Abstract
Introduction:
Recent publications frequently introduce new indexes to measure glycemic variability (GV), quality of glycemic control, or glycemic risk; however, there is a lack of evidence supporting the use of one particular parameter, especially in clinical practice.
Methods:
A cohort of type 2 diabetes mellitus (T2DM) patients in ambulatory care were followed using continuous glucose monitoring sensors (CGM). Mean glucose (MG), standard deviation, coefficient of variation (CV), interquartile range, CONGA1, 2, and 4, MAGE, M value, J index, high blood glucose index, and low blood glucose index (LBGI) were estimated. Hypoglycemia incidence (<54 mg/dl) was calculated. Area under the curve (AUC) was determined for different indexes as identifiers of patients with risk of hypoglycemia (IRH). Optimal cutoff thresholds were determined from analysis of the receiver operating characteristic curves.
Results:
CGM data for 657 days from 140 T2DM patients (4.69 average days per patient) were analyzed. Hypoglycemia was present in 50 patients with 144 hypoglycemic events in total (incidence rate of 0.22 events per patient/day). In the multivariate analysis, both CV (OR 1.20, 95% CI 1.12-1.28, P < .001) and LBGI (OR 4.83, 95% CI 2.41-9.71, P < .001) were shown to have a statistically significant association with hypoglycemia. The highest AUC were for CV (0.84; 95% CI 0.77-0.91) and LBGI (0.95; 95% CI 0.92-0.98). The optimal cutoff threshold for CV as IRH was 34%, and 3.4 for LBGI.
Conclusion:
This analysis shows that CV can be recommended as the preferred parameter of GV to be used in clinical practice for T2DM patients. LBGI is the preferred IRH between glycemic risk indexes.
Keywords: type 2 diabetes, hypoglycemia, continuous glucose monitoring, glucose variability
Multiple studies1-5 have consistently demonstrated reduction of microvascular complications with intensive therapy aimed at achieving stricter glycemic control in patients with type 2 diabetes mellitus (T2DM). This intensive therapy, however, carries an increased risk of hypoglycemia, which in turn can lead to an increased risk of arrhythmias, chronic neurological deterioration and mortality.6
The risk of hypoglycemia in patients receiving treatment for T2DM is associated with the type of medications received, the baseline level of glycated hemoglobin (HbA1c), advanced age, renal and hepatic function impairment, multiple comorbidities, and polypharmacy.7 An additional factor that has recently received increased attention as a predictor of the risk of hypoglycemia is glycemic variability (GV). With the advent of continuous glucose monitoring (CGM), it has been possible to assess comprehensively blood glucose levels, to document more accurately the actual incidence of hypoglycemia, especially in patients who are asymptomatic, and to recognize the importance of GV as a trigger factor for hypoglycemia.8
Monnier et al’s study demonstrated an association between the incidence of asymptomatic hypoglycemia and GV in patients with T2DM during management with oral antidiabetics and/or insulin using the standard deviation (SD) of the blood glucose level as the index to determine GV.9 There are, however, several additional methods for determining GV that may have potential utility as predictors of hypoglycemia, especially nocturnal hypoglycemia10 and other outcomes in diabetes.10 The most frequently GV indexes used are CV (coefficient of variation), MAGE (mean amplitude of glycemic excursions), IQR (interquartile range), CONGA (continuous overlapping net glycemic action), and the MODD (mean of daily differences). These methods were designed to measure GV based on the magnitude of glycemic excursions, the time of exposure to glycemic excursions, or both, but most of them have not been validated for use with CGM data. Likewise, quality of glycemic control (QGC) indexes such as the M value, and the J index, and glycemic risk metrics such as LBGI (low blood glucose index), HBGI (high blood glucose index), and average daily risk range have been described. None of these methods have been adequately standardized; therefore, they are not widely used clinically, leading to a lack of clarity as to which method is most appropriate for use.11
The aim of this study is to evaluate the association between the incidence of hypoglycemia and the GV determined by different methods and to attempt to determine which methods of determining GV and glycemic risk best reflect the incidence of hypoglycemia, seeking to offer the clinician better tools for decision making in the management of patients with T2DM.
Methods
A prospective cohort study including patients with T2DM in outpatient management was conducted at the Diabetes Clinic of the San Ignacio University Hospital, Renal Health Clinic in Bogotá, Colombia, and at the Colombian Diabetes Association. Recruitment was performed between July 2012 and November 2015. The inclusion criteria were as follows: over 18 years of age, use of oral antidiabetic drugs and/or insulin, and the presence of at least three episodes of hypoglycemia per week in the last three months. Pregnant patients were excluded. All patients gave informed consent to participation in the study. The study was approved by the ethics and institutional research committee.
The initial clinical characteristics of each patient were determined by direct interview and review of the patient’s clinical records, including the time since diagnosis of T2DM, the number and frequency of previous episodes of general and severe hypoglycemia, the medications and doses used, and history of macro- or microvascular complications and comorbidity. The levels of creatinine and the albumin/creatinine ratio were also recorded in the six months prior to the study. During the first visit, a HbA1c measurement was performed using high-performance liquid chromatography.
A CGM system was placed in each patient using CGMS iPro 2 equipment (Medtronic, Minneapolis, MN, USA) with the insertion of the Enlite® glucose sensor (Medtronic). The equipment was calibrated against four conventional capillary blood glucose measurement. The patient was instructed to perform strict preprandial capillary glucose monitoring and two-hour postprandial glucose monitoring after insertion of the device (six capillary glucose measurements per day). In addition, instructions were given for making additional blood glucose measurements should any symptom or sign suggestive of hypoglycemia be identified by the patient, including but not limited to dizziness, sweating, palpitations, or drowsiness, with a specific date and time record, as well as for determinations of asymptomatic capillary blood glucose levels less than 70 mg/dl. For standardization, capillary blood glucose measurements were performed using a single-capillary glucose metering system (Abbott FreeStyle Precision Pro, Alameda, CA) and recorded systematically in a format designed for data collection.
