Short abstract
Objectives
Lower serum melatonin levels are found in patients with ischaemic stroke compared with healthy controls. This study aimed to determine whether serum melatonin levels are associated with peroxidation status, antioxidant status, and mortality in patients with ischaemic stroke.
Methods
Patients with severe malignant middle cerebral artery infarction (MMCAI), defined as a Glasgow coma scale (GCS) score lower than 9, were included. Serum levels of melatonin, malondialdehyde (to assess lipid peroxidation), and total antioxidant capacity at the time of diagnosing MMCAI were determined. We chose 30-day mortality as the endpoint of the study.
Results
We found significantly higher serum levels of melatonin, total antioxidant capacity, and malondialdehyde in non-survivors (n = 32) than in survivors (n = 32) with MMCAI. Serum melatonin levels were associated with 30-day mortality (odds ratio = 2.205; 95% confidence interval = 1.294–3.759) after controlling for GCS score and age. We found a positive association between serum melatonin levels and total antioxidant capacity (rho = 0.36), and between serum melatonin and malondialdehyde levels (rho = 0.35).
Conclusions
Our study shows that serum melatonin levels are associated with peroxidation status, antioxidant status, and mortality in patients with MMCAI.
Keywords: Melatonin, cerebral infarction, mortality, stroke, malondialdehyde, antioxidant capacity
Introduction
Ischaemic stroke causes mortality, disability, and consumption of health resources.1 In cerebral infarction, there is cell death due to brain obstruction of the vasculature, which limits the supply of blood containing vital oxygen and substrates for neurons. Additionally, there can be a secondary injury due to different mechanisms, such as oxidative stress, inflammation, and apoptosis, which could contribute to increase cell death.2–6 Administration of melatonin on traumatic brain injury can have different beneficial effects, such as anti-oxidant, anti-inflammatory, and anti-apoptotic effects.7–9
Melatonin levels in patients with ischaemic stroke have not been well determined.10–15 Lower urinary levels of melatonin and/or its metabolite 6-sulfatoxymelatonin,10–14 and lower serum melatonin levels15 have been found in patients with ischaemic stroke compared with healthy controls. Therefore, this study aimed to determine whether serum melatonin levels are associated with peroxidation status, antioxidant status, and mortality in patients with ischaemic stroke.
Methods
Design and subjects
In this observational and prospective study, 64 patients with severe malignant middle cerebral artery infarction (MMCAI) were included. The diagnosis of ischaemic stroke was based on clinical and computed tomography findings.1 We considered a middle cerebral artery infarction territory as MMCAI when computed tomography findings indicated that there were ischaemic changes that affected more than 50% of the territory. Clinical severity of MMCAI was assessed using the Glasgow coma scale (GCS).16 We considered an MMCAI to be severe when the GCS score was < 9. Therefore, we included patients with MMCAI and a GCS score < 9. Exclusion criteria were pregnancy, inflammatory or malignant disease, and age younger than 18 years.
This multicentre study was carried out at six intensive care units (ICUs) in Spain. The study was approved by the Institutional Review Board of each of the following participating hospitals: Hospital Universitario Nuestra Señora de Candelaria from Santa Cruz de Tenerife, Hospital Universitario de Canarias from La Laguna, Hospital Universitario Dr. Negrín from Las Palmas de Gran Canaria, Hospital Insular from Las Palmas de Gran Canaria, Hospital General de La Palma from Breña Alta, and Hospital Clínico Universitario de Valencia from Valencia. Legal guardians of patients signed written informed consent.
Variables recorded
The following variables were recorded for each patient: sex, fibrinolytic therapy, decompressive craniectomy, age, temperature, sodium levels, glycaemia, leukocytes, pressure of arterial oxygen (PaO2), the PaO2/pressure of arterial oxygen/fraction inspired oxygen (FIO2) ratio, bilirubin levels, creatinine levels, haemoglobin levels, GCS, lactic acid levels, platelet count, international normalized ratio, activated partial thromboplastin time, fibrinogen levels, and Acute Physiology and Chronic Health Evaluation II score.17 The endpoint of the study was 30-day mortality.
