Short abstract
Inflammatory myofibroblastic tumors (IMTs) are neoplasms with low malignant potential, and the most common tumor in the lung and orbit. Their occurrence in the uterus is rare. Approximately 50% of IMT patients have anaplastic lymphoma kinase gene (ALK) rearrangements. Recent studies described novel fusions involving ROS1, platelet-derived growth factor receptor beta (PDGFR-β), and ETS translocation variant (ETV6) genes in a subset of ALK-negative patients. We report a 44-year-old woman with anemia and uterine IMT. Ultrasonography and magnetic resonance imaging revealed a myxoid degenerative myoma-like mass, 7.4 cm in maximum diameter, on the left uterine side wall. Hysterectomy was performed as a definitive treatment. Microscopic examination revealed spindle cell proliferation with numerous lymphocytes and plasma cells. Immunohistochemically, the spindle cells were negative for ALK-1, desmin, and smooth muscle actin. The pathological diagnosis was IMT arising from the uterus. Fluorescence in situ hybridization demonstrated an ETV6–neurotrophic tyrosine kinase, receptor, type 3 gene (NTRK3) translocation but no ALK, ROS1, or PDGFR-β translocations. Lung and abdomen computed tomography at 31 months postoperatively revealed no disease recurrence. This association of an ETV6–NTRK3 fusion oncogene with an ALK-negative uterine IMT increases our understanding of this neoplasm, which may help the development of specific therapies.
Keywords: Inflammatory myofibroblastic tumor, uterus, crizotinib, ETV6–NTRK3 fusion gene, fluorescence in situ hybridization, anaplastic lymphoma kinase
Introduction
Inflammatory myofibroblastic tumors (IMTs) are rare neoplasms with a wide spectrum of histological features that range from plasma cell-rich lesions to myofibroblast-predominant lesions. In the World Health Organization (WHO) classification of soft tissue tumors, IMTs are classed as intermediate malignancies.1 IMT primarily occurs in soft tissue and at any anatomical location. However, uterine involvement is extremely rare, with only 74 cases of uterine IMT reported to date. Regardless of the occurrence site, complete excision has been the mainstay of treatment.
In approximately 50% of IMT cases, a clonal rearrangement of chromosome 2p23 involving the anaplastic lymphoma kinase gene (ALK) is found, resulting in the overexpression and hyperactivation of this receptor tyrosine kinase.2 However, characterization of the remaining ALK-negative cases remains incomplete. Recent studies described novel fusions involving ROS1, platelet-derived growth factor receptor beta (PDGFR-β), and ETS translocation variant (ETV6) genes in a subset of ALK-negative patients.3–5
Here we present a case of a rare uterine IMT that was treated by hysterectomy. To our knowledge, this is the first report in which uterine IMT showed a novel rearrangement of ETV6.
Case report
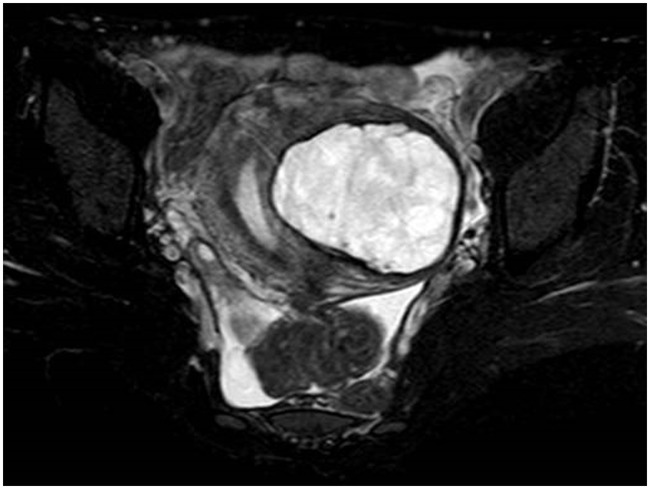
A 44-year-old woman with no relevant previous medical history presented with anemia. Laboratory test results revealed hemoglobin levels of 9.0 g/dL, and remaining basic laboratory work was unremarkable. Her physical examination was also normal. A pelvic ultrasound revealed an oval-shaped mass, measuring 7.2 × 6.8 cm, showing heterogeneous echogenicity on the posterior aspect of the uterus. Both ovaries were normal. Magnetic resonance imaging revealed a myxoid degenerative myoma-like mass, measuring 7.4 × 6.6 × 5.2 cm, on the left uterine side wall. The anatomical zone of the uterus was preserved, and the endometrium was enlarged. The parametrium was also normal (Figure 1). Following a diagnosis of hypermenorrhagia because of uterine myoma, total abdominal hysterectomy was performed as a definitive treatment. This study was approved by the research ethics board of Nagahama City Hospital. Written informed consent was obtained from the patient for study entry.
