Short abstract
Objectives
18F-choline is a useful tracer for detecting tumours with high lipogenesis. Knowledge of its biodistribution pattern is essential to recognise physiological variants. The aim of this study was to describe the physiologic distribution of 18F-choline and pitfalls in patients with breast cancer.
Methods
Twenty-one consecutive patients with breast cancer (10 premenopausal and 11 postmenopausal women; mean age, 52.82 ± 10.71 years) underwent 18F-choline positron emission tomography (PET)/computed tomography (CT) for staging. Whole-body PET/CT was acquired after 40 minutes of 18F-choline uptake. Acquired PET images were measured semiquantitatively.
Results
All patients showed pitfalls unrelated to breast cancer. These findings were predominantly caused by physiological glandular uptake in the liver, spleen, pancreas, bowels, axial skeleton (85%-100%), inflammation and benign changes (4.76%), appendicular skeleton (4.76%–19.049%), and site contamination (61.9%). In <1%, a concomitant metastatic neoplasm was found. The breast showed higher physiological uptake in premenopausal compared with postmenopausal woman (18F-choline maximum standardised uptake values [g/dL] of the right breast = 2.04 ± 0.404 vs 1.59 ± 0.97 and left breast = 2.00 ± 0.56 vs 1.93 ± 1.28, respectively).
Conclusion
18F-choline uptake was higher in premenopausal women. Physiological 18F-choline uptake was observed in many sites, representing possible pathologies.
Keywords: 18F-choline, positron-emission tomography/computed tomography, breast cancer, SUVmax, physiological variants, pitfalls, premenopausal women, HER-negative, lipogenesis, cell membrane metabolism
Introduction
Fluorodeoxyglucose (FDG) positron emission tomography (PET) is extensively used as an imaging biomarker for diagnosing and monitoring treatment responses of patients with cancer. Recently, newer imaging biomarkers such as fluorine 18 (18F)-choline1–3 and carbon 11 (11C)-choline2 have been evaluated for their greater specificity to assess proliferation and choline metabolism, respectively. The utility of 18F-choline in breast cancer has not been widely studied.4–6 For example, Aboagye and Bhujwalla7 showed a unique phenotype of increased choline metabolism in the transition of normal human mammary epithelial cells to immortalised, oncogene-transformed, and finally non-metastatic and metastatic cancer. In their study, the phosphocholine levels incrementally increased with progression such that breast cancer cells had the highest phosphocholine levels. Further studies in mammary epithelial cells found that the aberrant increases in phosphocholine metabolite levels are caused by the expression of the biosynthetic enzyme choline kinase-α7. Coincidentally, choline kinase activity and cellular phosphocholine levels are regulated by the growth factor receptor–mitogen-activated protein kinase pathway in mammalian cells—the same pathway that modulates oestrogen-independent growth.8 Considering the pharmacological features and properties of choline, the uptake of the tracer can occur in physiological or benign conditions such as cell membrane synthesis as well as in malignancies.3
Thus, the aim of the present study was to describe and discuss the pattern of physiological sites of 18F-choline uptake, common pitfalls, and variations in 18F-choline uptake in women.
Materials and methods
We conducted a prospective study of patients who were recruited based on mammographic criteria of having Breast Imaging Reporting and Data System class 4 or 5 for suspicious breast cancer. Patients with recurrent disease did not undergo treatment before positron emission tomography/computed tomography (PET/CT).
All patients received an intravenous injection of 3 mCi of 18F-choline and were subsequently rested for a mean of approximately 30–45 minutes before undergoing PET/CT. Image acquisition was performed using an integrated PET/CT device (Siemens Biograph-64; Siemens Health Care, Erlangen Germany) comprising a 3D-PET camera (lutetium oxyorthosilicate) and a 64-multi-detector CT scanner. Patients were allowed to take normal, shallow breaths during acquisition of the PET/CT. CT without contrast was performed first at 120 kV, 150 mAs transmission with a pitch of 0.8 (5.0-mm slice thickness) from the base of the skull to the proximal thighs for anatomical localisation and attenuation correction to rescale the FDG/PET image attenuation without delay at the second time point.
PET was performed contemporaneously with acquisition for 3 minutes per bed position. CT data were resized from a 512 × 512 matrix to a 128 × 128 matrix to match the PET data and allow image superposition followed by generation of CT transmission maps. PET image datasets were iteratively reconstructed using the ordered-subsets expectation maximisation algorithm with segmented measured attenuation correction (two iterations, 28 subsets) of the CT data. Coregistered images were displayed on a Siemens-Leonardo Workstation (Siemens Health Care). 18F-choline FDG PET/CT was performed 1 week following a successful 18F-choline PET/CT study. SUVmax values served as a surrogate marker. The significance of the difference between SUVmax values of the pre- and postmenopausal women was evaluated using the Student t test, and p≤0.05 indicates significance.
The ethics review boards of the University Putra, Malaysia and the Hospital of the University of Kebangsaan, Malaysia approved this study. All patients recruited into this study were granted written and verbal consent.