Measurement of melatonin, malondialdehyde and total antioxidant capacity levels
Serum samples were collected at the time of diagnosing MMCAI and were frozen at −80°C until measurement of melatonin, malondialdehyde, and total antioxidant capacity levels.
Serum melatonin levels were measured by the ELISA method using a commercial kit from Immuno Biological Laboratories (IBL Hamburg GmbH, Hamburg, Germany) in the Physiology Department of the Faculty of Medicine from La Laguna University (Tenerife, Spain). This kit has a detection limit of 0.13 pg/mL, and intra- and inter-assay coefficients of variation were 6.4% and 11.1%, respectively.
Serum levels of malondialdehyde, which is an end-product formed during lipid peroxidation that is released into the extracellular space and finally appears in the blood,18,19 were measured to assess oxidation. Malondialdehyde levels were measured using a thiobarbituric acid-reactive substance assay 20 in the Physiology Department of the Faculty of Medicine from La Laguna University (Tenerife, Spain). This kit has a detection limit of 0.079 nmol/mL, and intra- and inter-assay coefficients of variation were 1.82% and 4.01%, respectively.
Serum total antioxidant capacity provides better information on antioxidant status than individual antioxidant compounds21 because antioxidant compounds establish complex interactions with other antioxidant compounds.22 Therefore, we measured serum total antioxidant capacity to assess antioxidant status. Total antioxidant capacity measurements were performed using an antioxidant assay kit (Cayman Chemical Corporation, Ann Arbor, MI, USA) in the Laboratory Department of the Hospital Universitario de Canarias from La Laguna, (Tenerife, Spain). This kit has a detection limit of 0.04 mmol/L, and intra- and inter-assay coefficients of variation were 3.4% and 3.0%, respectively.
Statistical methods
We used medians and interquartile ranges to report continuous variables, and frequencies and percentages to report categorical variables. We used the Wilcoxon–Mann–Whitney test to compare continuous variables between groups and the chi-square test to compare categorical variables. Multiple logistic regression analysis was applied to determine the association between serum melatonin levels and 30-day mortality, controlling for the GCS score and age. We calculated odds ratios (ORs) and their 95% confidence intervals (CIs) to measure the clinical impact of each variable. We used receiver operating characteristic analysis to determine the 30-day mortality prediction capacity for serum melatonin levels. The Youden J index was used to select the optimal cut-off serum melatonin level for prognostic value (2.93 pg/mL). We carried out Kaplan–Meier analysis to compare 30-day survival between patients with serum melatonin levels ≤ 2.93 pg/mL and > 2.93 pg/mL. Spearman’s rank correlation coefficient was used to determine the association between serum levels of melatonin, total antioxidant capacity, and malondialdehyde. Bonferroni correction was used for multiple comparisons. All p values lower than 0.05 were considered as statistically significant. We performed statistical analyses using LogXact 4.1 (Cytel Co., Cambridge, MA, USA), NCSS 2000 (NCSS, Kaysville, UT, USA), and SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
A total of 32 of 64 patients died at 30 days of severe MMCAI. Non-survivors showed significantly higher serum levels of melatonin (p < 0.001), malondialdehyde (p < 0.001), and total antioxidant capacity (p < 0.001), and had a lower GCS score (p < 0.01) than did survivors (Table 1).
Table 1.
