Short abstract
Objectives
To assess the toxicopathologic effects of chronic exposure to the glyphosate-based herbicide Bushfire® on the pancreas of Wistar rats and the protective role of zinc.
Methods
We exposed the rats to daily doses of 14.4 to 750 mg/kg body weight of the glyphosate-based herbicide Bushfire® and to 50 or 100 mg/kg zinc, and measured blood glucose levels and serum insulin levels. Tissue samples were evaluated for histopathological alterations.
Results
Levels of both blood glucose and serum insulin increased in glyphosate-exposed rats, and moderate to severe degenerative changes were observed in both glandular pancreatic acinar cells and islets of Langerhans in all rats exposed to glyphosate. These effects were prevented by pretreatment with zinc.
Conclusion
Chronic exposure to glyphosate can alter pancreatic function and histoarchitecture, but zinc supplementation can mitigate these toxicopathologic effects.
Keywords: Glyphosate, zinc, mitigation, chronic, toxicopathology, pancreas, glucose, insulin
Introduction
Glyphosate (N-Phosphonomethyl-glycine) is a non-selective herbicide used worldwide to control weeds.1 Glyphosate-based formulations for commercial uses are primarily made up of an aqueous mixture of glyphosate in the form of a salt, a surfactant, and various minor constituents.1 Globally, glyphosate is the most widely used herbicide, and over 130 countries permit its extensive use; the US is the largest consumer, accounting for approximately 20% of the market.2 Over the past few years, concerns have been raised that environmental exposure to glyphosate-based herbicides may cause endocrine disruption and organ damage at doses below regulatory limits.3–5 Poisoning of domestic animals by pesticides and other agricultural chemicals is attributable to human error such as inaccuracies in calculating concentrations for spraying and dipping procedures, resulting in exposure to concentrations several times higher than recommended.6
The phytotoxicity of glyphosate is mediated by its action on various enzyme systems; the pesticide inhibits amino acid metabolism in what is known as the shikimic acid pathway.7,8 Its toxic mechanism of action in animals is not clear, although laboratory experiments have suggested that the toxicity is due primarily to the presence of surfactants in the formulation, and oxidative stress is the indicated molecular mechanism of glyphosate toxicity.1,9 Recent research has elucidated the toxicological effects of glyphosate-based herbicides in humans and animals.9–12 Altered glucose homeostasis and oxidative impairment in the pancreas of rats exposed to the organophosphate insecticide dimethoate have been reported.13 We previously found that zinc supplementation arrested glyphosate-mediated cellular degeneration in rat pancreas without altering the histoarchitecture of the organ.14 There is still, however, insufficient information on the effects of chronic glyphosate exposure on pancreas histology and function, or on the ameliorative effect of zinc.
Zinc is an essential trace element for a number of animal species. Under stress conditions, the liver synthesises large quantities of metallothionein, which then binds to zinc and can reduce its levels in the body, leading to a deficiency. Metallothionein is also synthesised by the non-glandular pancreatic acinar cells.15,16 Zinc has been shown to slow or delay the oxidative process14 by two mechanisms of action. The first mechanism involves the protection of sulphydryl groups from oxidation via inhibition of intramolecular disulphide formation.13 The second mechanism involves the prevention of free radical formation by transition metals.17–19 Oxidative stress has also been implicated in the molecular mechanisms of glyphosate toxicity.14 The objectives of this study were to investigate the effects of glyphosate on pancreas histology and function and to evaluate the mitigating role of zinc on alterations induced by chronic glyphosate exposure in rats.
Materials and methods
Animals
Approval of the study was obtained from the Ethics Committee on Animal Use and Care of Ahmadu Bello University (Zaria, Kaduna State, Nigeria). Eighty adult male Wistar rats weighing 140 to 150 g were purchased from the National Institute for Trypanosomosis and Onchocerciasis Research (Vom Office, Jos Plateau State, Nigeria). The animals were housed in the animal room of the Department of Veterinary Pathology, Ahmadu Bello University-Zaria for two weeks for acclimatisation prior to the experiment. The rats were fed standard rat chow and water was provided ad libitum.
