Short abstract
Pulmonary pleomorphic carcinoma (PPC) is rare, and the response of patients to conventional chemotherapy is very poor. Here we present a patient with anaplastic lymphoma kinase (ALK)-rearranged advanced PPC treated with crizotinib. Computed tomography revealed a mass in the left upper lung of a nonsmoking 60-year-old woman. Pathological findings using resected tissue were consistent with PPC stage 1A, T1bN0M0. Although the patient received adjuvant radiotherapy, the disease relapsed, quickly progressed, and remained PPC according to analysis of biopsied tissue. Although negative for epidermal growth factor receptor mutations, ALK rearrangements were detected in adenocarcinoma and spindle-cell components. The patient received crizotinib therapy and achieved a partial response for 7 months. This case indicates that patients with PPC, particularly those with adenocarcinoma, may harbor an epithelial component with the ALK rearrangement. Although the progression-free survival of patients treated with crizotinib is limited, they may obtain more benefit compared with conventional chemotherapy.
Keywords: Anaplastic lymphoma kinase (ALK), ALK rearrangement, pulmonary sarcomatoid carcinoma, pulmonary pleomorphic carcinoma, crizotinib, ALK inhibitor, chemotherapy
Introduction
Pulmonary pleomorphic carcinoma (PPC) is a rare subtype of pulmonary sarcomatoid carcinoma (PSC), which accounts for 0.1%–0.4% of lung malignancies.1 According to the 2015 World Health Organization’s (WHO) histological classification of lung tumors, PPCs comprise poorly differentiated adenocarcinomas, squamous cell carcinomas, adenosquamous carcinomas, or undifferentiated large-cell carcinomas that include a mesenchymal component of spindle cells, giant cells, or both, with a sarcomatoid tumor component ≥10%.2 The clinical course of PPC is more aggressive, and its outcome is worse compared with those of other histological types of non-small cell lung cancer (NSCLC).1,3–5 Several investigations found that the responses of advanced PPC to conventional cytotoxic chemotherapy are very poor (15%–17%), and median overall survival is only 5–8 months.6–8 It is therefore critically important to develop more effective treatments. To this end, here we report a patient with anaplastic lymphoma kinase (ALK)-rearranged advanced PPC comprising adenocarcinoma and spindle cell components treated with crizotinib.
Case report
A never-smoking 60-year-old woman was admitted to the Department of Thoracic surgery at Panyu Central Hospital in March 2015 with persistent pain for 2 months in the left side of her chest and back. The patient previously underwent modified radical mastectomy of the right chest caused by a right-breast invasive ductal carcinoma 5 years earlier. A 3.2 × 2.5-cm mass in the left upper lung was detected using computed tomography (Figure 1a). Bronchofiberoscopy and biopsy were negative before surgery. A left upper lobectomy and mediastinal lymph node dissection were performed through video-assisted thoracoscopy in April 2015, and during surgery, the tumor was observed obviously adhered to the chest wall. Pathological analysis found that the tumor specimen was 2.9 cm × 2.5 cm, and the histological findings showed that the tumor comprised an adenocarcinoma component (approximately 70%) and malignant spindle cells (30%) (Figure 2a).
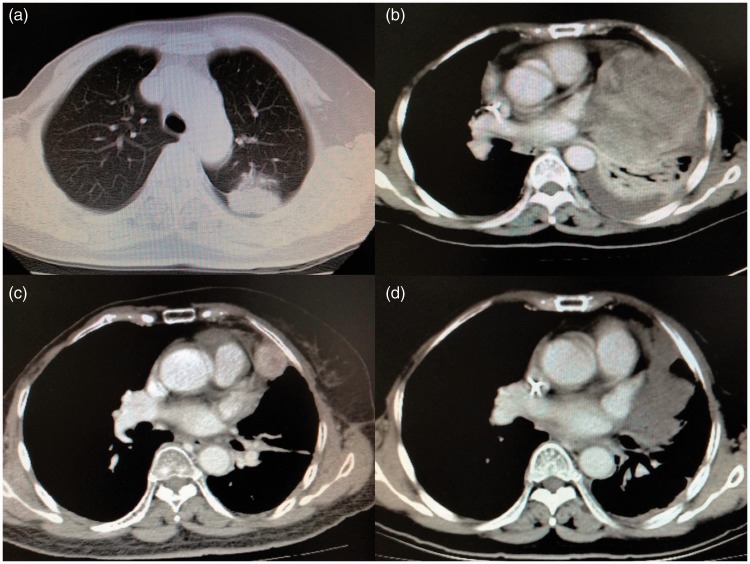
Figure 1.