Clinical and biochemical characteristics of patients with malignant middle cerebral artery infarction according to 30-day survival
| Survivors(n = 32) | Non-survivors(n = 32) | p value | |
|---|---|---|---|
| Sex, women | 13 (40.6%) | 12 (37.5%) | 0.99 |
| Age (years) | 59 (47–68) | 64 (54–70) | 0.30 |
| Volume of infarction (mL) | 173 (100–231) | 180 (60–277) | 0.64 |
| Thrombolysis | 10 (31.3%) | 10 (31.3%) | 0.99 |
| Temperature (°C) | 36.4 (35.8–37.0) | 37.0 (36.0–37.4) | 0.19 |
| TAC (mmol/L) | 2.28 (1.88–3.31) | 6.30 (3.44–12.31) | <0.001 |
| Platelet count (× 103/mm3) | 214 (170–280) | 170 (131–212) | 0.008 |
| PaO2 (mmHg) | 137 (104–207) | 114 (86–153) | 0.26 |
| PaO2/FIO2 ratio | 300 (197–372) | 248 (184–330) | 0.22 |
| Midline shift (mm) | 6 (2–12) | 10 (3–15) | 0.42 |
| Melatonin (pg/mL) | 2.46 (1.77–3.37) | 4.84 (3.83–11.61) | <0.001 |
| Malondialdehyde (nmol/mL) | 1.89 (1.28–2.29) | 2.95 (1.92–4.51) | <0.001 |
| Leukocytes-median × 103/mm3 (p 25–75) | 12.5 (9.5–17.0) | 13.9 (9.3–21.4) | 0.43 |
| Lactic acid (mmol/L) | 1.30 (0.90–1.70) | 1.40 (1.00–2.10) | 0.25 |
| INR | 1.09 (1.01–1.20) | 1.20 (1.05–1.31) | 0.10 |
| Haemoglobin (g/dL) | 12.2 (11.4–14.4) | 13.7 (11.0–15.0) | 0.78 |
| Haemorrhagic transformation | 7 (21.9%) | 6 (18.8%) | 0.99 |
| Glycaemia (g/dL) | 128 (100–170) | 135 (105–160) | 0.99 |
| GCS score | 7 (6–8) | 6 (3–7) | 0.01 |
| Fibrinogen (mg/dL) | 440 (335–494) | 419 (311–631) | 0.83 |
| Decompressive craniectomy | 8 (25.0%) | 5 (15.6%) | 0.54 |
| Creatinine (mg/dL) | 0.80 (0.60–1.15) | 1.00 (0.76–1.28) | 0.12 |
| Bilirubin (mg/dL) | 0.70 (0.40–0.95) | 0.70 (0.33–1.10) | 0.86 |
| aPTT (s) | 28 (26–30) | 27 (26–32) | 0.77 |
| APACHE-II score | 20 (16–25) | 22 (19–27) | 0.10 |
Data are presented as number of patients (percent), or median (interquartile range); PaO2 = pressure of arterial oxygen; FIO2 = pressure of arterial oxygen/fraction of inspired oxygen; INR = international normalized ratio; GCS= Glasgow coma scale; aPTT = activated partial thromboplastin time; APACHE = Acute Physiology and Chronic Health Evaluation; TAC = total antioxidant capacity.
There was no significant difference in serum melatonin levels between women and men (3.72 [2.11–6.58] vs 3.81 [2.43–6.18] pg/mL; p = 0.46), and no association with age (rho = 0.15; p = 0.22).