Results
Twenty-one consecutive patients underwent 18F-choline PET/CT imaging for presurgical staging of breast cancer (Table 1). Patients were 11 premenopausal and 10 postmenopausal women. The mean age of the patients was 54.48 ± 12.17 years. The majority of the patients showed biopsy-proven malignant breast tumours; 18 patients had invasive ductal carcinoma, and 3 patients had benign tumours. All patients with invasive ductal carcinoma showed increased 18F-choline uptake (maximum standardised uptake value [SUVmax] = 1.66 ± 0.26 g/dL), Figures 1–2. We observed physiological 18F-choline uptake in the liver, pancreas, spleen, salivary and lacrimal glands, and owing to renal excretion, in the urinary tract. Other sites of less intense tracer uptake were the bone marrow and intestines (Figure 3). The background 18F-choline uptake in premenopausal women was significantly higher than that in postmenopausal women (Table 2).
Table 1.
Patients' characteristics
| Patients (N = 21) | |
|---|---|
| Age, years (mean ± standard deviation) | 52.82 ± 10.71 |
| Head | |
| Choroid Plexus | 18 (85.7) |
| Pituitary Gland | 19 (90.4) |
| Lacrimal gland | 8 (38.0) |
| Falx cerebri | 1 (4.76) |
| Neck | |
| Salivary Gland | 21 (100) |
| Submandibular Gland | 21 (100) |
| Parotid Glands | 20 (95.2) |
| Tonsils | 6 (28.5) |
| Muscle of tongue | 1 (4.76) |
| Pharynx | 1 (4.76) |
| Thyroid | 1 (4.76) |
| Thorax | |
| Sternum/Manubrium | 7 (33.3) |
| Ribs | 2 (9.52) |
| Heart | 1 (4.76) |
| Clavicle | 2 (9.52) |