The use of the CGM system was programmed for a maximum period of six days. After this time, capillary blood glucose data were obtained on a second visit, and the interstitial monitoring data were downloaded using the iPro CareLink Personal version 3.0 (Medtronic) specific software for analysis of the data obtained with the device. These data remained blind for patients and physicians. The data were exported for calculations using a software prepared in MATLAB®. Preprocessing of the records was conducted to discard days with consecutive 50 or missing data points. Smaller losses were linearly interpolated. The data obtained from each patient were organized by calendar day (00:00 to 23:59 hours). Based on these data, GV was calculated using various metrics, including the SD, CV, IQR, MODD, CONGA (n = 1, 2, and 4 hours), and MAGE (mean MAGEup and MAGEdown using SD day-by-day and total recording). Quality of glycemic control was measured using M value and J index. Glycemic risk indexes calculated were LBGI and HBGI.
According to the recommendations of the American Diabetes Association and the European Association for the Study of Diabetes,12 a clinically significant episode of hypoglycemia was defined as a blood glucose level below 54 mg/dl in at least four consecutive interstitial glucose measurements (ie, for a duration of at least 20 minutes). Likewise, episodes of hypoglycemia alert with values below 70 mg/dl were identified. Only CGM tracings with correlation indexes between capillary and interstitial glucose >0.7 were analyzed.
To evaluate the correlation between the different GV metrics, Spearman’s coefficient of correlation was used. In a univariate analysis, the potential factors associated with hypoglycemia, including clinical and paraclinical variables, and the different metrics used to determine the GV, QGC, and glycemic risk were evaluated. A multivariate analysis was also performed, including in logistic regression models each metric individually, the clinical and paraclinical variables found as statistically significant in univariate analysis, and some additional variables considered as clinically relevant, such as baseline glycemia and the glomerular filtration rate. The area under the curve of the different metrics of GV, QGC, and glycemic risk was determined as identifiers of patients with risk of hypoglycemia, a sensitivity analysis was performed to evaluate if the conclusions were valid in different subgroups according to treatment received. The subgroups generated were insulin only regimens, insulin plus oral hypoglycemic agents (OHA), or oral treatment with metformin or DPP4 exclusively. The optimal cutoff point was determined from analysis of the receiver operating characteristic (ROC) curves, using the Liu method.13 Stata version 14.0 was used in the analysis.
Results
One hundred forty patients were included in the analysis. The demographic and clinical characteristics of the patients on admission to the cohort are presented in Table 1. The mean value of HbA1c was 7.71% ± 1.44%. The average percentage of time in hypoglycemic ranges (<54 mg/dL) was 1.66% and the average percentage of time in hypoglycemia alert (<70-54) was 3.44%. The average AUC <54 was 2.92 (mg/dL)*h. The glomerular filtration rate (GFR) was greater than 60 mL/min in 50.7% of patients and less than 30 ml/min in 12.8% of patients; 8.6% of patients had an albumin/creatinine ratio >300 mg/g.
Table 1.
Characteristics of Included Patients.
| Variable | n = 140 |
|---|---|
| Sex male, n (%) | 66 (47.1) |
| Age in years, mean (SD) | 68.9 (11.2) |
| BMI (kg/m2), mean (SD) | 27.4 (4.2) |
| Duration of diabetes in years, mean (SD) | 15.5 (9.7) |
| HbA1c (%), mean (SD) | 7.71 (1.44) |
| Creatinine (mg/dl), mean (SD) | 1.29 (0.77) |
| GFR (ml/min), mean (SD) | 64.3 (29.4) |
| Total cholesterol (mg/dl), mean (SD) | 167 (44.7) |
| LDL-C (mg/dl), mean (SD) | 92.6 (37.9) |
| Triglycerides (mg/dl), mean (SD) | 142.8 (70.0) |
| Microvascular complications | |
| Retinopathy, n (%) | 48 (34.2) |
| Nephropathy, n (%) | 24 (17.1) |
| Neuropathy, n (%) | 7 (5) |
| Macrovascular complications, n (%) | |
| Coronary heart disease, n (%) | 38 (27.1) |
| Cerebrovascular disease, n (%) | 3 (2.1) |
| Peripheral vascular disease, n (%) | 8 (5.7) |
| Neuropathic symptoms, n (%) | 70 (50) |
| Polypharmacy, n (%) | 117 (83.5) |
| Severe hypoglycemic episodes in last 3 months, patients, n (%) | 20 (14.4) |
| Treatment | |
| Basal insulin, n (%) | 11 (7.9) |
| Basal + bolus insulin, n (%) | 51 (36.4) |
| Insulin pump, n (%) | 2 (1.43) |
| Insulin + OHA, n (%) | 58 (41.4) |
| OHA exclusively, n (%) | 16 (11.4) |
| GLP1 + OHA, n (%) | 1 (0.71) |
Polypharmacy defined as the use of five or more medications. Severe hypoglycemic episodes defined as glucose <54 mg/dl or <70 mg/dl requiring assistance from another person.
Of the patients in the study 65 (46.4%) received only insulin in different regimens (Table 1), whereas 41.4% received OHA combined with insulin, 11.4% received OHA exclusively as a treatment for T2DM, and 1 patient received OHA plus GLP1 (glucagon-like peptide-1). Of the OHAs, the most frequently used were metformin (46.4%), DPP4 (dipeptidyl peptidase-4) inhibitors (22.8%), and sulfonylureas (8.6%). The basal insulin used was glargine or NPH (neutral protamine Hagedorn).