Characteristics of the PPC and response to therapy. (a) Computed tomography revealed a 3.2 cm × 2.5-cm mass in the left upper lung. (b) One of two metastases in the left lower lung after 1 month of adjuvant radiotherapy. (c) Tumor before crizotinib treatment. (d) A partial response after 1 month of crizotinib treatment.
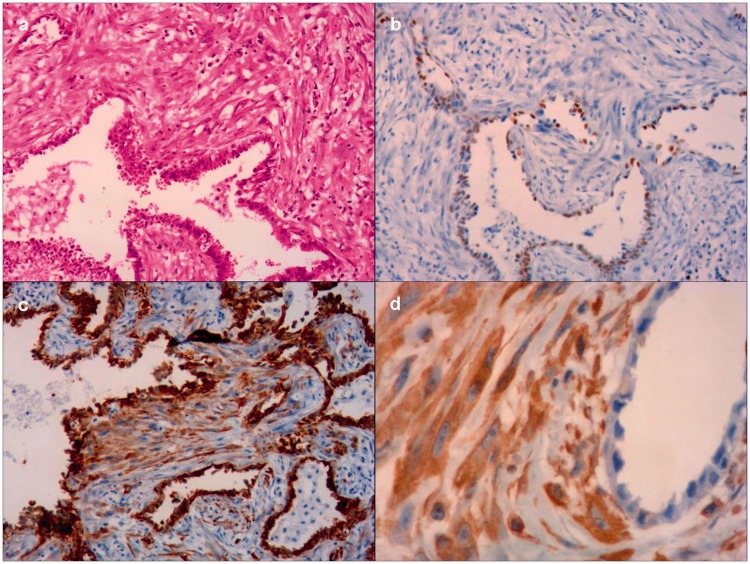
Figure 2.
Histological findings consistent with PPC. (a) Adenocarcinoma component (approximately 70%) and malignant spindle cells (30%) (hematoxylin–eosin staining, magnification 100×). The tumor cells expressed (b) thyroid transcription factor-1 (b), (c) creatine kinase and (d) vimentin (magnification 100×).
The results of immunohistochemistry showed that the tumor cells expressed thyroid transcription factor-1 (TTF-1) (Figure 2b), creatine kinase (Figure 2c), and vimentin (Figure 2d). Metastatic lesions were not detected in three aortopulmonary window lymph nodes. These findings are consistent with the features of PPC, and the pathological stage was diagnosed as stage 1A, T1bN0M0. The patient did not receive adjuvant chemotherapy and was only administered adjuvant radiotherapy to the area of adhesion. Unfortunately, two metastatic lesions were detected in the left lower lung in July 2015, 1 month after the cessation of adjuvant radiotherapy (Figure 1b). The patient refused chemotherapy, and the two metastatic lesions were only treated using radiofrequency ablation.
