Short abstract
Acetaminophen is absorbed rapidly after oral intake, and serum concentration peaks within 4 hours. The Rumack–Matthew (RM) nomogram is widely used to identify the potential risk of liver dysfunction. However, the RM nomogram was intended for use only when a single agent was ingested. We report the case of a patient with overdose ingestion of an over-the-counter combination cold medication that contained acetaminophen, where the patient’s serum concentration increased over time. Over-the-counter combination cold medications are designed to relieve cold symptoms. However, the possibility that other agents that were present in the drug may change gastrointestinal kinetics should also be considered. The risk of liver dysfunction cannot be accurately determined from a single serum acetaminophen concentration measurement. Because of the risk of a delayed increase in the serum acetaminophen concentration, monitoring for liver dysfunction and developing a treatment strategy that includes N-acetylcysteine are required. This case report is targeted to clinical physicians who treat patients with acetaminophen overdose resulting from ingestion of multiple agents, and it reviews points of consideration when using the RM nomogram in acute intoxication.
Keywords: Rumack–Matthew nomogram, delayed increase in acetaminophen concentration, multi-agent drug containing acetaminophen, N-acetylcysteine, liver dysfunction, acetaminophen overdose
Introduction
Acetaminophen is a widely used ingredient in antipyretic analgesics. Although high levels of this agent may also lead to liver dysfunction,1 over-the-counter combination medications, containing acetaminophen, for the common cold are readily available at many drugstores. Acetaminophen is rapidly absorbed from the gastrointestinal tract. A therapeutic dose is absorbed within 70 minutes, and an intoxicating dose is absorbed within 4 hours, at which time and serum concentration peaks.2 We report the case of a patient who ingested over-the-counter combination medications containing acetaminophen, where their serum concentration increased over time, resulting in an intoxicating dose after 4 hours. When a combination of medications has been taken, changes in the serum acetaminophen concentration may differ from those following ingestion of a single agent. However, there are no reports on the serum acetaminophen concentration over time for combination medications. The Rumack–Matthew (RM) nomogram can identify the risk of severe liver dysfunction occurrence by measuring the serum acetaminophen concentration. However, this nomogram was intended for use only following ingestion of a single agent. This case report is targeted to clinical physicians who treat patients with acetaminophen intoxication resulting from ingestion of multiple agents or combination medications, and reviews points of consideration when using the RM nomogram in acute intoxication.
Case report
Patient
The patient was a 20-year-old man who was diagnosed with dysthymic dysfunction 2 weeks previously, and had been prescribed a medication to treat the dysfunction. The patient’s wife went to bed earlier than her husband and found him unconscious when she woke up in the morning. There were empty medication containers from a prescription medication and an over-the-counter medication by his side, and he was taken to our hospital. The time of self-ingestion was estimated to be approximately 6 hours before arriving at the hospital.
Symptoms upon arrival at the hospital
The patient’s consciousness level was Glasgow coma scale E3V4M6, blood pressure was 117/65 mmHg, cardiac rate was 67/min, breathing rate was 27/min, SPO2 was 100% (Reserver mask 6 L/min), body temperature was 35.2°C, and body weight was 65 kg. No noteworthy physical findings were observed. Blood biochemical findings were as follows: white blood cell count, 9,900/mm3; red blood cell count, 5.4 million/mm3; hemoglobin, 16.5 g/dL; hematocrit, 48.7%; and platelets, 2.23 million/mm3, and no abnormality was observed except an increase in the white blood cell count. Blood biochemistry and blood coagulation test results were normal. All urinary drug qualitative reactions assessed by Triage® screening were negative. Additionally, vomiting was not observed during the initial treatment.
Course during hospital stay
The estimated contents of over-the-counter medicine X, which is a medication for the common cold, was 70 tablets (combination cold remedy; main ingredient, acetaminophen 100 mg/tablet), and benzodiazepine (11 mg). The ingredients in the over-the-counter medicine X are shown in Table 1.
Table 1.
The ingredients in medicine X (per 3 tablets).
| Dihydrocodeine phosphate* | 8 mg |
|---|---|
| dl-methyl ephedrine hydrochloride | 20 mg |
| Guaifenesin | 41.67 mg |
| Acetaminophen | 300 mg |
| Lysozyme hydrochloride | 20 mg |
| Carbinoxamine maleate | 2.5 mg |
| Anhydrous caffeine | 25 mg |
| Bisuibuchiamin | 8 mg |
| Riboflavin | 4 mg |
*anti-cholinergic agent.