The correlation between the different indexes is presented in Table 2. High correlation indexes were found between the SD and each of the GV and QGC metrics (r > .8); however, the correlation between the SD and LBGI was only moderate (r = .43). The correlation between the mean glucose and the various GV metrics was moderate, with values of r between .5 and .7.
Table 2.
Spearman’s Coefficient of Correlation Between Various Indexes of GV, QGC and Glycemic Risk.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. MG | 1.0000 | |||||||||||||
| 2. SD | .6994 | 1.0000 | ||||||||||||
| 3. CV | .2461 | .8244 | 1.0000 | |||||||||||
| 4. IQR | .6604 | .9532 | .8069 | 1.0000 | ||||||||||
| 5. CONGA 1 | .5366 | .8510 | .7580 | .7764 | 1.0000 | |||||||||
| 6. CONGA 2 | .5844 | .8973 | .7878 | .8256 | .9834 | 1.0000 | ||||||||
| 7. CONGA 4 | .6417 | .9484 | .8109 | .8847 | .9220 | .9642 | 1.0000 | |||||||
| 8. MODD | .6967 | .8597 | .6748 | .7932 | .7651 | .8093 | .8286 | 1.0000 | ||||||
| 9. MAGE-T | .6644 | .9594 | .8045 | .9041 | .8832 | .9243 | .9624 | .8189 | 1.0000 | |||||
| 10. MAGE D | .6197 | .9435 | .8235 | .8978 | .8879 | .9289 | .9656 | .7967 | .9856 | 1.0000 | ||||
| 11. M value | .9264 | .8887 | .5230 | .8411 | .7177 | .7690 | .8253 | .8238 | .8471 | .8112 | 1.0000 | |||
| 12. J index | .9428 | .8827 | .5068 | .8344 | .7151 | .7658 | .8229 | .8218 | .8425 | .8047 | .9939 | 1.0000 | ||
| 13. LBGI | −.1978 | .4318 | .7077 | .4317 | .4326 | .4399 | .4458 | .2878 | .4186 | .4596 | .0865 | .0568 | 1.0000 | |
| 14. HBGI | .8611 | .9426 | .6344 | .9073 | .7576 | .8130 | .8771 | .8509 | .9006 | .8724 | .9764 | .9726 | .1886 | 1.0000 |
Data were recorded for a total of 657 days, including 140 patients, for an average of 4.69 (±1.48) days and 852 (±426) samples per patient. Calibration of interstitial glucose measurements was adequate (r > .7). Fifty patients (35.7%) had at least one episode of hypoglycemia <54 mg/dl during follow-up. A total of 144 episodes were recorded for an incidence rate (IR) of 0.22 episodes per patient-day. The IR of events of hypoglycemia alert (<70 mg/dL) was 0.66 episodes per patient-day (433 events).
Univariate analysis of each clinical and laboratory factors potentially associated with hypoglycemia <54 mg/dL is presented in Table 3. Mean glucose (RR 0.98, 95% CI 0.965-0.997, P = .022), time since diagnosis (RR 1.04, 95% CI 1.00-1.08, P = .038), and body mass index (RR 1.04, 95% CI 0.83-0.99, P = .046) were significantly associated with hypoglycemia.
Table 3.
Univariate Analysis of Clinical and Paraclinical Factors Associated With Hypoglycemia <54 mg/dL.
| RR | 95% CI | P | |
|---|---|---|---|
| Clinical variable | |||
| Age | 1.00 | 0.97-1.03 | .830 |
| Female gender | 1.07 | 0.54-2.15 | .840 |
| BMI | 0.91 | 0.83-0.99 | .046 |
| Time since diagnosis (years) | 1.04 | 1.00-1.08 | .038 |
| Polypharmacy | 1.33 | 0.51-3.48 | .564 |
| Hypoglycemia history | |||
| History of severe hypoglycemia by clinic | 0.74 | 0.27-2.08 | .579 |
| History of hypoglycemia <54 mg/dl in the last 3 months | 1.31 | 0.65-2.63 | .451 |
| History of asymptomatic hypoglycemia in the last 3 months | 1.10 | 0.49-2.50 | .815 |
| Paraclinical variables | |||
| HbA1c | 0.91 | 0.72-1.17 | .473 |
| Mean glucose | 0.98 | 0.96-0.99 | .022 |
| Creatinine | 0.84 | 0.52-1.36 | .491 |
| GFR | 1 | 0.99-1.01 | .883 |
| Microalbumin/creatinine ratio | 0.99 | 0.99-1.00 | .552 |
Hypoglycemia history determined by direct interview and review of the patient’s clinical records. Polypharmacy defined as the use of five or more medications.
Comparison among patients with and without episodes of hypoglycemia <54 mg/dl showed significantly higher values of all GV indexes among patients with hypoglycemia. There were no differences for QGC indexes (M value and J index). Among the glycemic risk indexes, LBGI showed significant differences between the two groups (Table 4). A similar finding was found in subgroups analysis, according to treatment received (Table 4).
Table 4.
