Short abstract
We herein report two cases of Fanconi syndrome with refractory hypophosphatemic osteomalacia that was difficult to correct by phosphorus replacement therapy. The pathological result was a bony giant cell tumor and osteosarcoma, respectively. Interestingly, after resection of the tumors, the patient with osteosarcoma recovered completely but the patient with the bony giant cell tumor had a relapse. Although she underwent nine operations, her symptoms and laboratory tests did not improve. These findings indicate that Fanconi syndrome can result from a bone tumor.
Keywords: Osteomalacia, renal Fanconi syndrome, bone tumor, tumor-induced osteomalacia, osteosarcoma, phosphorus replacement
Introduction
Fanconi syndrome represents a generalized dysfunction of the proximal tubules with varying degrees of wasting of any substance normally reclaimed by the proximal tubular cells.1 Wasting of phosphate, glucose, amino acids, and bicarbonate produces specific clinical consequences. Osteomalacia associated with adult-acquired Fanconi syndrome is thought to result from hypophosphatemia and relative calcitriol deficiency.2 Few reports have described Fanconi syndrome caused by solid tumors.2 Tumor-induced osteomalacia is a rare metabolic bone disease that is caused by tumors producing fibroblast growth factor 23 (FGF-23).3 Overproduction of FGF-23 by the tumors induces hypophosphatemia and calcitriol deficiency, which results in defects in the mineralization of newly formed bone collagen.3 We herein describe two cases of hypophosphatemia, glycosuria, aminoaciduria, and bone tumors in two patients. These two cases are being reported to remind clinicians that Fanconi syndrome can be caused by bone tumors. Additionally, urine laboratory tests should be routinely performed in patients with oncogenic osteomalacia to determine the presence of glycosuria, aminoaciduria, or phosphaturia.
Case 1
A 43-year-old woman presented with a 10-year history of progressive bone pain, especially in the knee joint, beginning in 2003. Physical examination revealed kyphosis and valgus deformity of the ribs with obvious pain points. Her lower extremities were swollen, and her muscle strength was decreased (grade 3). Hypophosphatemia (0.21 mmol/L; reference range, 0.80–1.45 mmol/L), aminoaciduria, and glycosuria were detected. Her bone-type alkaline phosphatase level was high (>250 U/L; reference range, 40–150 U/L), as was her parathyroid hormone level (14.2 pmol/L; reference range, 1.1–7.5 pmol/L). However, her 1,25(OH)D3 level was normal at 24.65 pg/mL (reference range, 15.9–55.6 pg/mL). Renal glycosuria was confirmed by a simple oral glucose tolerance test. Her urine phosphorus level was 602 mg/24 hours (reference range, 713–1488 mg/24 hours) and did not increase as expected, but her percent tubular reabsorption of phosphate was 80% (reference range, 85%–90%), calculated as follows: 100 × [1 − (Up × Scr) / (Sp × Ucr)], where Up is urinary phosphorus, Scr is serum creatinine, Sp is serum phosphorus, and Ucr is urinary creatinine. A hip radiograph demonstrated low density of the superior branch of the pubis and blurred edge of the upper femur. The bone mineral density of the total lumbar spine (L2–L4) was 0.968 g/cm3 (T-score = −1.3), and that of the femoral neck was 0.697 g/cm3 (T-score = −1.7). The circulating immune complex and rheumatoid factor levels were 24 IU/mL (reference range, 0.0–13.1 IU/mL) and 357 IU/mL (reference range, 0.0–30 IU/mL), respectively. A muscle biopsy showed immunoglobulin G and fibrin-related antigen deposition along the muscle cell membrane, which indicated that the patient’s symptoms may have been related to immune factors. Therefore, the patient underwent immunosuppressive therapy with methylprednisolone at 40 mg/day for 2 weeks. However, this treatment had no effect. From 2003 to 2011, the patient was hospitalized eight times because of unrelieved bone pain. In 2011, the patient developed painful right ankle swelling and thus underwent amputation of her right lower extremity; postoperative pathologic examination revealed synovial sarcoma. The operation had marked beneficial effects in this patient. Her hypophosphatemia significantly improved from 0.64 to 1.51 mmol/L and did not recur, her urine phosphorus level decreased from 940.4 to 29.14 mg/24 hours (reference range, 713–1488 mg/24 hours), and her serum calcium level normalized. Her alkaline phosphatase (ALP) level gradually decreased from 250 to 90 U/L. Moreover, the glucose and amino acids in her urine disappeared. Her bone pain was completely ameliorated.