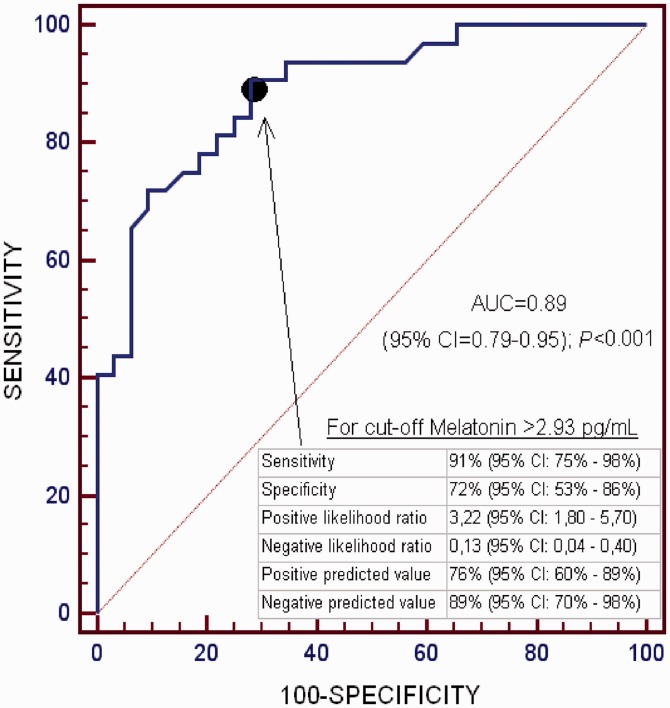
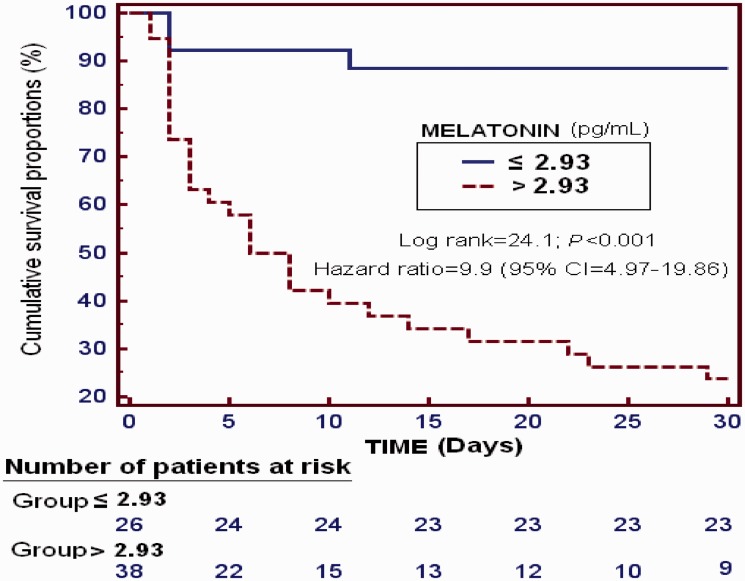
In multiple logistic regression analysis, serum melatonin levels predicted 30-day mortality (OR = 2.205; 95% CI = 1.294–3.759; p = 0.004) after controlling for GCS score and age (Table 2). The area under the curve of 30-day mortality prediction for serum melatonin levels was 0.89 (95% CI = 0.79–0.95; p < 0.001) (Figure 1). Survival analysis showed a higher 30-day mortality in patients with serum melatonin levels > 2.93 pg/mL than in those with lower levels (hazard ratio = 9.9; 95% CI = 4.97–19.86; p < 0.001) (Figure 2).
Table 2.
Multiple binomial logistic regression analysis to predict 30-day mortality
| Variable | Odds ratio | 95% Confidence interval | p value |
|---|---|---|---|
| Age (years) | 1.001 | 0.957–1.047 | 0.97 |
| Serum melatonin levels (pg/mL) | 2.205 | 1.294–3.759 | 0.004 |
| Glasgow coma scale score | 0.723 | 0.516–1.013 | 0.06 |
Figure 1.
Receiver operating characteristic analysis using serum melatonin levels as a predictor of mortality at 30 days
Figure 2.
Survival curves at 30 days using serum melatonin levels ≤ 2.93 pg/mL vs > 2.93 pg/mL
We found a positive association between serum levels of melatonin and total antioxidant capacity (rho = 0.36; p = 0.003), between serum levels of melatonin and malondialdehyde (rho = 0.35; p = 0.005), and between serum levels of total antioxidant capacity and malondialdehyde (rho = 0.43; p < 0.001). All of these p values were statistically significant after Bonferroni correction for multiple comparisons.
Discussion
To the best of our knowledge, our study is the first series to report data on serum melatonin levels in surviving and non-surviving patients with MMCAI. We found that patients with MMCAI who did not survive showed higher serum melatonin levels than did those who survived. We also showed that serum melatonin levels were associated with peroxidation status, antioxidant status, and mortality in patients with MMCAI.
