insulin feedback in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Technol Ther. 2016;18(6):377-384. [DOI] [PubMed] [Google Scholar]
- 30. Weisman A, Bai JW, Cardinez M, et al. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501-512. [DOI] [PubMed] [Google Scholar]
- 31. Haidar A, Messier V, Legault L, Ladouceur M, Rabasa-Lhoret R. Outpatient 60-hour day-and-night glucose control with dual-hormone artificial pancreas, single-hormone artificial pancreas, or sensor-augmented pump therapy in adults with type 1 diabetes: an open-label, randomised, crossover, controlled trial. Diabetes Obes Metab. 2017;19(5):713-720. [DOI] [PubMed] [Google Scholar]
- 32. El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389(10067):369-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kovatchev BP, Cheng P, Anderson SM, et al. Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther. 2017;19:18-24. [DOI] [PubMed] [Google Scholar]
- 34. Bally L, Thabit H, Kojzar H, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017;5(4):261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Forlenza GP, Deshpande S, Ly TT, et al. Application of zone model predictive control artificial pancreas during extended use of infusion set and sensor: a randomized crossover-controlled home-use trial. Diabetes Care. 2017;40(8):1096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Messori M, Kropff J, Del Favero S, et al. Individually adaptive artificial pancreas in subjects with type 1 diabetes: a one-month proof-of-concept trial in free-living conditions. Diabetes Technol Ther. 2017;19(10):560-571. [Google Scholar]
- 37. Parker R, Doyle FJ, III, Peppas N. A model-based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng. 1999;46(2):148-157. [DOI] [PubMed] [Google Scholar]
- 38. Ruiz-Velázquez E, Femat R, Campos-Delgado D. Blood glucose control for type I diabetes mellitus: a robust tracking H∞ problem. Control Eng Pr. 2004;12(9):1179-1195. [Google Scholar]
- 39. Kovács L, Benyó B, Bokor J, Benyó Z. Induced L2-norm minimization of glucose-insulin system for type 1 diabetic patients. Comput Methods Programs Biomed. 2011;102(2):105-118. [DOI] [PubMed] [Google Scholar]
- 40. Szalay P, Eigner G, Kovács LA. Linear matrix inequality-based robust controller design for type-1 diabetes model. IFAC Proc. 2014;47(3):9247-9252. [Google Scholar]
- 41. Colmegna P, Sanchez Peña RS, Gondhalekar R, Dassau E, Doyle FJ., III Reducing risks in type 1 diabetes using H∞ Control. IEEE Trans Biomed Eng. 2014;61(12):2939-2947. [DOI] [PubMed] [Google Scholar]
- 42. Colmegna P, Sánchez-Peña R, Gondhalekar R, Dassau E, Doyle FJ., III Switched LPV glucose control in type 1 diabetes. IEEE Trans Biomed Eng. 2016;63(6):1192-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steil GM, Rebrin K, Darwin C, Hariri F, Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):3344-3350. [DOI] [PubMed] [Google Scholar]
- 44. Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31(5):934-939. [DOI] [PubMed] [Google Scholar]
- 45. Dassau E, Zisser H, Harvey R, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36(4):801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mauseth R, Hirsch I, Bollyky J, et al. Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol Ther. 2013;15(8):628-633. [DOI] [PubMed] [Google Scholar]
- 47. van Bon AC, Luijf YM, Koebrugge R, Koops R, Hoekstra JBL, DeVries JH. Feasibility of a portable bihormonal closed-loop system to control glucose excursions at home under free-living conditions for 48 hours. Diabetes Technol Ther. 2014;16(3):131-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reddy M, Herrero P, Sharkawy ME, et al. Metabolic control with the bio-inspired artificial pancreas in adults with type 1 diabetes: a 24-hour randomized controlled crossover study. J Diabetes Sci Technol. 2015;10(2):405-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blauw H, van Bon AC, Koops R, DeVries JH; on behalf of the PCDIAB consortium. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016;18(7):671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cameron FM, Ly TT, Buckingham BA, et al. Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther. 2017;19(9):527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Turksoy K, Hajizadeh I, Samadi S, et al. Real-time insulin bolusing for unannounced meals with artificial pancreas. Control Eng Pr. 2017;59(suppl C):159-164. [Google Scholar]
- 52. Gómez-Díaz RA, Garibay-Nieto N, Wacher-Rodarte N, Aguilar-Salinas CA. Epidemiology of type 1 diabetes in Latin America. Curr Diabestes Rev. 2014;10(2):75-85. [DOI] [PubMed] [Google Scholar]
