Short abstract
Objective
This study was performed to evaluate the state of heavy metal contamination in soil and vegetables and assess the health risk of inhabitants in the mine-affected area and area far from the mine (reference area) in Daye, China.
Methods
The heavy metal concentrations in soil and vegetable samples were detected by inductively coupled plasma mass spectrometry. Residents’ exposure parameters were obtained through a questionnaire survey. A health risk assessment model recommended by the United States Environmental Protection Agency was used to evaluate the residents’ risk of oral exposure.
Results
The copper, lead, cadmium, and arsenic concentrations in soil and in vegetables were higher in the mine-affected area than in the reference area. The health risk of residents in the reference area was within the acceptable range (hazard index < 1, carcinogen risk < 10−4). In the contaminated area, however, the mean hazard index was 2.25 for children and 3.00 for adults, and the mean carcinogen risk was 4.749 × 10−4 for children and 0.587 × 10−4 for adults.
Conclusions
Potential health risks exist for inhabitants near the mine area. Cadmium and arsenic should be paid more attention as risk sources.
Keywords: Heavy metal, health risk assessment, soil, vegetable, Daye, hazard index, carcinogen risk
Introduction
The smelting process in metal mines can produce large amounts of waste, resulting in high accumulation of heavy metals in the soil and in the river and underground water surrounding the mining area.1 Similarly, the levels of heavy metal contamination in the soil, air, river, and crops from mining-affected areas are reportedly higher than those in non-mining areas in many countries including China,2,3 the United States,4 and South Korea.5 In addition to causing environmental damage, heavy metals in soil, air, and other media in mining-affected areas enter plants by absorption through the vegetable roots and dust on leaves.6,7 Ingestion of heavy metals can upset the body chemistry, especially because these metals do not undergo decomposition within the body and have a high affinity for many body systems.8 Increasing evidence indicates that oral exposure is the most important way for heavy metals in the environment to enter the body; contaminated soil can result in heavy metal translocation into the food chain, and high consumption of contaminated vegetables can pose a serious risk to the human body.9,10
Studies of the accumulation and origin of heavy metals in soil11 and the potential ecological hazards associated with this process12,13 have revealed an increasing trend. This intensive research has attracted attention to the effects of heavy metals on human health, especially in infants and children.14–17 Among many toxic elements, lead, arsenic, and cadmium are considered to be potential carcinogens and are associated with the development of several diseases, especially cardiovascular, kidney, nervous system, blood, and bone diseases.18 Although copper is an essential element for humans, a high concentration in soil and vegetables leads to human toxicity.19
China is one of few countries with a wide range of mineral resources and a high degree of self-sufficiency in minerals. Mining activities alone have generated about 1,500,000 hectare (ha) of wasteland in China,20 and this figure is increasing at a rate of 46,700 ha per year.21 Daye is one of the most important metal ore-concentrating areas in China and has a 3000-year metallurgical mining history since the Shang Dynasty. Heavy metal contamination of soil, surface water, and underground water in Daye caused by long-term mining activities has attracted extensive attention;22,23 however, research of the health risks to residents is still in the initial stage. Previous research of such health risks focused more on the contamination level of environmental media than on the risk of heavy metal exposure. This is because such studies more commonly used the exposure parameters from guidelines published by the United States Environmental Protection Agency (USEPA) or data from literature references for residents,24 which might not have been completely in line with the actual situation of the local population.
The aim of the present study was to estimate the environmental impact of heavy metals in farmland soil and vegetables sampled both from the mine-affected area and area away from mining activities (reference area) in Daye, China. The transformation characteristics of heavy metals from soil to vegetables in the reference and contaminated areas were studied in detail. At the initial stage, a questionnaire survey was administered to the residents in Daye to explore the exposure parameters of the local population specifically associated with vegetable consumption. The overriding concern was that the health risk of local inhabitants from the reference and contaminated areas are based on the actual inhabitant exposure parameters and heavy metal concentrations. The research techniques and results of this study can provide a reference for mining pollution control and health risk assessment in similar area around the world.
Materials and methods
Ethical approval
This study protocol was approved by a professional expert group (Expert Group on the Special Investigation Project of Environment and Health in Daye, Hubei Province) that was designated by the Hubei Provincial Center for Disease Control and Prevention and the Hubei Academy of Environmental Sciences.