There are scarce data regarding serum melatonin levels in patients with ischaemic stroke. A previous study reported lower serum melatonin levels in patients with ischaemic stroke compared with healthy controls.15 However, there are no previous data regarding serum melatonin levels in patients with ischaemic stroke according to survival. Therefore, our study is the first to report higher serum melatonin levels in non-surviving than in surviving patients with MMCAI. Additionally, we found that serum melatonin levels were associated with early mortality in patients with MMCAI after controlling for CGS and age in multiple logistic regression analysis. We also found that serum melatonin levels were a good predictor of 30-day mortality according to results of receiver operating characteristic analysis.
Another interesting novel finding of our study was that serum melatonin levels were associated with peroxidation status and antioxidant status in patients with MMCAI. A positive association was found between serum levels of melatonin and malondialdehyde, and between serum levels of melatonin and total antioxidant capacity. In our previous studies, we found an association between mortality of patients with MMCAI and serum levels of total antioxidant capacity23 and of malondialdehyde24
We speculate that an explanation for our findings is that non-survivors with MMCAI had increased reactive oxygen species production. This could have led to an increased oxidant state (according to higher serum malondialdehyde levels), higher circulating total antioxidant status (according to higher serum total antioxidant capacity), and higher serum melatonin levels to compensate for the increased production of oxidizing products. However, this attempt of compensation to maintain the balance of the oxidant and antioxidant states could have been insufficient in non-surviving patients with MMCAI. Consequently, these patients had higher peroxidation and finally died.
In animal models of ischaemic stroke, administration of melatonin has beneficial effects, such as reducing oxidation, inflammation, apoptosis, mitochondrial dysfunction, brain oedema, brain infarction volume, and neurological dysfunction, and increasing survival.25–34 Therefore, the findings of our study and those from animal models could lead to administration of melatonin in patients with ischaemic stroke.
Melatonin levels can decrease with age.35,36 However, we did not find any significant difference in serum melatonin levels according to age. Some studies have shown differences in serum melatonin secretion according to sex, with lower melatonin levels37 and higher melatonin levels 38,39 found in women. However, serum melatonin levels were not significantly different between women and men in our study.
We recognize that there are some limitations in our study. First, we did not report serum melatonin levels during follow-up in non-surviving and surviving patients. Second, blood samples were not obtained from all of the patients at the same time of day. Therefore, serum samples were collected at the time of diagnosing MMCAI. There is a circadian rhythm of melatonin, with lower values during the light than in the dark period. However, we measured ICU light intensity and we found a light intensity of 2.8 lux during the light period (corresponding to the period of greatest ICU activity) and of 0.2 lux during the dark period (corresponding to the period of the least ICU activity). The light intensity ratio between the light and dark periods in the ICU is approximately 14. However, outside of the hospital, the light intensity varies from 1000 lux during the light period to 0.1 lux during the dark period. Consequently, the light intensity ratio between the light and dark periods under normal conditions could be approximately 10,000. Therefore, there were almost no changes in the light intensity in the ICU throughout the day. Despite these limitations of our study, we believe that our novel and interesting findings and those from animal models could generate interest in research on melatonin in stroke patients regarding prediction of mortality and treatment.
Conclusions
Our series reported data on serum melatonin levels in surviving and non-surviving patients with MMCAI. Our study shows that non-survivors with MMCAI have higher serum melatonin levels than do survivors. Furthermore, serum melatonin levels are associated with peroxidation status, antioxidant status, and mortality in patients with MMCAI.
Key messages
Non-surviving patients with MMCAI have higher serum melatonin levels than do survivors.
Serum melatonin levels are associated with mortality of patients with MMCAI.
There is an association of serum melatonin levels with peroxidation and antioxidant status in patients with MMCAI.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by a grant from Grupo de Expertos Neurológicos de Canarias (GEN-Canarias, Santa Cruz de Tenerife, Spain) and by a grant from Instituto de Salud Carlos III (INT16/00165) (Madrid, Spain) co-financed with Fondo Europeo de Desarrollo Regional (FEDER).