Chemicals
A glyphosate-based herbicide (Bushfire®) containing 360 g glyphosate/L in the form of 441 g/L potassium salt, distilled water, and zinc chloride (BDH Chemicals Ltd.; Poole, UK), haematoxylin and eosin stain, and aldehyde fuchsin stain were obtained from a reputable chemical store in Zaria.
Experimental design
Chronic toxicity study
The rats were randomised into eight groups of 10. Group I (DW) served as the control and received 2 mL/kg of distilled water daily. Group II (Z) received 50 mg/kg body weight zinc.20 Group III (G1) received 14.4 mg/kg glyphosate (2% concentration in 2 mL of distilled water, the standard concentration used for agricultural spraying). Group IV (G2) received 375 mg/kg of the glyphosate-based herbicide Bushfire® (10% of the half-maximal lethal dose [LD50]).21 Group V (G3) received 750 mg/kg Bushfire® (20% of the LD50).21 Group VI (ZG1) was pretreated with zinc (50 mg/kg) and then administered Bushfire® (14.4 mg/kg) 1 hour later. Group VII (ZG2) was pretreated with zinc (50 mg/kg) and then administered Bushfire® (375 mg/kg) 1 hour later. Group VIII (ZG3) was pretreated with zinc (100 mg/kg) and then administered Bushfire® (750 mg/kg) 1 hour later.
The dose regimens were administered by gavage once daily for 36 weeks.22 Rats were weighed weekly using a digital electronic balance (Hangzhou Gongheng, Hangzhou, China) to monitor weight changes and ensure appropriate dosing. No rats died during the experimental period.
Determination of fasting blood glucose and insulin levels
Fasting blood glucose level was determined at the end of the study with a blood glucose metre (Accu-Check®) using blood from the tail vein after fasting the rats overnight. Insulin was measured in serum using an ultrasensitive insulin ELISA kit (Monobind Inc., Lake Forest, CA, USA).
Histopathological examination
Tissue samples from the pancreas were collected and fixed in 10% neutral buffered formalin. The samples were dehydrated in graded concentrations of alcohol (70%, 80%, 95%, and 100%), cleared using xylene, impregnated in paraffin wax, incubated in a vacuum oven at 60°C, embedded in plastic embedding rings, sectioned into 5-µm slices using a microtome, deparaffinised with xylene, rehydrated in graded concentrations of alcohol (100%, 95%, 80%, and 70%), stained with haematoxylin and eosin,23 and viewed under a light microscope. The histochemical features of the pancreas samples were also studied using aldehyde fuchsin staining.24
Data analysis
Data are expressed as the mean ± SEM and analysed by one-way ANOVA followed by Tukey’s post-hoc test with GraphPad Prism version 4.0 for Windows (La Jolla, CA, USA). p < 0.05 was considered statistically significant. Where there was no significant difference, the mean difference between groups, expressed as a percentage, is reported if the value was ≥10%.
Results
Effects of treatments on blood glucose levels
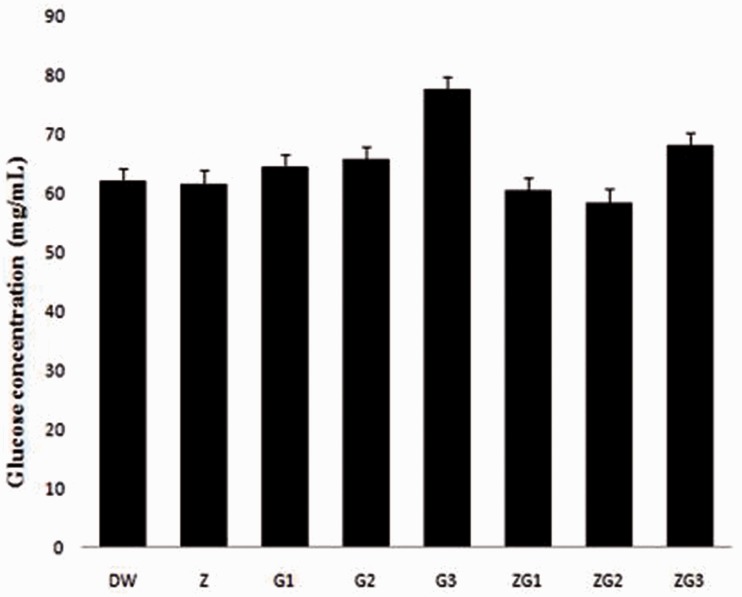
There was no significant difference (p > 0.05) in blood glucose levels between the treatment groups. An increase in glucose levels was observed in the ZG3 and G3 groups compared with the levels in the DW group (10% and 25%, respectively; Figure 1).