- 53. González L, Caporale JE, Elgart JF, Gagliardino JJ. The burden of diabetes in Argentina. Glob J Health Sci. 2015;7(3):124-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. International Diabetes Federation. IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 55. Colmegna P, Garelli F, De Battista H, Sánchez-Peña R. Automatic regulatory control in type 1 diabetes without carbohydrate counting. Control Eng Pr. 2018;74:22-32. [Google Scholar]
- 56. Keith-Hynes P, Mize B, Robert A, Place J. The diabetes assistant: a smartphone-based system for real-time control of blood glucose. Electronics. 2014;3(4):609-623. [Google Scholar]
- 57. Place J, Robert A, Ben N, et al. DiAs web monitoring: a real-time remote monitoring system designed for artificial pancreas outpatient trials. J Diabetes Sci Technol. 2013;7(6):1427-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hespanha JP, Morse AS. Switching between stabilizing controllers. Automatica. 2002;38(11):1905-1917. [Google Scholar]
- 59. Willinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type 1 diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng. 2015;52:3-12. [DOI] [PubMed] [Google Scholar]
- 60. Fushimi E, Rosales N, De Battista H, Garelli F. Artificial pancreas clinical trials: Moving towards closed-loop control using IOB constraints. Biomed Signal Process Control. 2018;45:1-9. [Google Scholar]
- 61. Clarke W, Kovatchev BP. Statistical tools to analyze continuous glucose monitor data. Diabetes Technol Ther. 2009;11(suppl 1):s45-s54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fabris C, Patek SD, Breton MD. Are risk indices derived from CGM interchangeable with SMBG-based indices? J Diabetes Sci Technol. 2016;10(1):50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Patek SD, Magni L, Dassau E, et al. Modular closed-loop control of diabetes. IEEE Trans Biomed Eng. 2012;59(11):2986-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sánchez-Peña R, Colmegna P, Grosembacher L, et al. Artificial pancreas: first clinical trials in Argentina. 20th IFAC World Congress. 2017;50:7997-8002. [Google Scholar]
- 65. Gingras V, Rabasa-Lhoret R, Messier V, Ladouceur M, Legault L, Haidar A. Efficacy of dual-hormone artificial pancreas to alleviate the carbohydrate-counting burden of type 1 diabetes: a randomized crossover trial. Diabetes Metab. 2016;42(1):47-54. [DOI] [PubMed] [Google Scholar]
- 66. Gingras V, Haidar A, Messier V, Legault L, Ladouceur M, Rabasa-Lhoret R. A simplified semiquantitative meal bolus strategy combined with single- and dual-hormone closed-loop delivery in patients with type 1 diabetes: a pilot study. Diabetes Technol Ther. 2016;18(8):464-471. [DOI] [PubMed] [Google Scholar]
- 67. Samadi S, Turksoy K, Hajizadeh I, Feng J, Sevil M, Cinar A. Meal detection and carbohydrate estimation using continuous glucose sensor data. IEEE J Biomed Heal Inf. 2017;21(3):619-627. [DOI] [PubMed] [Google Scholar]
- 68. Kawamura T, Takamura C, Hirose M, et al. The factors affecting on estimation of carbohydrate content of meals in carbohydrate counting. Clin Pediatr Endocrinol. 2015;24(4):153-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Davis FD. A Technology Acceptance Model for Empirically Testing New End-User Information Systems: Theory and Results. Cambridge, MA: MIT Sloan School of Management; 1986. [Google Scholar]
- 70. Colmegna P, Sánchez-Peña R, Gondhalekar R. Linear parameter-varying model to design control laws for an artificial pancreas. Biomed Signal Process Control. 2018;40:204-213. [Google Scholar]
- 71. Colmegna P, Sánchez-Peña R, Gondhalekar R, Dassau E, Doyle FJ., III Reducing glucose variability due to meals and postprandial exercise in T1DM using switched LPV control: in silico studies. J Diabetes Sci Technol. 2016;10(3):744-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dassau E, Bequette B, Buckingham B, Doyle FJ., III Detection of a meal using continuous glucose monitoring. Diabetes Care. 2008;31(2):295-300. [DOI] [PubMed] [Google Scholar]
- 73. Cameron FM, Niemeyer G, Bequette BW. Extended multiple model prediction with application to blood glucose regulation. J Process Contr. 2012;22(8):1422-1432. [Google Scholar]
- 74. Turksoy K, Samadi S, Feng J, Littlejohn E, Quinn L, Cinar A. Meal detection in patients with type 1 diabetes: a new module for the multivariable adaptive artificial pancreas control system. IEEE J Biomed Heal Inf. 2016;20(1):47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]