Five months later, the significantly enlarged tumor was biopsied and diagnosed as PPC. Genetic analyses were performed to detect mutations in the epidermal growth factor receptor gene and rearrangement of ALK. Specifically, the analyses of epidermal growth factor receptor expression were performed using a Scorpion Amplification-Refractory Mutation System. ALK expression was detected using immunohistochemistry (ALK D5F3, Ventana) and fluorescence in situ hybridization. Epidermal growth factor receptor was undetectable, whereas ALK was expressed by the adenocarcinoma and spindle cell components (Figure 3a) The ALK rearrangement was confirmed using fluorescence in situ hybridization (Figure 3b). The patient started subsequent therapy with crizotinib (500 mg daily) in January 2016 and achieved a partial response after one month (Figure 1d). However, the response lasted only 7 months, and computed tomography performed in August 2016 revealed that the tumor had significantly increased. She subsequently received best supportive care and died on January 31, 2017.
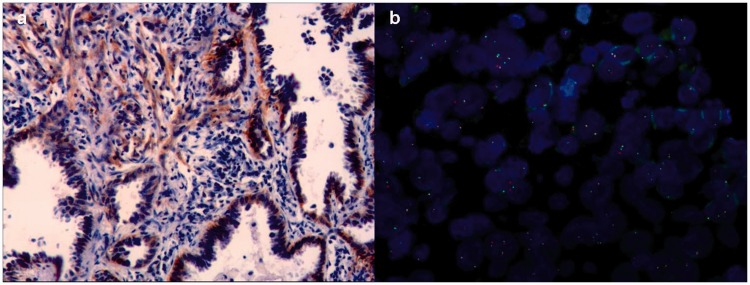
Figure 3.
Immunohistochemistry and fluorescence in situ hybridization analyses of the anaplastic lymphoma kinase (ALK) rearrangement. (a) Immunohistochemistry analysis detected ALK in the adenocarcinoma and spindle cell components of the tumor (magnification 100×). (b) Fluorescence in situ hybridization analysis detected a typical ALK rearrangement.
Subsequent to the patient’s death, her family provided written informed consent to publish our account of her treatment.
Discussion
Here we describe a rare case of PPC with ALK rearrangements in the adenocarcinoma and spindle cell components of the tumor of a 60-year-old woman patient treated with crizotinib. Inamura et al.9 initially reported the clinicopathological features of ALK rearrangements of patients with lung cancer. They found that the histotype of these patients was adenocarcinoma and that the ALK-rearranged adenocarcinoma was characterized by a TTF-1 cell lineage, an acinar structure with a mucin/signet-ring cell pattern, non-/light-smoking history, and early onset.9–11 Our present patient was a nonsmoker, and TTF-1 was expressed by the adenocarcinoma components. The age of our present patient and the pathological diagnosis of pleomorphic carcinoma are uncommon characteristics of patients with NSCLC with ALK rearrangements. Other studies describe three patients aged 50, 69, and 87 years with PPC with ALK rearrangements.12–14 These findings suggest that ALK-positive PPC patients may be older than patients with ALK-positive lung adenocarinoma.10 Moreover, it is notable that the epithelial components of our patients and those of the three aforementioned patients were all adenocarcinomas, consistent with the findings of Chen et al.15 that 4 of 5 ALK-positive patients with PSC had adenocarcinomas with epithelial components. These results indicate that ALK rearrangements in patients with PPC may likely arise in those with an adenocarcinoma epithelial component.
Greta et al.16 reported the first case of an EML4-ALK fusion in PSC in 2013; however, the frequency of ALK rearrangement in PSC or PPC is not as well established compared with that of lung adenocarcinoma. It is therefore unclear whether ALK testing should be performed routinely. Several investigations address this question. One study detected one ALK rearrangement in 33 sarcomatoid carcinomas (3%),17 and another found 5 (3.5%) of 141 PSCs with an ALK rearrangement.15 In contrast, three studies did not detect ALK rearrangements in 35 and 14 patients with PPC or 41 patients with PSC.18–20
Although the frequency is very low, ALK rearrangements are clinically significant. The response of patients with ALK rearrangements to conventional cytotoxic chemotherapy is poor, although ALK inhibitors such as crizotinib, ceritinib, and alectinib may affect treatment efficacy and improve outcomes of these patients.6–8 It is important to note that tumor biopsy specimens of patients with advanced NSCLC often tend to be small, and whether these specimens accurately represent tumor histology is unclear. Therefore, it is not possible to exclude the possibility that certain cells with ALK rearrangements may exist in tumors. If so, patients will lose the opportunity to undergo appropriate treatment if tests for the ALK rearrangement are not performed. Therefore, ALK testing should be recommended for patients with lung adenocarcinomas as well as those with lung cancers with an adenocarcinoma component, even for patients with squamous21 or small cell histology22 or large-cell neuroendocrine carcinomas.23 Testing will be particularly important if there is a possibility of an ALK rearrangement indicated by clinical characteristics such as age, sex, and smoking.