Figure 1.
Blood glucose levels in male Wistar rats treated with 2 mL/kg distilled water (DW), 50 mg/kg zinc (Z), 14.4 mg/kg glyphosate-based herbicide (Bushfire®) (G1), 375 mg/kg Bushfire® (G2), 750 mg/kg Bushfire® (G3), 50 mg/kg zinc + 14.4 mg/kg Bushfire® (ZG1), 50 mg/kg zinc + 375 mg/kg Bushfire® (ZG2), or 100 mg/kg zinc + 750 mg/kg Bushfire® (ZG3) for 36 weeks by gavage.
Effects of treatments on serum insulin levels
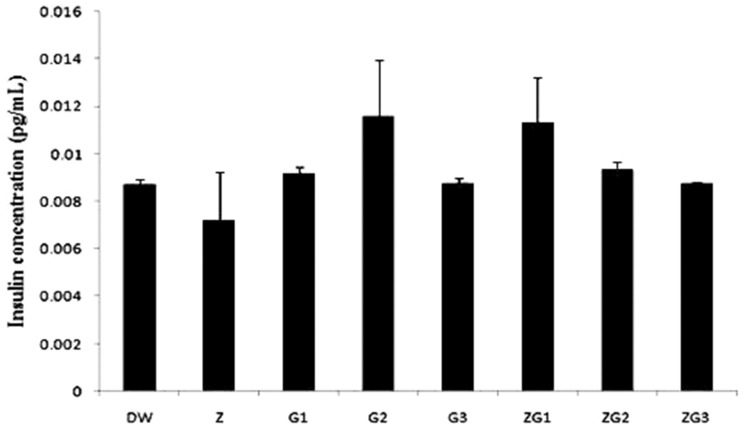
There was no significant difference (p > 0.05) in serum insulin levels between the treatment groups. An increase in serum insulin levels was observed in the ZG1 and G2 groups, compared with the levels in the DW group (30% and 33%, respectively; Figure 2).
Figure 2.
Serum insulin levels in male Wistar rats treated with 2 mL/kg distilled water (DW), 50 mg/kg zinc (Z), 14.4 mg/kg glyphosate-based herbicide (Bushfire®) (G1), 375 mg/kg Bushfire® (G2), 750 mg/kg Bushfire® (G3), 50 mg/kg zinc + 14.4 mg/kg Bushfire® (ZG1), 50 mg/kg zinc + 375 mg/kg Bushfire® (ZG2), or 100 mg/kg zinc + 750 mg/kg Bushfire® (ZG3) for 36 weeks by gavage.