| Abdomen/Pelvis | |
| Liver | 21 (100) |
| Spleen | 21 (100) |
| Pancreas | 21 (100) |
| Bowel | 21 (100) |
| Ovary | 1 (4.76) |
| Uterus | 2 (9.52) |
| Musculoskeletal | |
| Axial spine | 21 (100) |
| Peripheral skeleton | |
| Pubic bone | 4 (19.04) |
| Muscles | |
| Biceps | 3 (14.28) |
| Latissumus dorsi | 4 (19.04) |
| Pectoralis major | 1 (4.76) |
| Second pathology | |
| Lung Metastasis | 1 (4.76) |
| Axillary lymph node | 1 (4.76) |
| Degenerative bone disease | 3 (14.28) |
| Artefact | |
| Injection site contamination | 13 (61.9) |
All data are presented as number (percentage) of patients, unless otherwise indicated.
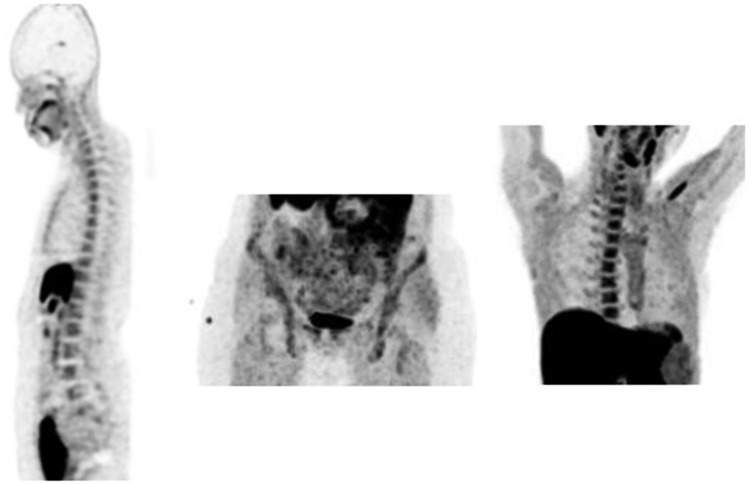
Figure 1.
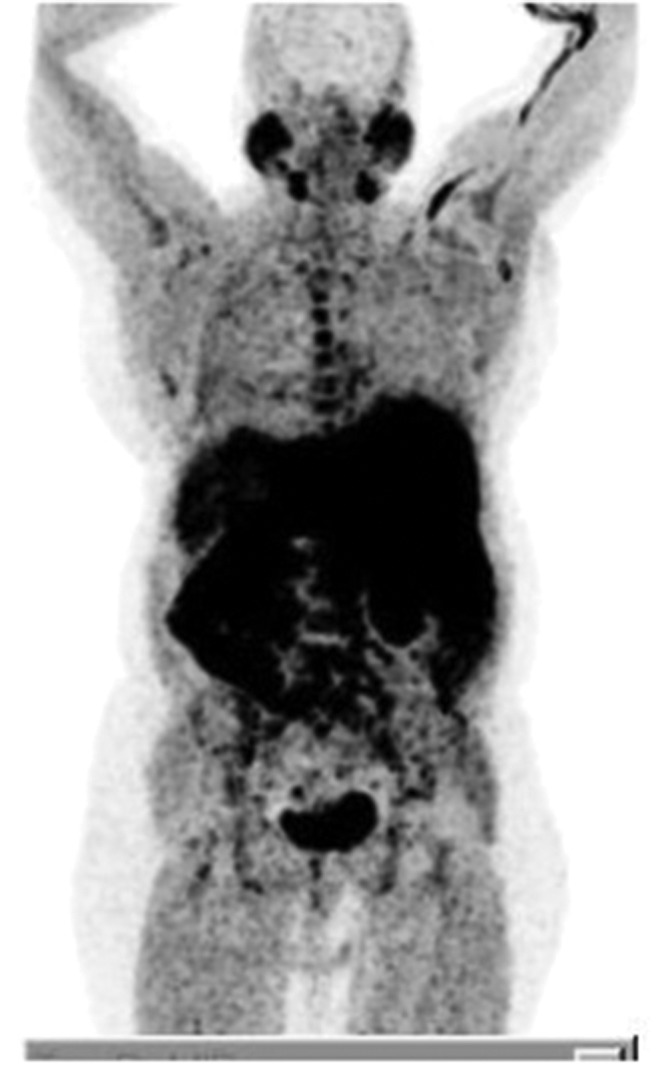
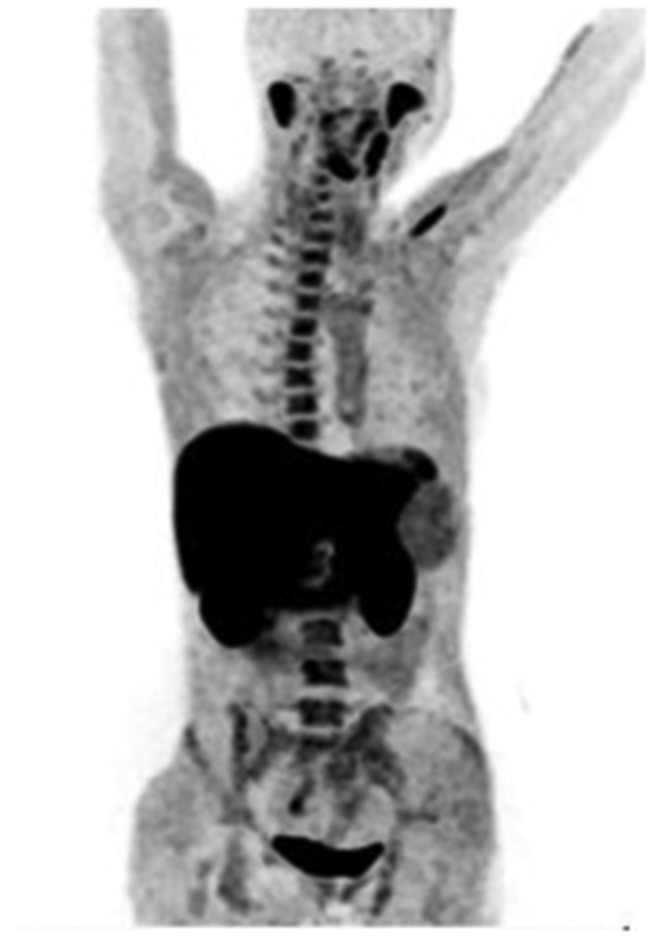
Maximum intensity projection (MIP)-positron emission tomography (PET) detected physiological 18F-choline uptake in the reticuloendothelial system and in the salivary glands.
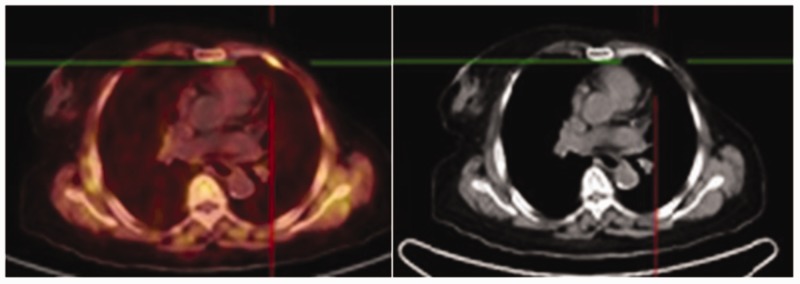
Figure 2.
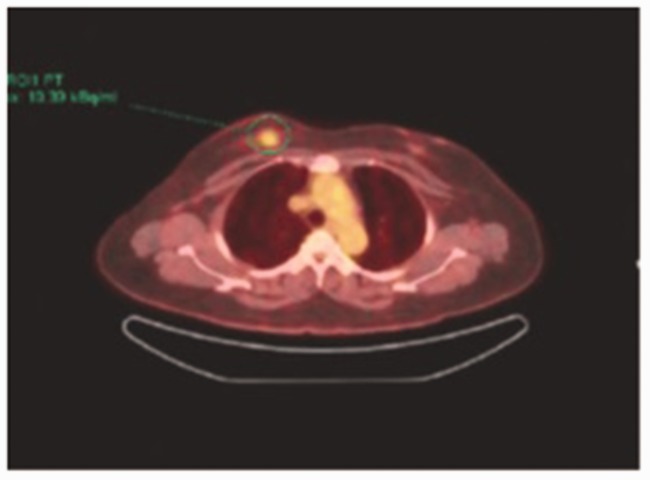
Axial-fused choline PET/CT detected avid choline (maximum standardized uptake volume, 13.3) uptake in the primary breast cancer lesion (circle).
Figure 3.
Maximum intensity projection (MIP) positron emission tomography (PET) 18F-choline images show physiological 18F-choline uptake in liver, pancreas, spleen, salivary and lacrimal glands and, owing to renal excretion, in the urinary tract. Other sites of less intense tracer uptake were bone marrow and intestines.