Crizotinib achieves remarkable efficacies (median progression-free survival for almost 10 months)24 for patients with ALK-positive advanced NSCLC, particularly those with an adenocarcinoma histology. However, because of the rarity of PPC, insufficient data are available that demonstrate the benefit of administering ALK inhibitors to patients with PPC with ALK rearrangements.24 The first case of ALK-rearranged advanced PPC treated using crizotinib was reported by Murakami et al.12 in 2015, in which the patient achieved a partial response for 1 month; however, crizotinib was discontinued 3 months after initiation because of new liver and brain metastases. The ALK rearrangement detected in the adenocarcinoma and spindle cell components in our present patient indicates that these two components may have a monoclonal origin from a single ancestor. The patient achieved a partial response to crizotinib of only 7 months, indicating that the antitumor activities of crizotinib for ALK-rearranged PPC may not be as effective as those for ALK-rearranged lung adenocarcinoma. However, with the introduction of the second-generation ALK inhibitor alectinib, the survival of patients with ALK-rearranged advanced NSCLC is better compared with that achieved using crizotinib. Two randomized phase III clinical trials found that alectinib achieves superior efficacy and lower toxicity in primary treatment of ALK-positive NSCLC compared with crizotinib.25,26 Therefore, alectinib therapy may confer increased benefits upon patients with ALK-rearranged PPC.
In summary, the present patient with ALK-rearranged PPC was treated with crizotinib for 7 months. The findings indicate that patients with PPC, particularly those with an adenocarcinoma epithelial component may possess ALK rearrangements. Therefore, tests for ALK rearrangements should be routinely recommended for patients with PPC. Moreover, although the progression-free survival of these patients is limited, they may obtain increased benefit from crizotinib therapy than conventional chemotherapy. In the future, second-generation ALK inhibitors such as alectinib may confer longer survival compared with crizotinib for patients with ALK-rearranged PPC.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Yendamuri S, Caty L, Pine M, et al. Outcomes of sarcomatoid carcinoma of the lung: a Surveillance, Epidemiology, and End Results Database analysis. Surgery 2012; 152: 397–402. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Burke AP, et al. WHO classification of tumours of the lung, pleura, thymus and heart. 4th ed Lyon: IARC Press, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Mochizuki T, Ishii G, Nagai K, et al. Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am J Surg Pathol 2008; 32: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 4.Ito K, Oizumi S, Fukumoto S, et al. Clinical characteristics of pleomorphic carcinoma of the lung. Lung Cancer 2010; 68: 204–210. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto S, Hamatake D, Ueno T, et al. Clinicopathological investigation of pulmonary pleomorphic carcinoma. Eur J Cardiothorac Surg 2007; 32: 873–876. [DOI] [PubMed] [Google Scholar]
- 6.Bae H-M, Min HS, Lee S-H, et al. Palliative chemotherapy for pulmonary pleomorphic carcinoma. Lung Cancer 2007; 58: 112–115. [DOI] [PubMed] [Google Scholar]
- 7.Hong JY, Choi MK, Uhm JE, et al. The role of palliative chemotherapy for advanced pulmonary pleomorphic carcinoma. Med Oncol 2009; 26: 287–291. [DOI] [PubMed] [Google Scholar]