Histopathological findings
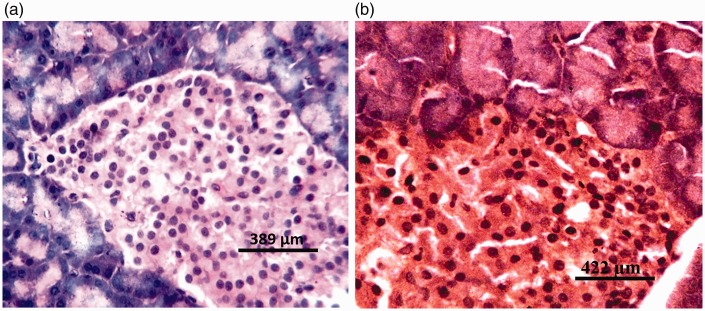
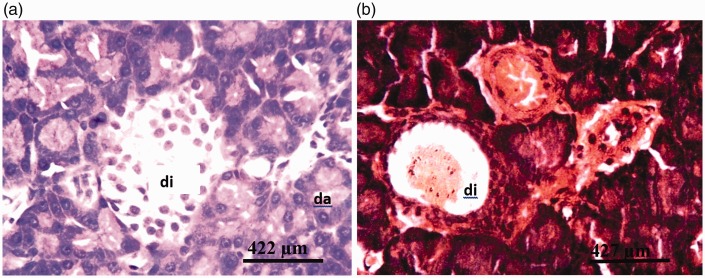
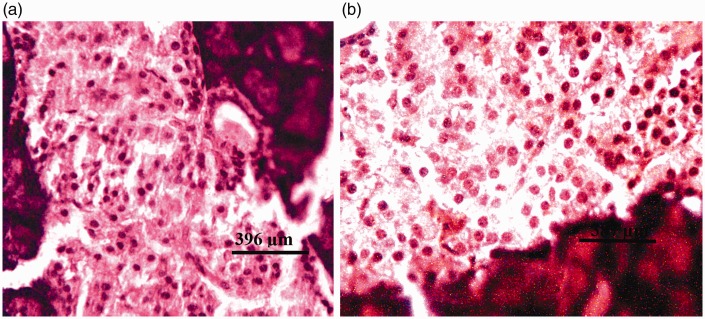
There were no visible lesions in the pancreatic tissues of rats from group I (DW) or group II (Z) (Figure 3a). Tissues from rats in group III (G1) and group IV (G2) showed degeneration of both pancreatic acinar cells and islets of Langerhans (Figures 4a and 5a, respectively). Severe degeneration of both pancreatic acinar cells and islets of Langerhans was observed in tissues from rats in group V (G3) (Figure 6a). Tissues from groups VI (ZG1), VII (ZG2), and VIII (ZG3) did not exhibit visible lesions (Figures 7a, 8a, and 9a, respectively). Histochemical analysis revealed morphologically normal islets of Langerhans in groups DW, Z, ZG1, ZG2, and ZG3 (Figures 3b, 7b, 8b, and 9b), whereas samples from groups G1, G2, and G3 revealed regions of depopulated and less deeply stained cells in the islets of Langerhans (Figures 4b, 5b, and 6b, respectively).
Figure 3.
Photomicrographs of pancreas of rat administered distilled water (DW) for 36 weeks by gavage, showing no visible lesions. (a) Haematoxylin and eosin staining; and (b) aldehyde fuchsin staining.
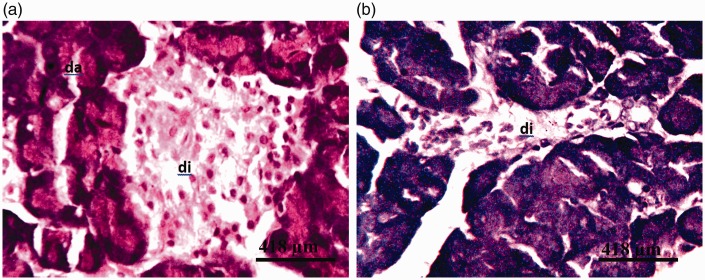
Figure 4.
Photomicrographs of pancreas of rat administered 14.4 mg/kg of the glyphosate-based herbicide Bushfire® for 36 weeks by gavage, showing (a) moderate degeneration of the islets of Langerhans (di) and degeneration of pancreatic acinar cells (da) (haematoxylin and eosin staining) and (b) severe cystic degeneration of the cells of the islets of Langerhans (di) (aldehyde fuchsin staining).