Table 2.
Mean 18F-choline uptake in both breasts of premenopausal and postmenopausal women
| PremenopausalMean SUVmax choline (g/dl) | Post-menopausalMean SUVmax choline (g/dl) | p | |
|---|---|---|---|
| Right Breast | 2.04 ± 0.404 | 1.59 ± 0.97 | <0.05 |
| Left Breast | 2.00 ± 0.56 | 1.93 ± 1.28 | <0.05 |
p<0.05, statistically significant (Student t test). SUVmax, maximum standardised uptake value.
Brain
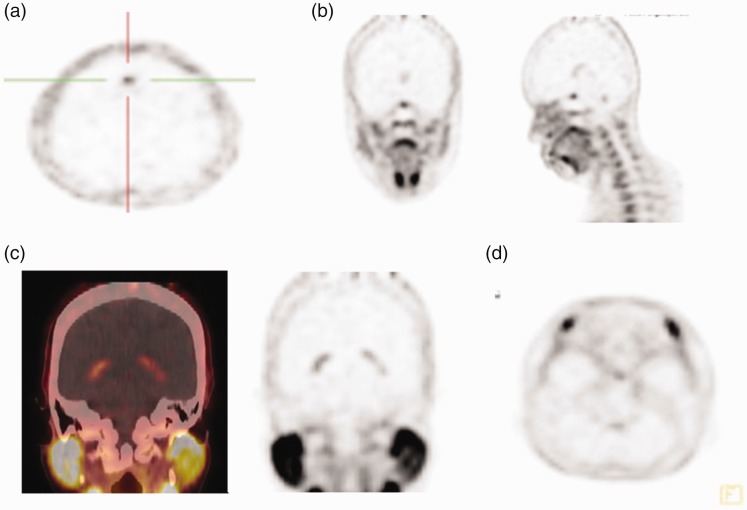
In the brain, choline uptake in the normal cerebral cortex and basal ganglia is less intense compared with FDG.6 In the present study, however, certain areas of the brain showed moderate to intense uptake in the falx cerebri (4.76%), choroid plexus (85.7%), and lacrimal gland (38.0%) (Figure 4). The pituitary gland showed the highest 18F-choline uptake in the brains of this group of subjects (Figure 4b).
Figure 4.
18F-choline positron emission tomography (PET) image (axial view) in a patient who had undergone 18F-choline PET/CT. (a) Increased activity is similar to that of the falx cerebri. (b, c, d) Uptake at the pituitary fossa (b). Increased activity in the choroid plexus (c) and lacrimal gland (d).
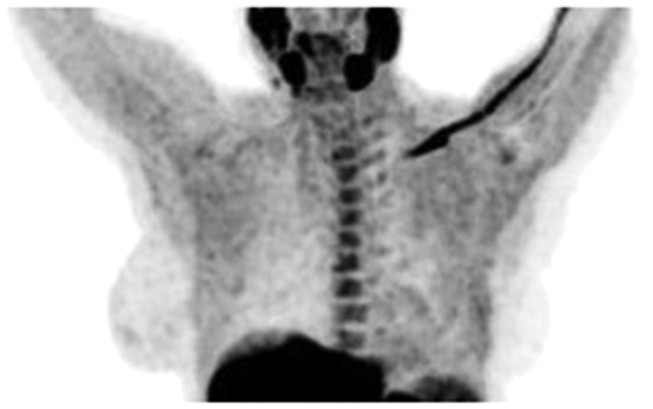
Neck
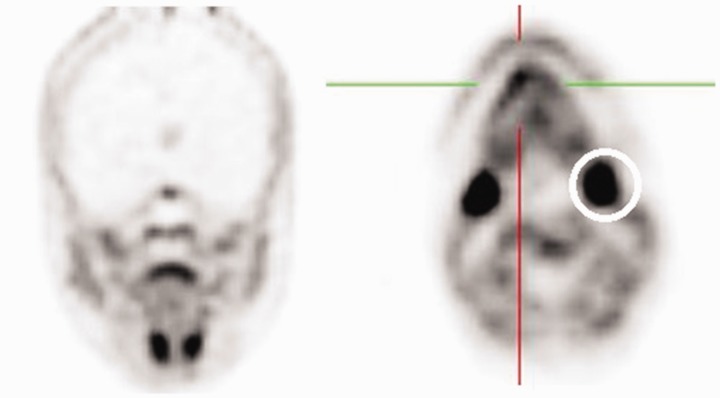
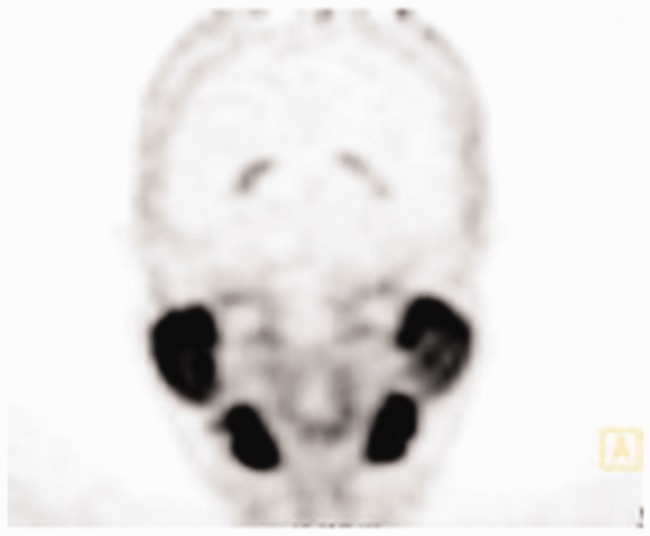
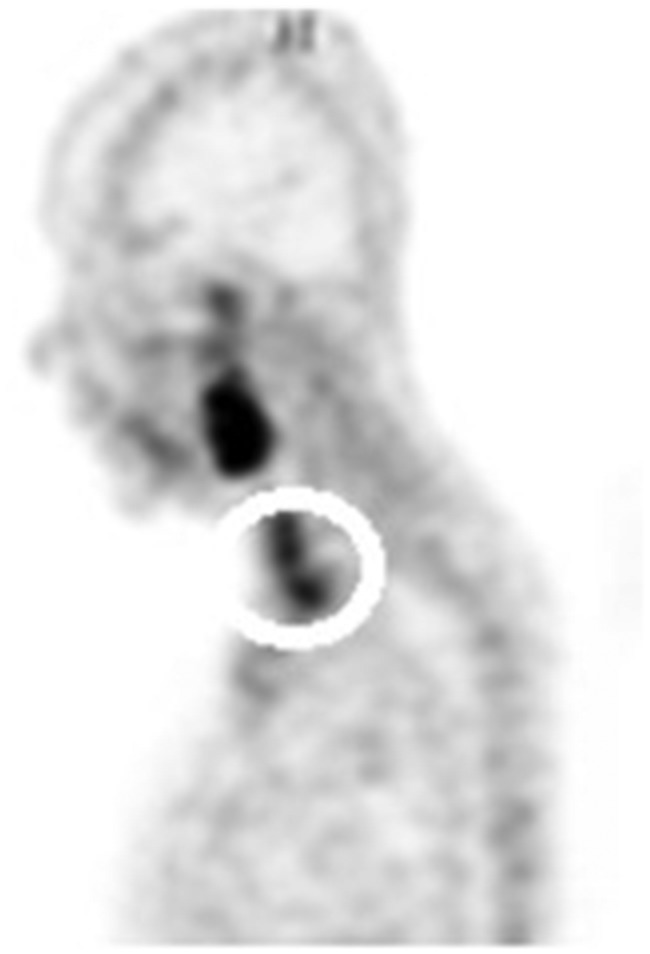
Normal lymphatic tissue may display low to moderate choline uptake in the head and neck, particularly in the salivary glands, lingual and palatine tonsils, and at the base of the tongue because of the physiological activity associated with the lymphatic tissue in Waldeyer’s ring.2 In the present study, we observed symmetrical 18F-choline uptake in the submandibular gland (100%) (Figure 5) and in the parotid