References
- 1.Adams HP, Jr, del Zoppo G, Alberts MJ, et al . Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007; 38: 1655–1711. [DOI] [PubMed] [Google Scholar]
- 2.Lewis KM, Turner RJ, Vink R. Blocking neurogenic inflammation for the treatment of acute disorders of the central nervous system. Int. J. Inflam 2013. , 2013, 578480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol 2006; 147(Suppl 1): S232–S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCall JM, Braughler JM, Hall ED. Lipid peroxidation and the role of oxygen radicals in CNS injury. Acta Anaesthesiol Belg 1987; 38: 373–379. [PubMed] [Google Scholar]
- 5.Warner DS, Sheng H, Batinić-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol 2004; 207: 3221–3231. [DOI] [PubMed] [Google Scholar]
- 6.Hall ED. Lipid antioxidants in acute central nervous system injury. Ann Emerg Med 1993; 22: 1022–1027. [DOI] [PubMed] [Google Scholar]
- 7.Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol 2010; 8: 228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter RJ, Sainz RM, Lopez-Burillo S, et al. Melatonin ameliorates neurologic damage and neurophysiologic deficits in experimental models of stroke. Ann N Y Acad Sci 2003; 993: 35–47. [DOI] [PubMed] [Google Scholar]
- 9.Andrabi SS, Parvez S, Tabassum H. Melatonin and Ischemic Stroke: Mechanistic Roles and Action. Adv Pharmacol Sci 2015. ; 2015: 384750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritzenthaler T, Nighoghossian N, Berthiller J, et al. Nocturnal urine melatonin and 6-sulphatoxymelatonin excretion at the acute stage of ischaemic stroke. J Pineal Res 2009; 46: 349–352. [DOI] [PubMed] [Google Scholar]
- 11.Ritzenthaler T, Lhommeau I, Douillard S, et al. Dynamics of oxidative stress and urinary excretion of melatonin and its metabolites during acuteischemic stroke. Neurosci Lett 2013; 544: 1–4. [DOI] [PubMed] [Google Scholar]
- 12.Fiorina P, Lattuada G, Silvestrini C, et al. Disruption of nocturnal melatonin rhythm and immunological involvement in ischaemic stroke patients. Scand J Immunol 1999; 50: 228–231. [DOI] [PubMed] [Google Scholar]
- 13.Kulesh AA, Shestakov VV. Secretion of melatonin and serum cholinesterase activity as biological markers of cognitive disorders in the acute stage of ischemic stroke. Zh Nevrol Psikhiatr Im S S Korsakova 2012; 112: 11–14. [PubMed] [Google Scholar]
- 14.Kulesh AA, Shestakov VV. Chronobiological parameters, cognitive emotional status and sleep quality in acute stroke. Zh Nevrol Psikhiatr Im S S Korsakova 2013; 113: 24–28. [PubMed] [Google Scholar]
- 15.Atanassova PA, Terzieva DD, Dimitrov BD. Impaired nocturnal melatonin in acute phase of ischaemic stroke: cross-sectional matched case-control analysis. J Neuroendocrinol 2009; 21: 657–663. [DOI] [PubMed] [Google Scholar]
- 16.Teasdale G, Jennett B. Assessement of coma and impaired conciousness. A practical scale. Lancet 1974; 2: 81–84. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 18.Dalle-Donne I, Rossi R, Colombo R, et al. Biomarkers of oxidative damage in human disease. Clin Chem 2006; 52: 601–623. [DOI] [PubMed] [Google Scholar]
- 19.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 1990; 186: 421–431. [DOI] [PubMed] [Google Scholar]
- 20.Kikugawa K, Kojima T, Yamaki S, et al. Interpretation of the thiobarbituric acid reactivity of rat liver and brain homogenates in the presence of ferric ion and ethylediaminotetraacetic acid. Anal Biochem 1992; 202: 249–255. [DOI] [PubMed] [Google Scholar]