Determinations of GV, QGC, and GR by Different Methods in Patients With and Without Episodes of Hypoglycemia <54 mg/dL According to Received Treatment.
| Total (n = 140) |
Insulin only (n = 65) |
Insulin + OHA (n = 58) |
Metformin or DPP4 (n = 17) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metric, mean (SD) | With HG (n = 50) | Without HG (n = 90) | P | With HG (n = 24) | Without HG (n = 41) | P | With HG (n = 19) | Without HG (n = 39) | P | With HG (n = 7) | Without HG (n = 10) | P |
| MG (mg/dl) | 142.0 (30.9) | 150.9 (40) | .14 | 145.5 (37.6) | 154.2 (36.6) | .36 | 139.9 (27.2) | 153.7 (38.3) | .16 | 135.6 (8.6) | 126.5 (17.8) | .23 |
| SD | 55.9 (15.3) | 44.1 (17.3) | <.001 | 57.8 (17.6) | 47.9 (16.2) | .02 | 53.7 (13.1) | 44.3 (16.9) | .04 | 55.3 (13.7) | 26.8 (13.7) | <.001 |
| CV | 39.4 (7.4) | 28.7 (8.2) | <.001 | 39.7 (7.5) | 31.0 (8.1) | <.001 | 38.2 (6.3) | 28.1 (6.9) | <.001 | 40.8 (9.6) | 20.8 (8.7) | <.001 |
| IQR | 80.8 (27.9) | 61.0 (27.3) | <.001 | 84.3 (33.4) | 66.1 (26.9) | .02 | 76.9 (20.7) | 61.5 (26.1) | .03 | 79.5 (26.3) | 37.9 (24.0) | .004 |
| CONGA 1 | 37.1 (11.0) | 28.5 (10.1) | <.001 | 37.3 (11.9) | 29.1 (8.6) | .002 | 36.4 (11.5) | 29.5 (11.6) | .03 | 37.7 (6.3) | 22.1 (7.2) | <.001 |
| CONGA 2 | 55.0 (15.5) | 42.1 (15.1) | <.001 | 55.7 (17.6) | 44.1 (14.2) | .005 | 53.6 (14.8) | 42.6 (15.9) | .014 | 55.9 (9.7) | 31.1 (11.4) | <.001 |
| CONGA 4 | 71.5 (20.3) | 55.0 (21.3) | <.001 | 73.2 (24.1) | 59.4 (21.1) | .02 | 68.1 (16.1) | 55.0 (20.7) | .018 | 74.6 (17.3) | 36.4 (15.8) | <.001 |
| MODD | 48.3 (14.5) | 40.7 (18.1) | .013 | 53.8 (16.5) | 44.7 (17.8) | .04 | 45.1 (11.5) | 41.1 (18.1) | .37 | 38.5 (5.4) | 23.5 (7.6) | <.001 |
| MAGE T | 127.0 (35.9) | 99.9 (41.3) | <.001 | 131.9 (44.2) | 109.1 (40.4) | .03 | 120.1 (26.7) | 99.6 (39.7) | .04 | 128.6 (25.4) | 63.7 (33.6) | <.001 |
| MAGE D | 125.0 (35.0) | 95.2 (39.18) | <.001 | 128.5 (41.1) | 103.8 (39.3) | .02 | 119.7 (28.4) | 94.7 (36.6) | .011 | 127.9 (29.9) | 61.5 (32.2) | <.001 |
| M value | 24.0 (18.8) | 23.6 (23.6) | .914 | 27.4 (23.2) | 25.8 (25.3) | .81 | 21.4 (14.8) | 25.2 (23.3) | .51 | 19.4 (8.1) | 7.8 (7.1) | .006 |
| J index | 41.0 (18.6) | 40.6 (21.6) | .900 | 43.9 (22.7) | 43.2 (22.4) | .90 | 38.9 (15.6) | 41.9 (21.6) | .58 | 36.7 (7.1) | 24.2 (8.9) | .007 |
| LBGI | 6.97 (3.6) | 1.87 (1.2) | <.001 | 6.96 (3.6) | 2.01 (1.2) | <.001 | 6.35 (3.05) | 1.87 (1.12) | <.001 | 8.66 (4.7) | 1.27 (1.1) | <.001 |
| HBGI | 8.22 (5.1) | 7.30 (5.8) | .356 | 9.24 (6.3) | 8.18 (6.0) | .50 | 7.38 (3.9) | 7.54 (5.8) | .90 | 7.02 (2.7) | 2.76 (2.9) | .009 |
HG : Hypoglycemia.
Univariate and multivariate analyses of each of the GV, QGC, and glycemic risk metrics as identifiers of patients with risk of hypoglycemia < 54 mg/dL are presented in Table 5. The univariate analysis showed a relationship between hypoglycemia and all the metrics of GV; there was no significant relationship with QGC indexes and HBGI. When the clinically relevant variables (mean values of glycemia, diabetes duration, BMI, and GFR) were included in the multivariate analysis, both the GV metrics and the glycemic risk metrics were significant (P < .0001).
Table 5.
Univariate and Multivariate Analysis of the Different Metrics of GV, QGC, and Glycemic Risk for Hypoglycemia < 54 mg/dL.
| Univariate analysis |
Multivariate analysisa |
||||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| MG (mg/dL) | 0.99 | 0.98, 1.00 | .148 | — | — | — | |
| Glycemic variability indexes | SD | 1.02 | 1.02-1.06 | <.0001 | 1.13 | 1.08-1.19 | <.0001 |
| CV | 1.19 | 1.11-1.26 | <.0001 | 1.20 | 1.12-1.28 | <.0001 | |
| IQR | 1.03 | 1.01-1.04 | <.0001 | 1.06 | 1.04-1.09 | <.0001 | |
| CONGA 1 | 1.08 | 1.04-1.12 | <.0001 | 1.16 | 1.10-1.25 | <.0001 | |
| CONGA 2 | 1.06 | 1.03-1.09 | <.0001 | 1.13 | 1.08-1.19 | <.0001 | |
| CONGA 4 | 1.04 | 1.02-1.06 | <.0001 | 1.09 | 1.05-1.13 | <.0001 | |
| MODD | 1.02 | 1.00-1.04 | .017 | 1.07 | 1.04-1.11 | <.0001 | |
| MAGE T | 1.02 | 1.01-1.03 | <.0001 | 1.04 | 1.02-1.06 | <.0001 | |
| MAGE D | 1.02 | 1.01-1.03 | <.0001 | 1.05 | 1.03-1.07 | <.0001 | |
| Quality of glycemic control indexes | M value | 1.00 | 0.98-1.02 | .914 | 1.19 | 1.10-1.30 | <.0001 |
| J index | 1.00 | 0.98-1.01 | .900 | 1.38 | 1.22-1.56 | <.0001 | |
| Glycemic risk indexes | LBGI | 3.77 | 2.28-6.24 | <.0001 | 4.83 | 2.41-9.71 | <.0001 |
| HBGI | 1.02 | 0.97-1.09 | .356 | 1.72 | 1.35-2.19 | <.0001 | |
Multivariate analysis controlled for glycemia mean, duration of diabetes, BMI, and GFR.