gland (100%) (Figure 6). Diffuse symmetric uptake can be seen in the normal thyroid gland (see Figure 7). Diffuse thyroid uptake can occur in association with inflammatory changes such a thyroiditis or Graves' disease.4 Focal thyroid uptake occurs in benign thyroid nodules or malignancies, and further work-up is warranted in such cases. Routinely, patients with focal uptake should be further evaluated due because of the higher risk that their findings are associated with a malignancy. 18F-choline was incorporated into the mylohyoid muscle (4.76%) and into the thyroid bed of one patient (4.76%) (Figure 4).
Figure 5.
18F-choline positron emission tomography (PET) image (coronal and axial views) with increased 18F-choline activity at the base of tongue muscle (hair-line) and in the submandibular glands (circle).
Figure 6.
18F-choline positron emission tomography (PET) image (coronal view) with 18F-choline avid parotids and submandibular gland.
Figure 7.
18F-choline positron emission tomography (PET) image (sagittal view) in a patient with 18F-choline avid thyroid gland in favour of thyroiditis (circle).
Abdomen/pelvis
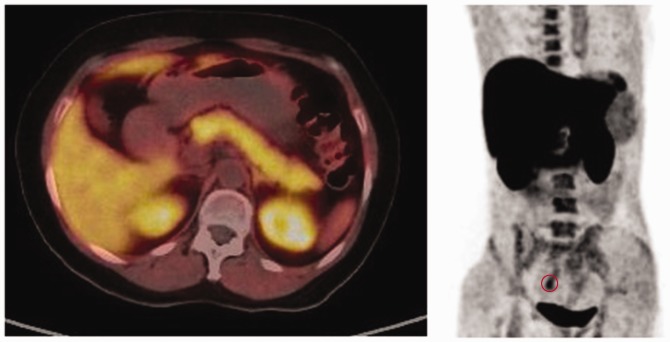
Avid 18F-choline uptake was detected in the liver, spleen, pancreas, and bowels of all patients. Good 18F-choline uptake and excretion were detected in the kidneys and bladder, respectively (Figure 8). Only one (4.76%) site with 18F-choline was noted in the ovaries and uterus of two patients (9.52%) (Figure 8). Detection of lymph node metastases may be hampered by high levels of physiological choline uptake in the gastrointestinal tract, commonly seen on 18F-choline PET/CT. Fasting is frequently proposed before patients undergo 18F-choline PET/CT.9
Figure 8.