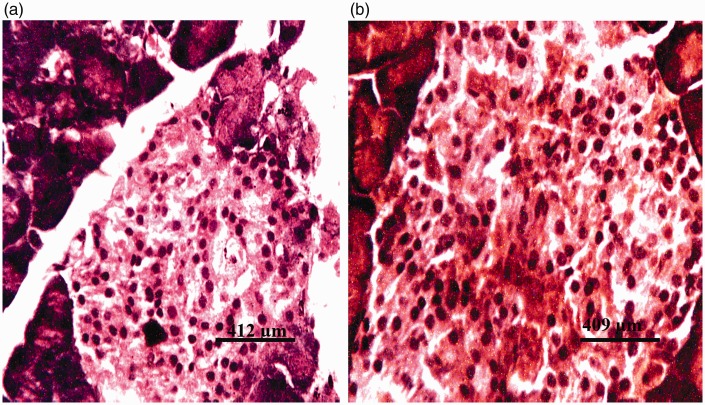
Figure 5.
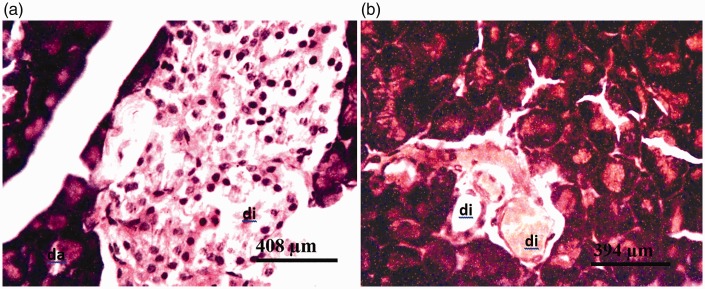
Photomicrographs of pancreas of rat administered 375 mg/kg of the glyphosate-based herbicide Bushfire® for 36 weeks by gavage, showing (a) moderate degeneration of the islets of Langerhans (di) and degeneration of pancreatic acinar cells (da) (haematoxylin and eosin staining) and (b) severe degeneration of the cells of the islets of Langerhans (di) (aldehyde fuchsin staining).
Figure 6.
Photomicrographs of pancreas of rat administered 750 mg/kg of the glyphosate-based herbicide Bushfire® for 36 weeks by gavage, showing (a) severe degeneration of the islets of Langerhans (di) and acinar cells (da) (haematoxylin and eosin staining) and (b) severe degeneration of the islets of Langerhans (di) (aldehyde fuchsin staining).
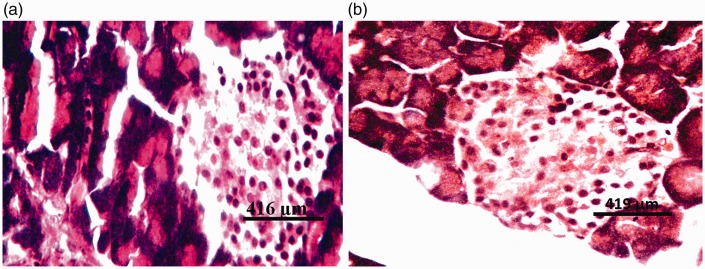
Figure 7.
Photomicrographs of pancreas of rat administered 50 mg/kg zinc and 14.4 mg/kg of the glyphosate-based herbicide Bushfire® for 36 weeks by gavage, showing no visible lesions. (a) Haematoxylin and eosin staining; and (b) aldehyde fuchsin staining.
Figure 8.
Photomicrographs of pancreas of rat administered 50 mg/kg zinc and 375 mg/kg of the glyphosate-based herbicide Bushfire® for 36 weeks by gavage, showing no visible lesions. (a) Haematoxylin and eosin staining; and (b) aldehyde fuchsin staining.
Figure 9.
Photomicrographs of pancreas of rat administered 100 mg/kg zinc and 750 mg/kg of the glyphosate-based herbicide Bushfire® for 36 weeks by gavage, showing no visible lesions. (a) Haematoxylin and eosin staining; and (b) aldehyde fuchsin staining.