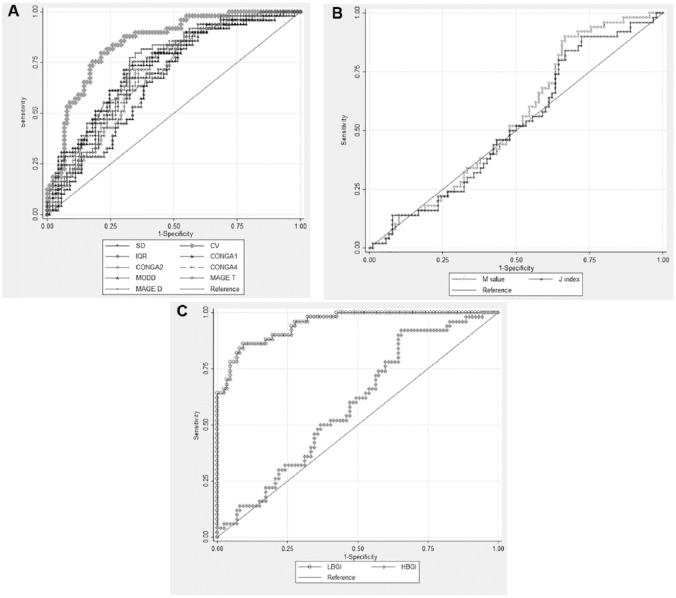
Analysis of the ROC curves of each index as identifier of patients with risk of hypoglycemia <54 mg/dL showed that, among the GV metrics, the CV yielded the best AUC (0.84, 95% CI 0.77-0.90; Table 6 and Figure 1). For the glycemic risk metrics, the best AUC was found for LBGI (0.9525; 95% CI 0.92-0.98). A similar finding was done for events of hypoglycemia alert (<70 mg/dL) but with a lower discriminatory ability. Among the GV metrics, the CV yielded the best AUC (0.77, 95% CI 0.69-0.86), and LBGI was the best glycemic risk metric (AUC 0.97; 95% CI 0.94-0.99) (Table 6).
Table 6.
Comparison of AUC (ROC Curves) for Various GV, QGC, and Glycemic Risk Indexes.
| Index | <54 mg/dL |
<70 mg/dL |
||||
|---|---|---|---|---|---|---|
| Area ROC | 95% CI | Optimal cutoff point | Area ROC | 95% CI | ||
| Glycemic variability indexes | SD | 0.7155 | 0.62812-0.80283 | 51.1 | 0.5769 | 0.46364-0.69011 |
| CV | 0.8420 | 0.77482-0.90909 | 34.02% | 0.7781 | 0.69049-0.86572 | |
| IQR | 0.7181 | 0.63162-0.80454 | 66.5 | 0.5913 | 0.48101-0.70158 | |
| CONGA 1 | 0.7439 | 0.65785-0.83005 | 31.68 | 0.6235 | 0.51501-0.73193 | |
| CONGA 2 | 0.7430 | 0.65799-0.82801 | 47.78 | 0.6174 | 0.50958-0.72513 | |
| CONGA 4 | 0.7342 | 0.64921-0.81923 | 61.68 | 0.6092 | 0.50073-0.71767 | |
| MODD | 0.6649 | 0.57365-0.75620 | 39.1 | 0.5437 | 0.42767-0.65969 | |
| MAGE T | 0.7174 | 0.63165-0.80309 | 112.24 | 0.5877 | 0.47737-0.69794 | |
| MAGE D | 0.7442 | 0.66120 0.82717 | 107.57 | 0.6144 | 0.50812-0.72077 | |
| Quality of glycemic control indexes | M value | 0.5695 | 0.47237-0.66669 | 9.95 | 0.3672 | 0.25354-0.48088 |
| J index | 0.5484 | 0.44949-0.64733 | 29.07 | 0.3695 | 0.25737-0.48171 | |
| Glycemic risk indexes | LBGI | 0.9525 | 0.92038-0.98470 | 3.38 | 0.9659 | 0.93691-0.99495 |
| HBGI | 0.6058 | 0.51011-0.70156 | 4.04 | 0.4412 | 0.32399-0.55836 | |
Optimal cutoff points estimated with Liu method.
Figure 1.
Comparison of ROC curves among different Glycemic variability, quality of glycemic control, and glycemic risk metrics as identifiers of patients with risk of hypoglycemia. (a) Glycemic variability indexes. (b) Quality of glycemic control indexes: M value, J index. (c) Glycemic risk indexes.
The comparison of AUC (ROC curves) by subgroups according to received treatment, showed that CV was the best GV metric for patients receiving insulin exclusively and for patients treated with insulin plus OHA (AUC 0.81 and 0.85, respectively). For patients receiving metformin or DPP4 exclusively, all GV metrics had AUC > 0.90. LBGI was the best glycemic risk index in each subgroup evaluated with AUCs > 0.94 (Table 7).