18F-choline positron emission tomography (PET) Image (axial and coronal views) in a patient who had undergone 18F-choline PET/CT. Increased activity is seen liver, spleen, pancreas, gastrointestinal tract, genital system ○, urinary bladder and bone marrow.
Soft tissue, skeleton, and marrow
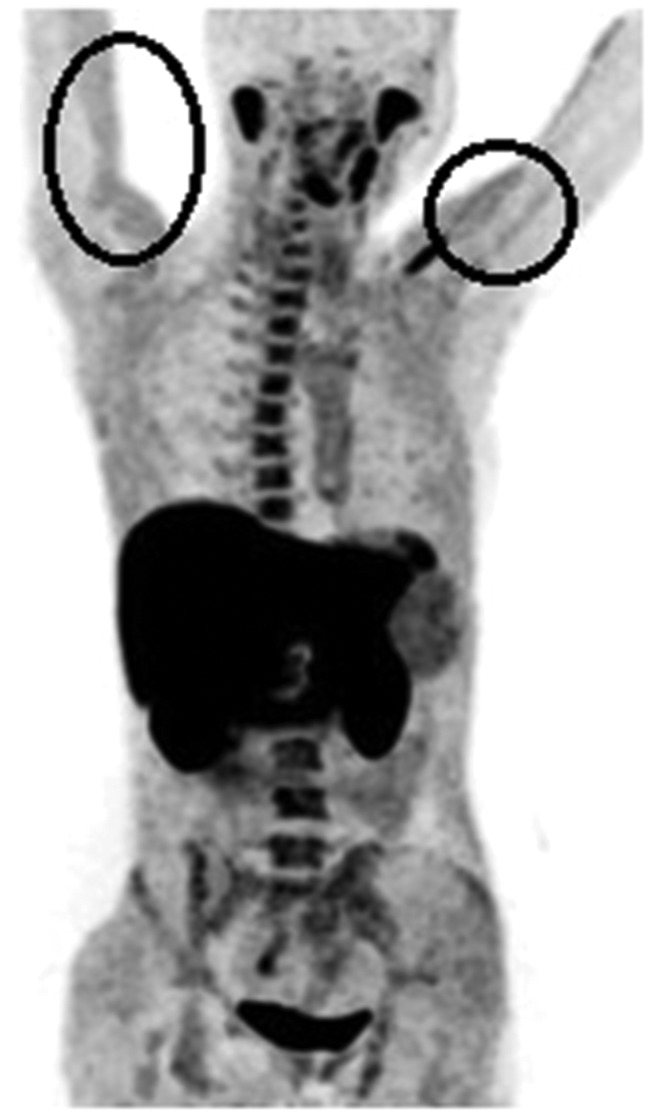
After recognising normal choline uptake, there is relatively low choline uptake in soft tissues, but conditions of muscular imbalance, e.g. postsurgery or scoliosis, may result in increased FDG uptake in affected muscles.2 In the present study, we detected 18F-choline uptake in the muscles (biceps, latissimus dorsi, and pectoralis major) was the lowest among the other organ systems (Figure 9). In all patients, 18F-choline uptake in the axial skeleton was recorded as avid, with scattered uptake involving the ribs and clavicle, accounting for <0.05% (Figure 10). Nevertheless, 18F-choline uptake in the sternum was relatively high (30%) (Figure 10). One site of uptake documented in the rib was likely attributable to an existing fracture (Figure 11). Pubic bone uptake (19.04%) was likely related to post-chemotherapy or radiotherapy effects.
Figure 9.
18F-choline positron emission tomography (PET) image (coronal view) showing symmetrical 18F-choline uptake in the biceps muscle (circle).
Figure 10.
Maximum intensity projection (MIP) positron emission tomography (PET) image (sagittal, coronal views) showing prevalence of 18F-choline uptake in the axial skeleton, pubic bone and in the sternum. The increased 18F-choline uptake in the hip joints indicate degenerative changes.
Figure 11.
Axial-fused 18F-choline positron emission tomography (PET)/ computed tomography (CT) images showing (left) increased 18F-choline uptake at an old rib fracture, (right) exhibiting callous union.
Miscellaneous
Some benign conditions showed a moderate to intense uptake of choline, which included healing of the surgical incision sites, sites of previous radiation therapy (no or low uptake), joint prosthesis (not infected), degenerative joint disease, and stomal sites, (Figure 10). Further, we documented 18F-choline contamination at the injection site in 61.9% of the patients (Figure 12) and an 18F-choline avid flow artefact due to a bolus of venous flow (Figure 13). We also detected 18F-choline uptake in lung metastasis (4.76%) and axillary lymph nodes (4.76%).
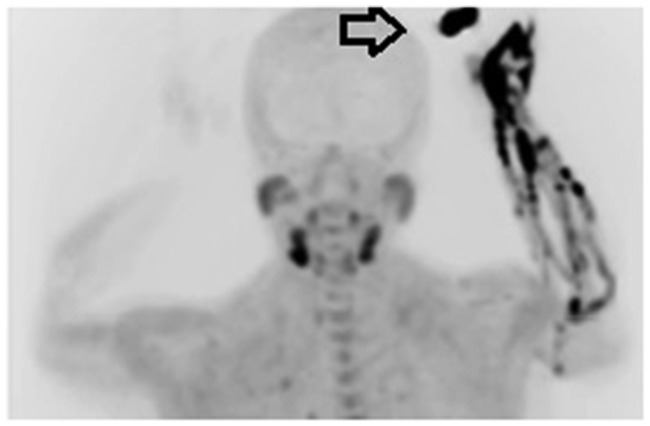
Figure 12.