- 21.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol 2001; 54: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghiselli A, Serafini M, Natella F, et al. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med 2000; 29: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 23.Lorente L, Martín MM, Pérez-Cejas A, et al. Association between total antioxidant capacity and mortality in ischemic stroke patients. Ann Intensive Care 2016; 6: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorente L, Martín MM, Abreu-González P, et al. Serum malondialdehyde levels in patients with malignant middle cerebral artery infarction are associated with mortality. PLoS One 2015; 10: e0125893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh PO. Melatonin attenuates the cerebral ischemic injury via the MEK/ERK/p90RSK/bad signaling cascade. J Vet Med Sci 2008; 70: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 26.Cuzzocrea S, Costantino G, Gitto E, et al. Protective effects of melatonin in ischemic brain injury. J Pineal Res 2000; 29: 217–227. [DOI] [PubMed] [Google Scholar]
- 27.Koh PO. Melatonin regulates the calcium-buffering proteins, parvalbumin and hippocalcin, in ischemic brain injury. J Pineal Res 2012; 53: 358–365. [DOI] [PubMed] [Google Scholar]
- 28.Tai SH, Hung YC, Lee EJ, et al. Melatonin protects against transient focal cerebral ischemia in both reproductively active and estrogen-deficient female rats: the impact of circulating estrogen on its hormetic dose-response. J Pineal Res 2011; 50: 292–303. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Jiang S, Dong Y, et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J Pineal Res 2015; 58: 61–70. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Wang Y, Feng D, et al. Alterations in the time course of expression of the Nox family in the brain in a rat experimental cerebral ischemia and reperfusion model: effects of melatonin. J Pineal Res 2014; 57: 110–119. [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharya P, Pandey AK, Paul S, et al. Melatonin renders by protein kinase C mediated aquaporin-4 inhibition in animal model of focal cerebral ischemia. Life Sci 2014; 100: 97–109. [DOI] [PubMed] [Google Scholar]
- 32.Paredes SD, Rancan L, Kireev R, et al. Melatonin Counteracts at a Transcriptional Level the Inflammatory and Apoptotic Response Secondary to Ischemic Brain Injury Induced by Middle Cerebral Artery Blockade in Aging Rats. Biores Open Access 2015; 4: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Y, Feng Q, Ma J, et al. Melatonin ameliorates injury and specific responses of ischemic striatal neurons in rats. J Histochem Cytochem 2013; 61: 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balduini W, Carloni S, Perrone S, et al. The use of melatonin in hypoxic-ischemic brain damage: an experimental study. J Matern Fetal Neonatal Med 2012; 25(Suppl 1): 119–124. [DOI] [PubMed] [Google Scholar]
- 35.Ermolenko KS, Rapoport SI, Solov'eva AV. Melatonin secretion in women of advanced reproductive age. Klin Med (Mosk) 2013; 91: 52–54. [PubMed] [Google Scholar]
- 36.Wetterberg L, Aperia B, Gorelick DA, et al. Age, alcoholism and depression are associated with low levels of urinary melatonin. J Psychiatry Neurosci 1992; 17: 215–224. [PMC free article] [PubMed] [Google Scholar]
- 37.Obayashi K, Saeki K, Tone N, et al. Lower melatonin secretion in older females: gender differences independent of light exposure profiles. J Epidemiol 2015; 25: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wada K, Nakamura K, Tamai Y, et al. Associations of urinary 6-sulfatoxymelatonin with demographics, body mass, sex steroids, and lifestyle factors in preschool Japanese children. Ann Epidemiol 2013; 23: 60–65. [DOI] [PubMed] [Google Scholar]
- 39.Touitou Y, Fevre-Montange M, Proust J, et al. Age- and sex-associated modification of plasma melatonin concentrations in man. Relationship to pathology, malignant or not, and autopsy findings. Acta Endocrinol (Copenh) 1985; 108: 135–144. [DOI] [PubMed] [Google Scholar]