Table 7.
Comparison of AUC (ROC Curves) for Various GV, QGC, and Glycemic Risk Indexes by Subgroups According to Received Treatment.
| Index | Insulin only (n = 65) |
Insulin + OHA (n = 58) |
Metformin or DPP4 (n = 17) |
||||
|---|---|---|---|---|---|---|---|
| Area ROC | 95% CI | Area ROC | 95% CI | Area ROC | 95% CI | ||
| Glycemic variability indexes | SD | 0.6934 | 0.55365-0.83308 | 0.6814 | 0.53652-0.82637 | 0.9286 | 0.78398-1 |
| CV | 0.8135 | 0.70185-0.92515 | 0.8518 | 0.74668-0.95692 | 0.9286 | 0.78398-1 | |
| IQR | 0.7008 | 0.56312-0.83848 | 0.6884 | 0.54532-0.83141 | 0.9286 | 0.78398-1 | |
| CONGA 1 | 0.7460 | 0.60734-0.88465 | 0.6773 | 0.53605-0.81852 | 0.9429 | 0.82407-1 | |
| CONGA 2 | 0.7323 | 0.59315-0.87138 | 0.6787 | 0.53646-0.82088 | 0.9286 | 0.78398-1 | |
| CONGA 4 | 0.7151 | 0.57803-0.85217 | 0.6870 | 0.54629-0.82767 | 0.9286 | 0.78398-1 | |
| MODD | 0.6728 | 0.53408-0.81146 | 0.6247 | 0.47541-0.77390 | 0.9429 | 0.83851-1 | |
| MAGE T | 0.7014 | 0.56542-0.83733 | 0.6717 | 0.53118-0.81231 | 0.9143 | 0.74397-1 | |
| MAGE D | 0.7185 | 0.58391-0.85316 | 0.7022 | 0.56577-0.83866 | 0.9143 | 0.74397-1 | |
| Quality of glycemic control indexes | M value | 0.5606 | 0.40861-0.71267 | 0.5111 | 0.35850-0.66366 | 0.8571 | 0.65697-1 |
| J index | 0.5400 | 0.38610-0.69399 | 0.4875 | 0.33191-0.64316 | 0.8857 | 0.71694-1 | |
| Glycemic risk indexes | LBGI | 0.9531 | 0.90668-0.99950 | 0.9460 | 0.88739-1.00000 | 0.9714 | 0.90413-1 |
| HBGI | 0.6087 | 0.45910-0.75829 | 0.5485 | 0.39621-0.70074 | 0.9143 | 0.76554-1 | |
The optimal cutoff points for each identifier of patients with risk of hypoglycemia <54 mg/dL, were determined from the analysis of the ROC curves, and are presented in Table 6. A cutoff point of 34% was the optimal for the CV, with a sensitivity of 0.80 and a specificity of 0.78 at cutpoint. For LBGI was 3.4, with a sensitivity of 0.86 and a specificity of 0.91 at cutpoint. Using these cut points the percentage of time in hypoglycemic ranges (<54 mg/dL) was significantly higher in patients with CV >34% (3.55% vs 0.25%, P < .001), and in patients with LBGI >3.4 (4.5% vs 0.04%, P < .001). The average AUC <54 was significantly higher in patients with CV >34% (5.67 vs 0.86 (mg/dL)*h, P < .001), and in patients with LBGI >3.4 (7.17 vs 0.50 (mg/dL)*h, P < .001).
Discussion
Determination of HbA1c levels has proven to be useful in both the diagnosis and monitoring of diabetes, making this indicator a key tool in clinical control. However, although the HbA1c value has greater stability in the presence of day-to-day disturbances and physiological stressors than many other methods used to estimate blood glucose levels, this advantage limits its role as a predictor of hypoglycemia,14 which is the main barrier to achieving adequate glycemic control.
GV has been evaluated in theoretical reviews and in studies based on both CGM and capillary records using a variety of mathematical methods. Although GV has been shown to be associated with the risk of hypoglycemia, it has not been possible to standardize a particular metric to generate unified recommendations for wide use within clinical practice;11 also, there are multiple QGC and glycemic risk indexes used, generating confusion for health professionals. Although there are precedents in the literature for defining a specific method to assess GV, most clinical studies limit their evaluation to only some of the possible metrics, and others only consider capillary measurements in the mathematical estimation.15,16 Our study is the first in which data obtained from CGM were used to estimate GV, QGC, and glycemic risk by most of the available methods and in which the outcome of this estimation was directly compared and correlated with a major clinical outcome (in this case, hypoglycemia) to validate its applicability in the daily clinical context. The strengths of this study is that the analysis was performed considering the new definition of clinically significant hypoglycemia, and the use of the blinded data of the CGM for patients and physicians, it eliminates bias from behavioral changes of patients.
In recent decades, a number of research groups have attempted to determine the ideal method of measuring GV. In one of the most relevant studies, conducted by Monnier and colleagues, they evaluated the average blood glucose and its SD;9 their results showed that the risk of hypoglycemia was virtually eliminated when the SD was less than 30 mg/dl (1.7 mmol/L). Subsequently, in the work of Saisho et al,17 the correlation between variability and hypoglycemia was again demonstrated, finding that the best ROC curve correlations were for SD and MODD, however they did not evaluate CV as a metric of GV. A study by Jin et al18 that included a significant Asian sample of both T1DM and T2DM patients showed that CV was better correlated than SD with a risk of blood glucose values below 70 mg/dl regardless of the type of diabetes. Similar results were reported by Qu et al,19 who found that intraday glucose CV was significantly correlated with the rate of hypoglycemia events.