Maximum intensity projection (MIP) PET image. Contamination at the injection site occurs during 18F-choline injection (arrow).
Figure 13.
Maximum intensity projection (MIP) PET image: 18F-choline intravenous flow artefact due to bolus of venous flow.
Discussion
Choline metabolism is elevated in the majority of tumours, and accounts for increased phospholipid synthesis required for cell membrane turnover.8 In mammary epithelial cells, increased intracellular phosphocholine levels are associated with malignancy, increasing with malignant transformation and progression.7 Thus, elevated choline levels in breast cancer are well documented. For the 21 patients in the present study, median choline uptake values were 10-fold lower in normal breast and lung tissues compared with those of tumours and were higher in more aggressive malignant lesions. These findings are consistent with the in vitro findings of Aboagye and Bhujwalla,7 who showed elevated phosphocholine levels associated with malignant transformation and progression in patients with an underlying malignant tumour.
A range of avid choline uptake values were observed in the cerebral cortex, falx cerebri, choroid plexus, pituitary and lacrimal glands, whereas choline was conspicuous in the salivary glands, and to some extent, in tonsillar glands. 18F-choline is also feasible as a tracer for imaging brain tumours.10,11 The distribution rate of choline is low in normal white and grey matter and in other regions of the brain, with the exceptions of the choroid plexus and the pituitary gland.12
The physiological choline uptake patterns in female organs are different from those in male organs. One particularly notable attribute is elevated choline kinase-α expression, which promotes cell division and is regulated by the growth factor receptor–mitogen-activated protein kinase pathway.13 Compared with choline uptake in men, the prevalence of thyroid inflammatory disease in women is more frequently encountered as an incidental finding on 18F-choline PET/CT.8
Here we detected significant differences in the values of 18F-choline SUVmax of the breasts between premenopausal and postmenopausal women. This was substantiated by findings that the efficiency of endogenous choline uptake is greater among premenopausal women because of the up-regulation of oestrogen production.14–16
The possibility of 18F-choline uptake into benign tumours may be explained by an overall increased rate of cell proliferation, which requires a high rate of synthesis of cell membranes, which may influence the uptake of the tracer.10 These differences in 18F-choline uptake were observed in the three benign breast lesions analysed here. Further, we detected sites of abnormal uptake of 18F-choline in inflammatory sites in 16%17 of patients, which suggests that certain inflammatory processes show a relatively high uptake of 18F-choline. The uptake of 18F-choline associated with inflammatory conditions may be explained by the proliferation of certain cells involved in the genesis of phlogosis, such as white cells.13
In the abdomen and pelvis, 18F-choline uptake detected in the liver, spleen, and pancreas was comparable with that detected in men. Nonetheless, uptake by gonadal glands was more frequently observed in women (ovaries, uterus) compared with in men (testis). High uptake of 11C-choline is normally observed in the liver and kidney (cortex), where choline is converted into betaine as well as in the pancreas and duodenum because of the secretion of phospholipid-rich pancreatic juice.18
In the spine, 18F-choline avidity was detected in men and women, although to a greater extent, and its uptake by the sternum and manubrium sterni is more frequently detected following chemotherapy or radiotherapy. Choline may serve as a predictive marker for chemotherapy-responsive patients with prostate cancer who express high levels of choline kinase. This hypothesis is based on findings that the cell-free androgen receptor expression and choline uptake occurs among those treated with abiraterone and enzalutamide.19 Thus, choline is a theranostic biomarker for women with breast cancer and is influenced by hormonal expression (progesterone or oestrogen). The possibility that choline serves a surrogate marker for hormone-dependent breast cancer that predicts tumour aggressiveness will be addressed by future research, because total reliance on determining the immunohistochemical subtypes of breast cancer lineages hampers treatment efficacy. This hypothesis is supported by the role of choline as a predictive marker, because it reflects the early response to abiraterone, detected using PET/CT, among patients with metastatic castration-resistant prostate cancer.20 Other incidental findings such as rib fractures were present in these patients.
Liu et al reported increased uptake of 18F-choline in some granulomatous diseases such as sarcoidosis and tuberculosis.17 In the present study, one patient with choline-negative postradiation pneumonitis was unique, because most inflammatory conditions should accumulate choline.
Conclusion
18F-choline PET/CT is useful for differentiating the levels of tumour aggressiveness associated with a tumour's biochemistry, physical and chemical properties, as well as its metabolism. These properties of choline are unique, specifically reflecting tumour phenotypes. These unique properties distinguish choline from FDG, because choline will localise to any part of the body with high physiological activity. Detailed knowledge of the technique and interpretation of 18F-choline PET/CT studies are required for data interpretation. Not knowing these potential causes of misinterpretation can lead to inaccurate interpretation of PET images or false-positive results. Therefore, the new knowledge presented here of the distribution pattern of 18F-choline in women may subsequently decrease the number of unnecessary follow-up studies or procedures that improve patient management plans and monitoring.