Figure 1.
Axial MRI T2-weighted fat-suppressed sequence demonstrating the local lesion with an inhomogeneous hyperintensity on the left uterine side wall.
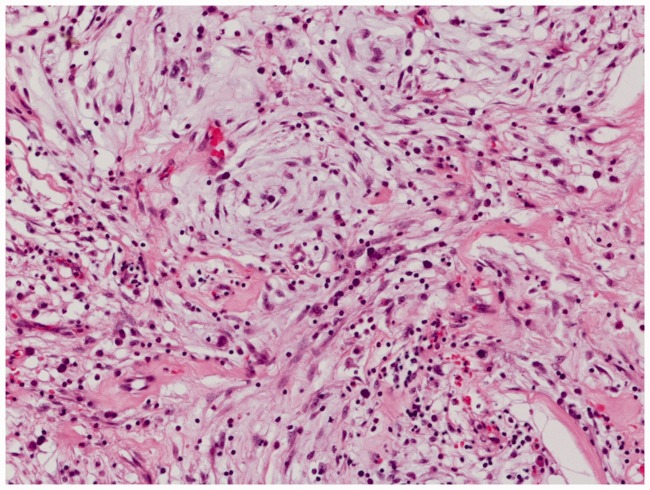
Microscopic examination of the excised specimen showed proliferation of spindle cells embedded in a storiform pattern together with numerous lymphocytes, plasma cells, and histiocytes in variable amounts of myxoid matrix (Figure 2). Overall, mitotic activity was scant, and necrosis was absent. Focal immunoreactivity for estrogen receptor, progesterone receptor, and cytokeratin AE1/AE3 was present. Immunostaining for ALK-1, ROS1, desmin, vimentin, smooth muscle actin, S-100 protein, CD34, CD31, and h-caldesmon was negative. A diagnosis of IMT was made, based on morphological and immunohistochemical findings.
Figure 2.
Histopathological findings showing plump spindle and epithelioid cells admixed with lymphocytes and plasma cells in an abundant myxoid extracellular matrix. Cells were stained with hematoxylin and eosin (magnification, ×200).
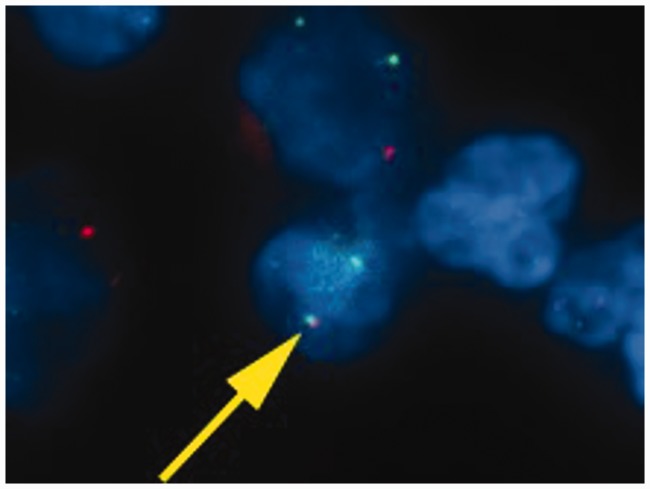
To confirm the diagnosis, we conducted fluorescence in situ hybridization studies to assess the characteristic genetic rearrangements for IMT using ALK, ROS1, PDGFR-β, and ETV6 break-apart assays.3,4 These studies were positive for ETV6 rearrangement, i.e., the ETV6–neurotrophic tyrosine kinase, receptor, type 3 gene (NTRK3) fusion oncogene (Figure 3), and negative for ALK, ROS1, and PDGFR-β.