Case 2
In 1993, an 8-year-old girl with whole-body weakness and an inability to walk was diagnosed with hypophosphatemia and vitamin D-resistant rickets, and she began treatment with neutral phosphorus and calcitriol. In 1995, tenderness of the ulnar side of the distal right forearm was evident, and bone biopsy specimens showed a grade III giant cell tumor (Figure 1). The patient underwent tumor resection. The ALP level decreased from 915 U/L preoperatively to 529 U/L postoperatively. In 1999, the patient’s laboratory findings were as follows: hypophosphatemia (1.99 mmol/L), high serum ALP (843 U/L), high urinary phosphorus (1363.2 mg/24 hours), low urinary calcium (20.4 mg/24 hours; reference range, 150–250 mg/24 hours), and positive urine glucose. Urine amino acid analysis suggested aminoaciduria, and renal acidification function testing suggested renal tubular acidosis. The patient also exhibited scoliosis, genu varus of the left lower extremity, and knee valgus of the right lower extremity. In X-ray, there were thoracic scoliosis and enlargement of the costal head; thus, the patient was diagnosed with Fanconi syndrome. In 2002, the patient underwent right forearm mass resection, and postoperative pathologic examination showed a grade II to III giant cell tumor of bone. From 2004 to 2010, the patient underwent eight operations for the giant cell tumor of bone in the right forearm. In 2010, a radiograph of the right forearm showed an irregular cortex of the distal radius and a wavy ulnar edge with a strip of dense shadow (Figure 2). However, her serum phosphorus level was still below the reference range, and her aminoaciduria and glycosuria persisted.
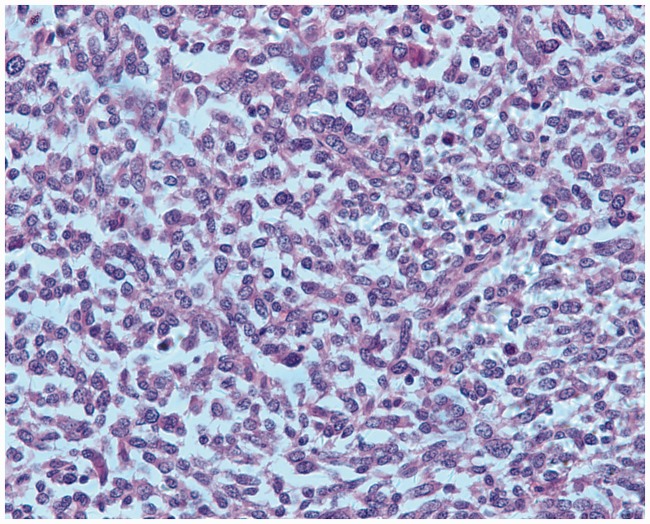
Figure 1.
Pathological findings of bone biopsy. A large number of spindle cells and partially visible mitosis of bone cells led to a diagnosis of a grade III giant cell tumor of bone (hematoxylin and eosin, 400×).
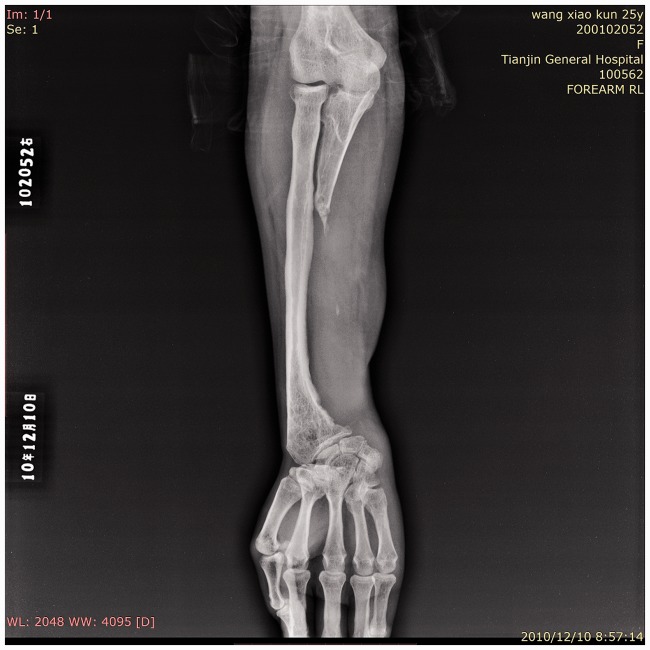
Figure 2.