Discussion
We found a relative increase in blood glucose levels in glyphosate-exposed rats that did not reach statistical significance. This increase may be attributable to the oxidative damage induced in the pancreas by glyphosate. Stress is known to activate the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system, resulting in hyperglycaemia.25–28 Activation of the HPA axis causes increased secretion of glucocorticoids from the adrenal cortex, eventually resulting in increased gluconeogenesis. The activation of the HPA axis has also been reported to impair glucose uptake in skeletal muscle.29 Similarly, the stimulation of the sympathetic nervous system under stress conditions has been reported to lead to increased secretion of catecholamines, glucagon, and growth hormone, promoting gluconeogenesis, glycogenolysis, insulin resistance, and hyperglycaemia.30,31 Previous studies have shown that organophosphate pesticides induce insulin resistance by inhibiting glucose transport in skeletal muscle via alterations in the insulin signalling pathway.32,33 It can therefore be deduced that the increased glucose levels observed in this study, along with the corresponding increase in insulin secretion, may be attributable to insulin resistance induced by activation of the HPA axis and/or oxidative stress associated with reduced peripheral tissue uptake of glucose and a chronic exposure. Zinc supplementation exerted a protective effect on serum glucose levels, possibly by preventing oxidative stress and decreasing insulin resistance.17,34
Rats exposed to 14.4 and 375 mg/kg glyphosate exhibited a relative increase in serum insulin levels, perhaps because of oxidative damage, while rats exposed to 750 mg/kg glyphosate did not. This finding may be due partly to the degenerative changes in the islets of Langerhans that were observed in these groups; the damage would be expected to limit insulin secretion. Previous studies have shown that organophosphate pesticides can elevate insulin levels and lead to insulin resistance by inhibiting glucose transport and dysregulating the insulin signalling pathway.32,33 Zinc treatment alone caused a relative decrease in serum insulin levels, possibly because of the pro-oxidant effect of zinc. Zinc supplementation prior to treatment with the lowest glyphosate dose resulted in an apparent increase in serum insulin levels compared with levels in the control group. The pro-oxidant effect of zinc has been documented in earlier studies,35,36 but zinc supplementation in the groups that received 375 and 750 mg/kg glyphosate restored serum insulin levels to near normal.
Degeneration of both pancreatic acinar cells and islets of Langerhans were observed, probably as a result of oxidative damage. Similarly, in our previous study,14 we observed degeneration of pancreatic acinar cells following subchronic (8-week) exposure to the glyphosate-based herbicide Bushfire® in rats. The damage to the islets of Langerhans observed in this study may be attributable to the increased duration of exposure. Zinc supplementation in the present study prevented any visible histopathological damage, indicating that zinc may exert an ameliorative effect on the pancreas. Zinc has been reported to play an important role in the maintenance of structure, function, and integrity of biological membranes,37,38 and to protect sulphydryl groups against oxidation, thereby stabilising the cellular thiol pools.39
We did not conduct an oral glucose tolerance test, which would have determined the rate at which glucose was cleared from the blood, so we could not verify whether the rats had developed insulin resistance. In addition, we did not identify whether β-cells or α-cells in the islets of Langerhans were most affected by the exposure. This information would have been beneficial in elucidating why serum insulin levels increased as blood glucose increased.
In summary, chronic exposure to the glyphosate-based herbicide Bushfire® can alter blood glucose homeostasis and influence insulin secretion in rats by damaging pancreatic islet and acinar cells, and zinc supplementation can ameliorate these effects.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work did not receive any form of support from individuals, institutions, or organisations. The study was funded by the corresponding author from his personal savings.
References
- 1.Bradberry SM, Proudfoot AT, Vale JA. Glyphosate poisoning. Toxicol Rev 2004; 23: 159–167. [DOI] [PubMed] [Google Scholar]
- 2.Baden-Mayer A. Monsanto’s Roundup®; enough to make you sick. Orgc Cx Assoc 2015; January 21st, 2015.