- 8.Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013; 8: 1574–1577. [DOI] [PubMed] [Google Scholar]
- 9.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK Fusion Is Linked to Histological Characteristics in a Subset of Lung Cancers. J Thorac Oncol 2008; 3: 13–17. [DOI] [PubMed] [Google Scholar]
- 10.Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009; 22: 508–515. [DOI] [PubMed] [Google Scholar]
- 11.Inamura K. Lung cancer: understanding its molecular pathology and the 2015 WHO Classification. Front Oncol 2017, 7: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami Y, Saka H, Oki M. Response to Crizotinib and Clinical Outcome in ALK-Rearranged Pulmonary Pleomorphic Carcinoma. J Thorac Oncol 2015; 10: e28–e29. [DOI] [PubMed] [Google Scholar]
- 13.Shiroyama T, Tanaka A, Tamiya M, et al. A Rare Case of Pleomorphic Carcinoma of the Lung Harboring an Anaplastic Lymphoma Kinase (ALK) Rearrangement. Intern Med 2015; 54: 2741–2743. [DOI] [PubMed] [Google Scholar]
- 14.Maruyama R, Matsumura F, Shibata Y, et al. Detection of ALK rearrangement in an octogenarian patient with pleomorphic carcinoma of the lung. Gen Thorac Cardiovasc Surg 2016; 64: 167–169. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Zhang Y, Lu J, et al. Pulmonary Sarcomatoid Carcinoma with ALK Rearrangement: frequency, clinical-pathologic characteristics, and response to ALK Inhibitor. Transl Oncol 2017; 10: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alì G, Proietti A, Niccoli C, et al. EML4-ALK translocation in both metachronous second primary lung sarcomatoid carcinoma and lung adenocarcinoma: a case report. Lung Cancer 2013; 81: 297–301. [DOI] [PubMed] [Google Scholar]
- 17.Terra SB, Jang JS, Bi L, et al. Molecular characterization of pulmonary sarcomatoid carcinoma: analysis of 33 cases. Mod Pathol 2016; 29: 824–831. [DOI] [PubMed] [Google Scholar]
- 18.Forest F, Yvorel V, Karpathiou G, et al. Histomolecular profiling of pleomorphic, spindle cell, and giant cell carcinoma of the lung for targeted therapies. Hum Pathol 2016; 49: 99–106. [DOI] [PubMed] [Google Scholar]
- 19.Tamura Y, Fujiwara Y, Yamamoto N, et al. Retrospective analysis of the efficacy of chemotherapy and molecular targeted therapy for advanced pulmonary pleomorphic carcinoma. BMC Res Notes 2015; 8: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Jia Y, Stoopler MB, et al. Next-Generation Sequencing of Pulmonary Sarcomatoid Carcinoma Reveals High Frequency of Actionable MET Gene Mutations. J Clin Oncol 2016; 34: 794–802. [DOI] [PubMed] [Google Scholar]
- 21.Toyokawa G, Taguchi K, Ohba T, et al. First case of combined small-cell lung cancer with adenocarcinoma harboring EML4-ALK fusion and an exon 19 EGFR mutation in each histological component. J Thorac Oncol 2012; 7: e39–e41. [DOI] [PubMed] [Google Scholar]
- 22.Omachi N, Shimizu S, Kawaguchi T, et al. A case of large-cell neuroendocrine carcinoma harboring an EML4-ALK rearrangement with resistance to the ALK inhibitor crizotinib. J Thorac Oncol 2014; 9: e40–e42. [DOI] [PubMed] [Google Scholar]
- 23.Caliò A, Nottegar A, Gilioli E, et al. ALK/EML4 fusion gene may be found in pure squamous carcinoma of the lung. J Thorac Oncol 2014; 9: 729–732. [DOI] [PubMed] [Google Scholar]
- 24.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014; 371: 2167–2177. [DOI] [PubMed] [Google Scholar]
- 25.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017; 390: 29–39. [DOI] [PubMed] [Google Scholar]
- 26.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017; 377: 829–838. [DOI] [PubMed] [Google Scholar]