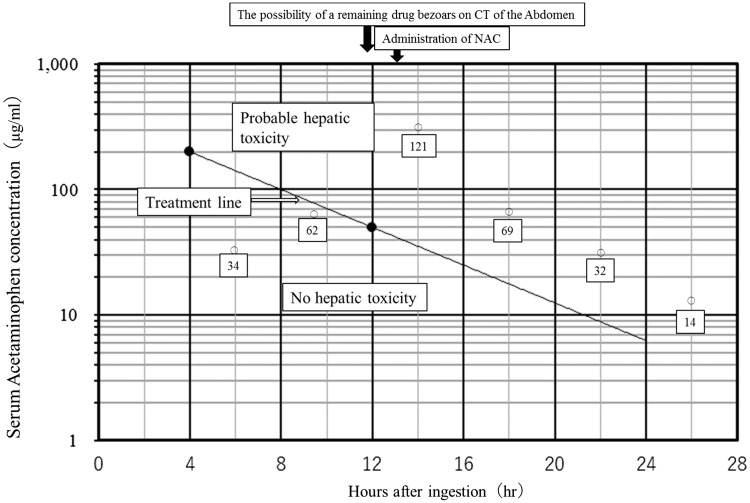
At the time of hospital admission, a gastric tube was not inserted because patient consent could not be obtained. The acetaminophen serum concentration, estimated 6 hours after administration, was 34 µg/mL. This serum concentration is not considered to be a risk factor for liver dysfunction because the acetaminophen serum concentration at the 6-hour value that is associated with liver dysfunction is more than 100 µg/ mL, based on the RM nomogram. The RM nomogram shows the correlation between acetaminophen serum concentration and liver dysfunction level, as observed over time after administration. Thus, N-acetylcysteine, which is an antidote for acetaminophen poisoning, was not administered. The acetaminophen serum concentration was measured every 4 hours after hospital admission, and an increase to 62 µg/mL was observed 10 hours after estimated administration (N-acetylcysteine administration is recommended at an acetaminophen serum concentration higher than 50 µg/mL at 10 hours after ingestion). Plain X-ray and CT of the abdomen were performed 12 hours after estimated administration to investigate the possibility that drug bezoars remained in the stomach, and a region of high absorption was observed in the stomach. These findings were explained to the patient, and a gastric tube was inserted after obtaining consent. N-acetylcysteine (20 mL) was administered every 4 hours thereafter. The serum concentration continued to increase until 14 hours after the estimated time of ingestion. The peak acetaminophen serum concentration was 121 µg/mL at 14 hours after the estimated time of ingestion (N-acetylcysteine administration is recommended at an acetaminophen serum concentration higher than 25 µg/mL at 14 hours after ingestion; Figure 1). This corresponded to the danger zone for liver dysfunction according to the RM nomogram, but no liver dysfunction or other coexisting illness occurred by the third day after administration. The patient was released from the hospital with a good outcome.
Figure 1.
Serum acetaminophen concentration vs. time after acetaminophen ingestion. We replicated our own version of the Rumack–Matthew nomogram. If the acetaminophen concentration level is above the treatment line, NAC therapy should be administered. If the concentration level is below the treatment line, NAC therapy is not required. The black arrow shows the course during the patient’s hospital stay. The white arrow indicates the treatment line. The white frame shows the serum acetaminophen concentration in this patient according to the number of hours after ingestion. NAC, N-acetylcysteine.