Figure 3.
Fluorescent in situ hybridization analysis. Yellow arrow indicates ETV6 arrangement. The presence of the ETV6-NTRK3 fusion is demonstrated by the close proximity of a green signal (ETV6 from chromosome 12) with a red signal (NTRK3 from chromosome 15) in multiple tumor cells.
The patient did not undergo any adjuvant therapies, including radiotherapy. Lung and abdomen computed tomography at 31 months postoperatively showed no disease recurrence.
Discussion
To our knowledge, this is the first study to genetically confirm the presence of the ETV6-NTRK3 fusion oncogene in an ALK-negative IMT of the uterus.
The WHO classification of soft tissues defines IMT as a distinctive neoplasm comprising myofibroblastic and fibroblastic spindle cells accompanied by an inflammatory infiltrate of plasma cells, lymphocytes, and/or eosinophils.1 IMT most commonly occurs in the lung but can also be observed in the soft tissue, retroperitoneum, head, laryngeal region, and central nervous system.6 The uterine body is an extremely rare location for IMTs, with even the largest series of uterine IMT reporting only 13 cases.7 Thus, IMTs may often be misdiagnosed because difficulties are associated with their histological differentiation from uterine smooth muscle tumors such as leiomyomas and leiomyosarcomas. Three microscopic patterns have been described: myxoid, fascicular, and hyalinized,6 and the tumor in our patient showed a myxoid pattern.
ALK expression in IMTs of the female genital tract appears to be frequent, similar to other anatomical sites.8 Indeed, a review article reported that 58 of 62 (93.6%) uterine IMT patients were positive for ALK immunostaining.9 Immunohistochemical expression of the ALK protein is therefore a very important hallmark for differentiating IMTs from other tumors. The review also stated that only four of the 47 ALK-positive immunostaining patients with follow-up information died of disease, and had a mean overall survival time of 52.8 months.9 Therefore, if spindle cell lesions are detected in the uterus, IMTs should be included in the differential diagnosis, and an immunohistochemical examination of ALK protein expression is required.
We confirmed that IMT in our case did not have the ALK fusion gene. Typically, 50%–60% of IMTs carry translocations involving ALK on chromosome 2p23,10,11 resulting in the constitutive activation of ALK tyrosine kinase. Clinically, the presence of ALK expression is determined by immunohistochemistry, and the lack of ALK expression is associated with a higher age overall, subtle histological differences, and death from disease or distant metastases.10 However, the characterization of ALK-negative IMT remains incomplete. A recent study using targeted next-generation sequencing described novel fusions involving ROS1, PDGFRβ, and ETV6 in ALK-negative cases.3,4 In a previous study, only two cases of IMT contained the ETV6–NTRK3 fusion gene: one in the lung and the other in the liver.4 To our knowledge, our study is the fourth to report IMTs with the ETV6–NTRK3 fusion gene, and the first to report IMT of the uterus.
ETV6–NTRK3 is an oncogenic chimeric tyrosine kinase. This fusion gene has been discovered in various tumors such as congenital infantile fibrosarcoma, acute myeloblastic leukemia, secretory breast cancer, mammary analog secretory carcinoma of the salivary gland, and papillary thyroid carcinoma.12–16 Tumors with ETV6–NTRK3 expression demonstrated downstream hyperactivation of phosphoinositide 3-kinase and RAS signaling pathways,17 and only show a limited response to cytotoxic anticancer agents;18 however, acute lymphoblastic leukemia cell lines with ETV6–NTRK3 fusions were sensitive to crizotinib.19 Although crizotinib may be a promising therapeutic agent for IMT with the ETV6–NTRK3 fusion gene, the effect of crizotinib on this tumor is unknown. Therefore, the therapeutic relevance of identifying fusion genes for ALK-negative IMT is unclear. In general, uterine IMTs require surgical removal, but there is a propensity for local recurrence. When surgical resection is difficult, drug therapy may be necessary, but no effective drugs have been identified. Therefore, we believe that our findings will contribute to the development of novel targeted therapeutic agents against IMT.