In conclusion, we show here that the uptake of 18F-choline was higher in premenopausal subjects, and the physiological uptake of 18F-choline was detected in many sites of the body, which may masquerade as cancers. Our knowledge of the biodistribution of 18F-choline in female patients with breast cancers is limited. It is therefore essential to recognize physiologic variations to avert false-positive results.
Declaration of conflicting interest
The author(s) declare that there is no conflict of interest.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Fundamental research Grant Scheme (FRGS), Ministry of Education, Malaysia. (Grant number: 28612-61021).
References
- 1.Fathinul F, Nordin AJ, Lau WEF. 18[F] FDG-PET/CT is a useful molecular marker in evaluating tumour aggressiveness; a revised understanding of an in- vivo FDGPET imaging that alludes the alteration of cancer biology. Cell Biochem Biophys 2014; 55: 631–640. [DOI] [PubMed] [Google Scholar]
- 2.Mertens K, Slaets D, Lambert B, et al. PET with (18)F-labelled choline-based tracers for tumuor imaging: a review of the literature. Eur J Nucl Med Mol Imaging 2010; 37: 2188–2193. [DOI] [PubMed] [Google Scholar]
- 3.Schillaci O, Calabria F, Tavolozza M, et al. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate cancer. Nucl Med Commun. 2010; 31: 39–45. [DOI] [PubMed] [Google Scholar]
- 4.Calabria F, D’Auria S, Sannino P, et al. A case of thymoma detected by 18Fcholine positron emission tomography/computed tomography. Eur J Nucl Med Mol Imaging. 2011; 38: 602. [DOI] [PubMed] [Google Scholar]
- 5.Fallanca F, Giovacchini G, Picchio M, et al. Incidental detection by [11C]choline PET/CT of meningiomas in prostate cancer patients. Q J Nucl Med Mol Imaging 2009; 53: 417–421. [PubMed] [Google Scholar]
- 6.Mertens K, Ham H, Deblaere K, et al. Distribution patterns of 18F-labelled fluoromethylcholine in normal structures and tumors of the head: a PET/MRI evaluation. Clin Nucl Med. 2012; 37: 196–203. [DOI] [PubMed] [Google Scholar]
- 7.Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res 1999; 59: 80–84 [PubMed] [Google Scholar]
- 8.Ramirez de Molina A, Rodriguez-Gonzalez A, Gutierrez R, et al. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun 2002; 296: 580–583. [DOI] [PubMed] [Google Scholar]
- 9.Chondrogiannis S, Marzola MC, Grassetto G, et al. New acquisition protocol of 18F-choline PET/CT in prostate cancer patients: review of the literature about methodology and proposal of standardization. BioMed Res Int 2014; 2014: 215650. doi 10.1155/2014/215650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabria F, Chiaravalloti A, Schillaci O. 18F-Choline PET/CT Pitfalls in Image Interpretation An Update on 300 Examined Patients With Prostate Cancer. Clin Nucl Med 2014; 39: 122–130. [DOI] [PubMed] [Google Scholar]
- 11.Cook GJ, Maisey MN, Fogelman I. Normal variants, artefacts and interpretative pitfalls in PET imaging with 18-fluoro-2- deoxyglucose and carbon-11 methionine. Eur J Nucl Med 1999; 26: 1363–1378. [DOI] [PubMed] [Google Scholar]
- 12.Schillaci O, Calabria F, Tavolozza M, et al. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate cancer. Nucl Med Commun 2010; 31: 39–45. [DOI] [PubMed] [Google Scholar]
- 13.Roivainen A, Forsback S, Gronroos T, et al. Blood metabolism of [methyl-11C]choline; implications for in vivo imaging with positron emission tomography. Eur J Nucl Med. 2000; 27: 25–32. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and C. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press (US): Washington, DC, USA, 1998. [PubMed]
- 15.Yan J, Wang W, Gregory JF, 3rd, et al. MTHFR C677T genotype influences the isotopic enrichment of one-carbon metabolites in folate-compromised men consuming. Am. J. Clin. Nutr. 2011, 93, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Jiang X, West AA, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am. J. Clin. Nutr 2012, 95, 1060–1071. [DOI] [PubMed] [Google Scholar]
- 17.Schillaci O, Calabria F, Tavolozza M, et al. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate cancer. Nuclear Medicine Communications 2010, 31: 39–45. [DOI] [PubMed] [Google Scholar]
- 18.Roivainen A, Forsback S, Gronroos T, et al. Blood metabolism of [methyl-11C]choline; implications for in vivo imaging with positron emission tomography. Eur J Nucl Med. 2000; 27: 25–32. [DOI] [PubMed] [Google Scholar]
- 19.Conteduca V, Scarpi E, Caroli P, et al. Circulating androgen receptor combined with 18F-fluorocholine PET/CT metabolic activity and outcome to androgen receptor signalling-directed therapies in castration-resistant prostate cancer. Sci Rep 2017; 7: 15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Giorgi U, Caroli P, Burgio SL, et al. Early outcome prediction on 18F-fluorocholine PET/CT in metastatic castration-resistant prostate cancer patients treated with abiraterone. Oncotarget. 2014; 5: 12448–12458. [DOI] [PMC free article] [PubMed] [Google Scholar]