Radiograph of the right forearm. The radiograph showed an irregular cortex of the distal radius and a wavy ulnar edge with a strip of dense shadow.
Ethics approval and patient consent
This study was performed in accordance with the Declaration of Helsinki (1975). Written informed consent was obtained from both patients.
Discussion
Fanconi syndrome may be inherited or acquired. Inborn errors in patients with inherited Fanconi syndrome include infantile nephropathic cystinosis, Lowe syndrome, and others. Causes of acquired Fanconi syndrome include some drugs such as valproic acid, ifosfamide, and tenofovir; multiple myeloma; amyloidosis; and acute tubular necrosis.4 However, few reports have described Fanconi syndrome caused by solid tumors that secrete FGF-23, causing increased renal phosphate wasting and resultant bone demineralization.5 In addition to FGF-23, some phosphatonins such as secreted frizzled-related protein 4, FGF-7, and matrix extracellular phosphoglycoprotein have been identified from gene expression studies in patients with tumor-induced osteomalacia.6–8 However, the serum FGF-23 level is not always high. In one study, the FGF-23 level was elevated in 16 (94.1%) of 17 Chinese patients with tumor-induced osteomalacia.5 In our two cases, we found no typical features of phosphaturic mesenchymal tumor, mixed connective tissue variant in the pathologic examination. Unfortunately, we were unable to test the patients’ FGF-23 levels. Interestingly, the first patient’s Fanconi syndrome was cured after removal of the synovial sarcoma, while the second patient developed several recurrences of her bone giant cell tumor and her Fanconi syndrome did not improve. Therefore, we hypothesized that Fanconi syndrome can be caused by a bone tumor. In fact, ours is not the first report to describe tumor-induced Fanconi syndrome. Norden et al.9 reported an old female patient with osteomalacia due to a paraspinal tumor of mesenchymal origin. The authors considered that either plasma FGF-23 or other humoral factors highly correlated with FGF-23 had caused the renal Fanconi syndrome; however, there is no proof that a specific factor results in Fanconi syndrome.9 Based on our cases, we consider that patients can develop Fanconi syndrome secondary to a tumor, which can produce some factors that affect proximal tubular cells. We believe that our patients had tubular transport defects in addition to phosphorus wasting and that their tumors may have produced substances with more generalized effects on proximal tubular cells. Possibilities include interference with intracellular generation of ATP and/or inhibition of sodium-potassium ATPase, resulting in a decrease in sodium-coupled solute transport. However, the factors that may induce such phenomena are unknown.
Authors’ contributions
Manting Gou collected and analyzed the case data and wrote the report. Zhongshu Ma analyzed the cases.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Solano A, Lew SQ, Ing TS. Dent–Wrong disease and other rare causes of the Fanconi syndrome. Clin Kidney J 2014; 7: 344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke BL, Wynne AG, Wilson DM, et al. Osteomalacia associated with adult Fanconi’s syndrome: clinical and diagnostic features. Clin Endocrinol (Oxf) 1995; 43: 479–490. [DOI] [PubMed] [Google Scholar]
- 3.Chong WH, Molinolo AA, Chen CC, et al. Tumor-induced osteomalacia. Endocr Relat Cancer 2011; 18: R53–R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke BL, Wynne AG, Wilson DM. Osteomalacia associated with adult Fanconi’s syndrome: clinical and diagnostic feature. Clin Endocrinol (Oxf) 1995; 43: 479–490. [DOI] [PubMed] [Google Scholar]
- 5.Yu WJ, He JW, Fu WZ, et al. Reports of 17 Chinese patients with tumor-induced osteomalacia. J Bone Miner Metab 2017; 35: 298–307. [DOI] [PubMed] [Google Scholar]
- 6.Quarles LD. Evidence for a bone–kidney axis regulating phosphate homeostasis. J Clin Invest 2003; 112: 642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter TO, Ellis BK, Insogna KL, et al. Fibroblast growth factor 7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab 2005; 90: 1012–1020. [DOI] [PubMed] [Google Scholar]
- 8.Habra MA, Jimenez C, Huang SC, et al. Expression analysis of fibroblast growth factor-23, matrix extracellular phosphoglycoprotein, secreted frizzled-related protein-4, and fibroblast growth factor-7: identification of fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein as major factors involved in tumor-induced osteomalacia. Endocr Pract 2008; 14: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 9.Norden AG, Laing RJ, Rowe P, et al. Oncogenic osteomalacia, raised FGF-23, and renal Fanconi syndrome. QJM 2014; 107: 139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]