In summary, we demonstrated to our knowledge the first occurrence of the ETV6–NTRK3 fusion oncogene in an ALK-negative IMT of the uterus. These results suggest that a subset of ALK-negative IMTs have ETV6–NTRK3 rearrangements as a possible oncogenic mechanism, and that detection of these alterations may be of diagnostic value and help for determining promising therapeutic strategies.
Acknowledgments
The authors thank Chromosome Science Labo Inc. (Hokkaido, Japan) for in situ hybridization studies. The authors would also like to thank Enago (www.enago.jp) for their English language review.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014; 46: 95–104. [DOI] [PubMed] [Google Scholar]
- 2.Coffin CM, Patel A, Perkins S, et al. ALK1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Patho 2001; 14: 569–576. [DOI] [PubMed] [Google Scholar]
- 3.Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 2014; 4: 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alassiri AH, Ali RH, Shen Y, et al. ETV6-NTRK3 is expressed in a subset of ALK-negative inflammatory myofibroblastic tumors. Am J Surg Pathol 2016; 40: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto H, Yoshida A, Taguchi K, et al. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology 2016; 69: 72–83. [DOI] [PubMed] [Google Scholar]
- 6.Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995; 19: 859–872. [DOI] [PubMed] [Google Scholar]
- 7.Bennett JA, Nardi V, Rouzbahman M, et al. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Mod Pathol 2017; 30: 1489–1503. [DOI] [PubMed] [Google Scholar]
- 8.Fuehrer NE, Keeney GL, Ketterling RP, et al. ALK-1 protein expression and ALK gene rearrangements aid in the diagnosis of inflammatory myofibroblastic tumors of the female genital tract. Arch Pathol Lab Med 2012; 136: 623–626. [DOI] [PubMed] [Google Scholar]
- 9.Mandato VD, Valli R, Mastrofilippo V, et al. Uterine inflammatory myofibroblastic tumor: more common than expected: Case report and review. Medicine 2017; 96: e8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007; 31: 509–520. [DOI] [PubMed] [Google Scholar]
- 11.Saab ST, Hornick JL, Fletcher CD, et al. IgG4 plasma cells in inflammatory myofibroblastic tumor: inflammatory marker or pathogenic link? Mod Pathol 2011; 24: 606–612. [DOI] [PubMed] [Google Scholar]
- 12.Knezevich SR, Garnett MJ, Pysher TJ, et al. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res 1998; 58: 5046–5048. [PubMed] [Google Scholar]
- 13.Eguchi M, Eguchi-Ishimae M, Tojo A, et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25). Blood 1999; 93: 1355–1363. [PubMed] [Google Scholar]
- 14.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2002; 2: 367–376. [DOI] [PubMed] [Google Scholar]
- 15.Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010; 34: 599–608. [DOI] [PubMed] [Google Scholar]
- 16.Leeman-Neill RJ, Kelly LM, Liu P, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer 2014; 120: 799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tognon C, Garnett M, Kenward E, et al . The chimeric protein tyrosine kinase ETV6-NTRK3 requires both Ras-Erk1/2 and PI3-kinase-Akt signaling for fibroblast transformation. Cancer Res 2001; 61: 8909–8916. [PubMed] [Google Scholar]
- 18.Arce C, Cortes-Padilla D, Huntsman DG, et al. Secretory carcinoma of the breast containing the ETV6-NTRK3 fusion gene in a male: case report and review of the literature. World J Surg Oncol 2005; 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts KG, Li Y, Payne-Turner D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 2014; 371: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]