- 3.Mesnage R, Arno M, Costanzo M, et al. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup® exposure. Environ Health 2015; 14: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers JP, Antoniou MN, Blumberg B, et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: a concensus statement. Environ Health 2016; 15: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesnage R, Renney G, Séralini GE, et al. Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of Roundup® herbicide. Sci Rep 2017; 7: 39328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bursian SJ. The effects of agric-chemicals on animals. Department of Animal Science, Michigan State University. East Lansing, M.I. Gen Sess–Environ Issues 1993; 77–82. [Google Scholar]
- 7.Cox C. Herbicide factsheet: Glyphosate, Part 1. Toxicol J Pest Ref 1995; 15: 3. [Google Scholar]
- 8.Malik J, Barry G, Kishore G. The herbicide glyphosate. Biofactor 1989; 2: 17–25. [PubMed] [Google Scholar]
- 9.Beuret CJ, Zirulnik F, Giménez MS. Effects of the herbicide glyphosate on liver lipoperoxidation in pregnant rats and their fetuses. Reprod Toxicol 2005; 19: 501–504. [DOI] [PubMed] [Google Scholar]
- 10.Cortinovis C, Davanzo F, Rivolta M, et al. Glyphosate-surfactant herbicide poisoning in domestic animals: an epidemiological survey. Vet Rec 2015; 176: 413. [DOI] [PubMed] [Google Scholar]
- 11.Kamijo Y, Takai M, Fujita Y, et al. roundup multicenter retrospective survey of poisoning after ingestion of herbicides containing glyphosate potassium salts or other glyphosate salts in Japan. Clin Toxicol 2016; 54: 147–151. [DOI] [PubMed] [Google Scholar]
- 12.Gill JPK, Sethi N, Mohan A, et al. Glyphosate toxicity for animals. Environ Chem Letters 2017; 4: 1–26. [Google Scholar]
- 13.Kamath V, Rajini PS. Altered glucose homeostasis and oxidative impairment in pancreas of rats subjected to dimethoate intoxication. Toxicol 2007; 231: 137–146. [DOI] [PubMed] [Google Scholar]
- 14.Tizhe EV, Ibrahim NDG, Fatihu MY, et al. Influence of zinc supplementation on histopathological changes in the stomach, liver, kidney, brain, pancreas, and spleen during subchronic exposure of Wistar rats to glyphosate. J Comp Clin Path 2014; 23: 1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hambidge KM, Casey CE, Krebs NF. Zinc In: Mertz W. (ed.) Trace elements in human and animal nutrition. 5th ed NY: Acad Press, 1986, 6, pp.1–109. [Google Scholar]
- 16.De Lisle RC, Sarras MP, Jr, Hidalgo J, et al. Metallothionein is a component of exocrine pancreas secretion: implications for zinc homeostasis. Am J Physiol 1996; 27: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 17.Powell SR. The antioxidant properties of zinc. J Nutr 2000; 130: 1447S–1454S. [DOI] [PubMed] [Google Scholar]
- 18.Gibbs PN, Gore MG, Jordan PM. Investigation of the effect of metal ions on the activity of thiol groups in human 5-aminolevulinate dehydratase. Biochem J 1985; 225: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Searle AJF, Tomasi A. Hydroxyl free radical production in iron-cysteine solutions and protection by zinc. J Inorg Biochem 1982; 17: 161–166. [Google Scholar]
- 20.Ambali SF, Abubakar AT, Shittu M, et al. Chlorpyrifos-induced alteration of haematological parameters in Wistar rats: Ameliorative Effects of Zinc. Res J Environ Toxicol 2010; 4: 55–66. [Google Scholar]
- 21.Tizhe EV, Ibrahim NDG, Fatihu MY, et al. Haematological changes induced by subchronic glyphosate exposure: ameliorative effect of zinc in Wistar rats. Sok J Vet Sc 2013; 11: 28–35. [Google Scholar]
- 22.OECD (Organisation for Economic Cooperation and Development). Chronic toxicity studies- duration of the study. Org Eco Co-op Dev 2009; 8(452): 1–15. [Google Scholar]
- 23.Baker J, Silverton RE and Pallister CJ. Dehydration, impregnation, embedding technique and section preparation. Introduction to Medical Laboratory Technology. 7th ed. London: Arnold, 2001, pp. 199–242.