The analysis conducted in the present study includes the greatest number of GV metrics available from CGM data that might be associated with a relevant clinical outcome, hypoglycemia, in a high-risk population of T2DM patients. It should be noted that when comparing the data obtained in patients with and without hypoglycemia during the study period, a statistically significant difference was found in almost all measures of variability between the two groups of patients. This demonstrates that although the methods estimate the variability based on different temporal intervals (interday, intraday, total, etc), there is a consistent relationship with the occurrence of hypoglycemia, as reported in previous studies,17,19 is remarkable that this finding was evident even in the subgroups of patient treated with metformin or DPP4, as these OHAs have lower risk of hypoglycemia. Even when all these metrics were highly correlated as it was expected,20 CV was a better metric of GV to identify patients with risk of events of hypoglycemia <54 mg/dL and of events of hypoglycemia alert (<70 mg/dL) in T2DM patients. The ability to discriminate these patients could be explained by the fact that CV is less affected by average glucose and HbA1c levels than other GV metrics.21 In addition, CV has been associated with the presence of cardiovascular autonomic neuropathy in patients with inadequately controlled T2DM,22 is reported in CGM, and is easy to calculate and to use in general practice. That is why we propose that the CV represents a key tool for not only research purposes but also routine clinical use.
A recent study by Monnier et al23 proposed the extensive use of the CV for the estimation of GV in clinical practice, and proposed a cutoff point of 36% for distinguishing high and low variability in the framework of stable or unstable dysglycemia. The methodology to define the threshold was different between that and the present study; in ours, patients with high hypoglycemia risk (including patients with history of recurrent or severe hypoglycemia, and those who were receiving insulin or insulin secretion management) were evaluated using ROC curves analysis, whereas in Monnier et al’s study23 patients without risk of hypoglycemia were used as controls for setting the cutoff point, however our findings suggest a similar value of 34% as marker of risk of hypoglycemia.
Beside the GV indexes, the present study evaluates most of QGC and glycemic risk measurements available from CGM. It is important to emphasize that the intention of these metrics is different. GV evaluates not only the fluctuations of glucose around a mean but also the deleterious cellular processes attributed to both the hyperglycemic spikes and the hypoglycemic troughs, while QGC is an hybrid measure of both variability and mean glycemia, since the intent of the creators was to determine “the difference between the observed blood sugar and normal blood sugar.”24 As expected, our data showed that QGC indexes had a poor ability to discriminate patients with risk of hypoglycemia. Similar results were described by Le Floch and Kessler,25 who found that different glucose variability indexes and ratios provide complementary pieces of information associated with high and low glucose values.
Glycemic risk measurements want to evaluate exclusively the risk of events of hypoglycemia or hyperglycemia, based in the characteristics of previous episodes and a mathematic correction of the skewness of glycemia, but they do not evaluate the fluctuations of glycemia levels by itself. Although originally developed from self-monitored BG data,26 these parameters have been adapted to continuous interstitial glucose monitoring and have demonstrated a good ability to identify patients with risk of hypoglycemia in some settings.27 Our data are compatible with these findings and demonstrate that LBGI was the best metric to identify high risk patients, between all the evaluated indexes.
One of the limitations of our study is that despite the selection of a population at risk of hypoglycemia, the number of hypoglycemic events was not very high. Part of the explanation for this phenomenon is the use of the currently established cutoff point for the definition of clinically significant hypoglycemia (less than 54 mg/dl); this is much lower than the conventional value that was used in most previous studies. The lower value has now become the standard for evaluation, partly because of the data obtained from CGM and their correlation with the physiological response to hypoglycemia.12
Considering the strong correlation of GV with the mean glucose,20 it could be seen as a limitation that in our study we did not consider the basal levels of HbA1c or the mean glucose to adjust our analysis. However it better reflects the way how the health professionals use the GV measures and its thresholds, as they do not use charts with individual values for each metric and each HbA1c level.
An additional potential limitation is the duration of CGM. Recent consensus have suggested a minimum of 14 days of data must be collected for accurate analysis.28 However a previous study evaluating the amount of data required to measure GV examined 50 days of CGM data from 68 participants and concluded that 6 days was a sufficient time period to approximate GV using SD or MAGE;29 the study was conducted in adult participants with T1DM. In the present study we evaluated patients with T2DM who may exhibit a more stable glycemic profile than T1D patients; the average of 4.7 days duration of CGM should not affect seriously the analysis.
Our study, similar to most of those previously mentioned, included patients with T2DM. Overall, analyses of this type have not been performed independently for the consideration of patients with T1DM, although in the study of Jin et al, an adequate correlation was also found for CV independent of the type of diabetes and the general risk of hypoglycemia (<70 mg/dl).18 Further studies are needed to further evaluate the GV metrics in patients with T1DM or exclusively on insulin therapy.
Conclusion
The present analysis shows a strong correlation of the different metrics of GV, and a consistent relationship between GV indexes and the occurrence of hypoglycemia. CV was the measure of GV with the best ability to discriminate patients with risk of hypoglycemia < 54 mg/dL in T2DM. Combined with the practicality and ease of calculation of this parameter, the findings suggest that the CV of blood glucose levels is an ideal measure for the evaluation of GV in the context of clinical practice at all levels of care in T2DM patients. LBGI was the best glycemic risk index.