Discussion
The speed at which an orally ingested drug passes through the stomach and moves to the small intestine is called the gastric emptying rate (GER). Acetaminophen, an analgesic antipyretic, has a fast absorption rate in the gastrointestinal tract (small intestine). However, absorption is affected by GER.3 Thus, inclusion of other agents that change the GER may affect the acetaminophen absorption rate. In this case, residue was observed in the stomach on an abdominal CT, and this residue could have formed drug bezoars in the stomach.4 Over-the-counter combination medications for the common cold are designed to relieve cold symptoms. Thus, these medications contain many different drugs such as a pain reliever, fever reducer, cough suppressant, or an antihistamine or nasal decongestant for symptomatic relief. Generally, anticholinergic agents slow the GER, but the over-the-counter medicine X contained acetaminophen and dihydrocodeine phosphate as a cough suppressant, which has an anticholinergic action. Thus, GER was reduced and there was a greater risk of drug bezoar formation. Formation of a drug bezoar may also change the absorption kinetics. Because of changes in gastrointestinal tract motility caused by the drug interaction, drug absorption kinetics may differ from those of pure acetaminophen. This combination cold medication also included ethenzamide and caffeine. Caffeine is present as a supplementary analgesic, but it also promotes gastrointestinal absorption of acetaminophen.5 Consequently, changes in drug absorption kinetics resulting from the effect of the agents’ mutual interaction should be considered. The RM nomogram is a plot graph with elapsed time (h) after acetaminophen ingestion on the horizontal axis, and acetaminophen serum concentration (µg/mL) on the vertical axis (semi-logarithm). It predicts hepatotoxicity, which may occur later, by drawing from the probable-risk line and treatment line, and determines whether to start administration of N-acetylcysteine, which is the antidote to acetaminophen.2, 6 The principles used to apply the RM nomogram in treatment are as follows: 1) samples 4 hours after administration and later can be obtained; 2) serum acetaminophen concentration from 4 hours to 24 hours after administration can be obtained; and 3) in principle, a single agent of acetaminophen is administered at one time. This is because the peak of serum acetaminophen concentration is determined to be 4 hours after administration, and its value is estimated by the data collected up to 24 hours.2 The RM nomogram is an estimate of the degree of liver dysfunction according to the serum acetaminophen concentration from a single agent. However, cases of intoxication caused by over-the-counter combination cold medications containing acetaminophen are often observed. Thus, the risk of hepatotoxicity cannot be predicted using the RM nomogram. For the treatment in response to predicted liver dysfunction based on an estimated administered volume, which indicates a high intake of acetaminophen (a single dose of more than 7.5 g in adults or 150 mg/kg in children is defined as “at risk”), disassociation from serum acetaminophen concentration has been reported.6
Additionally, the approximation formula that was established by Edwards et al.,7 which estimates indications of standard treatment when serum concentration cannot be measured, is convenient, but predictability varies when the intake volume cannot be accurately assessed because of vomiting or in cases of combined use of multiple agents that influence acetaminophen metabolism. Although there has been no discussion about combination medications that include acetaminophen, the risk of liver dysfunction cannot be accurately determined from a single measurement of serum acetaminophen concentration. The RM nomogram should be used only when a single acetaminophen dose was ingested. When patients overdose on combination medications that include acetaminophen, treatment with N-acetylcysteine should be initiated, serum concentration of acetaminophen should be repeatedly measured, and liver function tests should be performed.
Conclusion
We report the case of a patient who ingested an overdose of an over-the-counter combination medication containing acetaminophen, where the serum acetaminophen concentration increased over time. When combination medications that contain acetaminophen are ingested and changes in the serum concentration are unclear, the risk of liver dysfunction should not be determined based on a single measurement of the serum acetaminophen concentration. Liver function should be repeatedly tested and a treatment strategy that includes N-acetylcysteine should be prepared to address of the risk of a delayed increase in the serum acetaminophen concentration.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Rumack BH, Peterson RC, Koch GG, et al. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment . Arch Intern Med 1981; 141: 380–385. [DOI] [PubMed] [Google Scholar]
- 2.Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975; 55: 871–876. [PubMed] [Google Scholar]
- 3.Heading RC, Nimmo J, Prescott LF. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. The dependence of paracetamol absorption on the rate of gastric emptying. Br J Pharmacol 1973; 47: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts DM, Buckley NA. Prolonged absorption and delayed peak paracetamol concentration following poisoning with extended-release formulation. Med J Aust 2008; 188: 310–311. [DOI] [PubMed] [Google Scholar]
- 5.Renner B, Clarke G, Grattan T, et al. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. J Clin Pharmacol 2007; 47: 715–726. [DOI] [PubMed] [Google Scholar]
- 6.Smilkstein MJ, Knapp GL, Kulig KW, et al. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). New Engl J Med 1988; 319: 1557–1562. [DOI] [PubMed] [Google Scholar]
- 7.Edwards DA, Fish SF, Lamson MJ, et al. Prediction of acetaminophen level from clinical history of overdose using a pharmacokinetic model. Ann Emerg Med. 1986; 15: 1314–1319. [DOI] [PubMed] [Google Scholar]