- 24.Bancroft JD and Gamble M. Carbohydrates. Theory and Practice of Histological Techniques. 6th ed. Edinburgh: Churchill Livingstone, 2008, pp. 170–376
- 25.Bateman A, Singh A, Kral T, et al. The immune-hypothalamic-pituitary-adrenal axis. Endocrinol Rev 1989; 10: 92–112. [DOI] [PubMed] [Google Scholar]
- 26.Blalock JE. Harnessing a neural-immune circuit to control inflammation and shock. J Exp Med 2002; 195: 25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mechanik JI. Metabolic mechanisms of stress hyperglycaemia. J Parenter and Enter Nutr 2006; 30: 157–163. [DOI] [PubMed] [Google Scholar]
- 28.Rahimi R, Nikfar S, Larijani B, et al. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother 2005; 59: 365–373. [DOI] [PubMed] [Google Scholar]
- 29.Oda N, Nakai A, Mokuno T, et al. Dexamethasone-induced changes in glucose transporter-4 in rat heart muscles, skeletal muscle and adipocytes. Eur J Endocrinol 1995; 133: 121–126. [DOI] [PubMed] [Google Scholar]
- 30.Gustavson SM, Chu CA, Nishizawa M, et al. Interaction of glucagon and epinephrine in the contral of hepatic glucose production in the conscious dog. Am J Physiol Endocrinol Metab 2003; 284: 695–707. [DOI] [PubMed] [Google Scholar]
- 31.Gearthart MM, Parbhoo SK. Hyperglycaemia in critically ill patient. Clin Issues 2006; 17: 50–55. [DOI] [PubMed] [Google Scholar]
- 32.Chiasson JL, Shikama H, Chu DT, et al. Inhibitory effect of epinephrine insulin-stimulated glucose uptake by rat skeletal muscle. J Clin Invest 1981; 68: 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt DG, Ivy JL. Epinephrine inhibits insulin-stimulated muscle glucose transport. J Appl Physiol 2002; 93: 1638–1643. [DOI] [PubMed] [Google Scholar]
- 34.Goel A, Danni V, Dhawan DK. Role of zinc in mitigating toxic effects of chlorpyrifos on haematological alterations and electron microscopic observations in rat blood. Biometals 2006; 19: 483–492. [DOI] [PubMed] [Google Scholar]
- 35.Abdallah SM, Samman S. The effect of increasing dietary zinc on the activity of superoxide dismutase and zinc concentration in erythrocytes of healthy female subjects. Eur J Clin Nutr 1993; 47: 327–332. [PubMed] [Google Scholar]
- 36.Sidhu P, Gorg ML, Morgenstern P, et al. The role of zinc in regulating the levels of hepatic elements following nickel toxicity in rats. Biol Trace Elem Res 2004; 102: 161–172. [DOI] [PubMed] [Google Scholar]
- 37.Bettger WJ, O’Dell BL. The physiological role of zinc in the plasma membrane of mammalian cells. J Nutr Biochem 1993; 4: 197–207. [Google Scholar]
- 38.Sahin A, Kucuk O. Zinc supplementation alleviates heat stress in laying Japanese quail. J Nutr 2003; 133: 2808–2811. [DOI] [PubMed] [Google Scholar]
- 39.Kraus A Roth HP, andKirchgessner M.. Supplementation with vitamin C, vitamin E or β- carotene influences osmotic fragility and oxidative damage of erythrocytes of zinc deficient rats. J Nutr 1997; 127: 1290–1296. [DOI] [PubMed] [Google Scholar]