Footnotes
Abbreviations: BMI, body mass index; CGM, continuous glucose monitoring; CONGA, continuous overlapping net glycemic action; CV, coefficient of variation; GFR, glomerular filtration rate; GLP1, glucagon-like peptide-1; GV, glycemic variability; HbA1c, glycated hemoglobin; HBGI, high blood glucose index; HG, hypoglycemia; IQR, interquartile range; IRH, identifiers of patients with risk of hypoglycemia; LBGI, low blood glucose index; LDL-C, low-density lipoprotein cholesterol; MAGE D, daily mean amplitude of glycemic excursions; MAGE T, total mean amplitude of glycemic excursions; MG, mean glucose; MODD, mean of daily differences; OHA, oral hypoglycemic agents; QGC, quality of glycemic control; ROC, receiver operating characteristic; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AMG has acted as speaker for Novo-Nordisk, MSD, Novartis, Astra Zeneca, Boeringher, and Medtronic and has received financial support for research from Medtronic, Sanofi Aventis, Abbott, and Novartis. OMM has received financial support for research from Medtronic and Novo-Nordisk. AM has acted as speaker for Medtronic, Sanofi, Novartis, Eli Lilly, Bayer, Novo Nordisk, Boehringer, and Abbott and has received financial support for research from Medtronic and Novartis. DL has acted as speaker for Eli Lilly, Novo Nordisk, and Astra Zeneca and has received financial support for research from Novartis. The other authors declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AMG has acted as speaker for Novo-Nordisk, MSD, Novartis, Astra Zeneca, Boeringher, and Medtronic and has received financial support for research from Medtronic, Sanofi Aventis, Abbott, and Novartis. OMM has received financial support for research from Medtronic and Novo-Nordisk. AM has acted as speaker for Medtronic, Sanofi, Novartis, Eli Lilly, Bayer, Novo Nordisk, Boehringer, and Abbott and has received financial support for research from Medtronic and Novartis. DL has acted as speaker for Eli Lilly, Novo Nordisk, and Astra Zeneca and has received financial support for research from Novartis.
ORCID iD: Oscar M. Muñoz  https://orcid.org/0000-0001-5401-0018
https://orcid.org/0000-0001-5401-0018
References
- 1. Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2013;(11):CD008143. [DOI] [PubMed] [Google Scholar]
- 2. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589. [DOI] [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837-853. [PubMed] [Google Scholar]
- 4. UK Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854-865. [PubMed] [Google Scholar]
- 5. Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(suppl 2):B21-B29. [PubMed] [Google Scholar]
- 6. Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shorr RI, Ray WA, Daugherty JR, Griffin MR. Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas. Arch Intern Med. 1997;157(15):1681-1686. [PubMed] [Google Scholar]
- 8. Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39(4):273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monnier L, Wojtusciszyn A, Colette C, Owens D. The contribution of glucose variability to asymptomatic hypoglycemia in persons with type 2 diabetes. Diabetes Technol Ther. 2011;13(8):813-818. [DOI] [PubMed] [Google Scholar]
- 10. Kohnert K-D, Heinke P, Vogt L, Salzsieder E. Utility of different glycemic control metrics for optimizing management of diabetes. World J Diabetes. 2015;6(1):17-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. J Diabetes Sci Technol. 2013;7(2):562-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American diabetes association and the European association for the study of diabetes. Diabetes Care. 2017;40:155-157. [DOI] [PubMed] [Google Scholar]
- 13. Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31(23):2676-2686. [DOI] [PubMed] [Google Scholar]
- 14. Khunti K, Alsifri S, Aronson R, et al. Rates and predictors of hypoglycaemia in 27 585 people from 24 countries with insulin-treated type 1 and type 2 diabetes: the global HAT study. Diabetes Obes Metab. 2016;18(9):907-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cox DJ, Kovatchev BP, Julian DM, et al. 1994 Frequency of severe hypoglycemia in insulin-dependent diabetes mellitus can be predicted from self-monitoring blood glucose data. J Clin Endocrinol Metab. 1994;79(6):1659-1662. [DOI] [PubMed] [Google Scholar]
- 16. Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29(11):2433-2438. [DOI] [PubMed] [Google Scholar]
- 17. Saisho Y, Tanaka C, Tanaka K, et al. Relationships among different glycemic variability indexes obtained by continuous glucose monitoring. Prim Care Diabetes. 2015;9(4):290-296. [DOI] [PubMed] [Google Scholar]
- 18. Jin S-M, Kim T-H, Bae JC, et al. Clinical factors associated with absolute and relative measures of glycemic variability determined by continuous glucose monitoring: an analysis of 480 subjects. Diabetes Res Clin Pract. 2014;104(2):266-272. [DOI] [PubMed] [Google Scholar]
- 19. Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulin-treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther. 2012;14(11):1008-1012. [DOI] [PubMed] [Google Scholar]
- 20. Rodbard D. The challenges of measuring glycemic variability. J Diabetes Sci Technol. 2012;6(3):712-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodbard D. Clinical interpretation of indices of quality of glycemic control and glycemic variability. Postgrad Med. 2011;123(4):107-118. [DOI] [PubMed] [Google Scholar]
- 22. Jun JE, Jin SM, Baek J, et al. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monnier L, Colette C, Wojtusciszyn A, et al. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017;40(7):832-838. [DOI] [PubMed] [Google Scholar]
- 24. Service FJ. Glucose variability. Diabetes. 2013;62(5):1398-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Le Floch J-P, Kessler L. Glucose variability. J Diabetes Sci Technol. 2016;10(4):885-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870-1875. [DOI] [PubMed] [Google Scholar]
- 27. Crenier L, Abou-Elias C, Corvilain B. Glucose variability assessed by low blood glucose index is predictive of hypoglycemic events in patients with type 1 diabetes switched to pump therapy. Diabetes Care. 2013;36(8):2148-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bugler J. An estimation of the amount of data required to measure glycaemic variability (Abstract). In: Proceedings 1st ATTD. Meeting, Prague: 2008;55. [Google Scholar]