Study site description
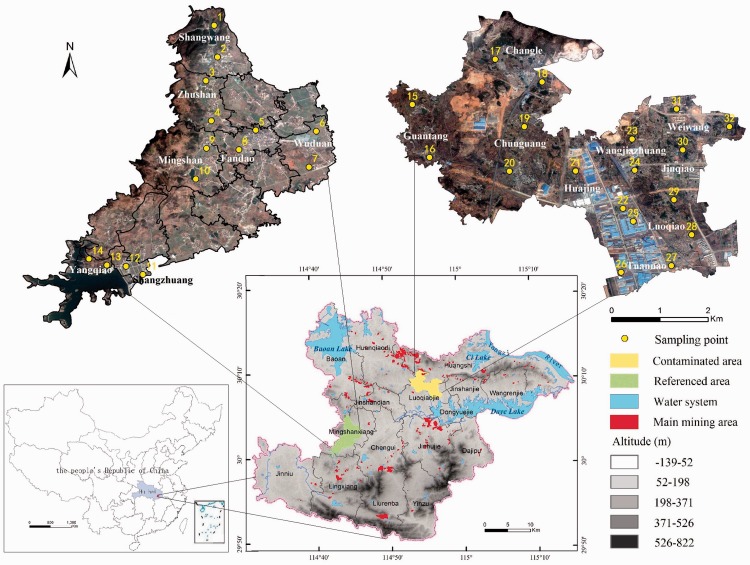
Daye city lies to the southeast of Hubei province in central China. The present study included two areas, namely the mine-affected area (i.e., contaminated area) in the northeast of Daye city and an area away from the mining activities (i.e., reference area) in the west of Daye city. (Figure 1). In total, 32 sampling sites are randomly distributed in farmland in the selected villages, with 2 sampling sites in every village. The reference area includes 14 sampling sites in 7 villages: Shangwang (sampling sites 1 and 2), Zhushan (sampling sites 3 and 4), Fandao (sampling sites 5 and 8), Wuduan (sampling sites 6 and 7), Mingshan (sampling sites 9 and 10), Shangzhuang (sampling sites 11 and 12), and Yangqiao (sampling sites 13 and 14) (Figure 1). The contaminated area includes 18 sampling sites in 9 villages: Guantang (sampling sites 15 and 16), Changle (sampling sites 17 and 18), Chunguang (sampling sites 19 and 20), Huajing (sampling sites 21 and 22), Wangjiazhuang (sampling sites 23 and 24), Luoqiao (sampling sites 25 and 28), Tuannao (sampling sites 26 and 27), Jinqiao (sampling sites 29 and 30), and Weiwang (sampling sites 31 and 32). The villages and sampling sites in the two study areas were selected with consideration of the inhabitants’ settlement and distribution. The reference area has socioeconomic characteristics similar to those of the contaminated area. The geographical locations of the sampling points are shown in Figure 1.
Figure 1.
Distribution of sampling points.
Sampling and pretreatment
Sample collection
In total, 32 surface soil samples (1 kg each) were collected from the top 0- to 20-cm layer at the sampling sites with reference to HJ/166-2004 (China, 2004).25 This is because most crops’ roots are present in the top 20 cm of soil,26 which is regularly turned over by local farmers. At each sampling site, one subsample was collected within a distance of 5 m surrounding the site to form a composite sample. A total of 1 kg of soil was selected from the mixed samples by the quartile method, stored in a polyethylene zip-bag, and immediately transported to the laboratory. In total, 204 vegetable samples were collected directly from the land from which the soil samples were collected, including 7 kinds of vegetables: cowpea (Vigna unguiculata (Linn.) Walp), water spinach (Ipomoea aquatica Forsk), amaranth (Amaranthus tricolor L), sweet potato leaves (Ipomoea batatas Lam), tomato (Lycopersicon esculentum Miller), eggplant (Solanum melongena Linn), and pepper (Capsicum annuum Linn. var. grossum (L.) Sendt). The specific sampling quantities were 21 cowpea, 21 water spinach, 6 amaranth, 24 sweet potato leaves, 18 tomato, 15 eggplant, and 18 pepper samples in the contaminated area and 15 cowpea, 12 water spinach, 6 amaranth, 12 sweet potato leaves, 6 tomato, 15 eggplant, and 15 pepper samples in the reference area.
Sample pretreatment and analysis
The soil samples were air-dried at room temperature, then pulverized and sieved through a 1-mm stainless steel mesh to obtain a fine homogenous powder. The samples were stored in polyethylene bags at 4°C prior to analysis. The edible portions of the vegetables were thoroughly washed with tap water and deionized. After being primarily air-dried at room temperature and milled by a ceramic-coated grinder, the vegetable samples were frozen at 18°C until chemical analysis.
The soil pH was measured in ultrapure water (1.0:2.5 soil:solution ratio, dry w/v). The soil samples were digested according to “Acid Digestion of Sediments, Sludges and Soils” (USEPA Method 3050b).27 The vegetable samples were digested according to “Determination of Lead in Food, Arsenic, Iron, Calcium, Zinc, Aluminium, Sodium, Magnesium, Boron, Manganese, Copper, Barium, Strontium, Titanium, Tin, Cadmium, Chromium and Vanadium Content by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) Method” (DB53/T 288-2009).28
The concentrations of copper (Cu), lead (Pb), arsenic (As), and cadmium (Cd) in vegetables and soil were measured by inductively coupled plasma mass spectrometry (NexION 350x; PerkinElmer, Waltham, MA, USA). Each batch of testing blank and parallel samples were analyzed simultaneously. The relative deviation between the parallel samples was controlled within 10%.
Population survey
The exposure parameters from the Exposure Factors Handbook,29 such as the inhabitants’ weight and vegetable intake, are not suitable for calculation of the residents’ health risk. Thus, the questionnaire regarding the exposure parameters of the Daye area mainly covered the problems related to calculation of dietary route exposure, including sex, age, height, weight, and other individual characteristics as well as food types, consumption, frequency, time, and other dietary exposure parameters. Four hundred questionnaires were issued, and 389 valid responses were obtained. After data input, PASW Statistics, version 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Data analysis
Transfer factor
The transfer factor (TF) of heavy metals was calculated by Equation (1), which represents the potential capability of heavy metal transmission from soil to the edible parts of a vegetable:30
| (1) |
where Cveg is the heavy metal concentration (on a fresh weight basis) in the edible parts of the vegetable, and Csoil is the total heavy metal concentration (on a dry weight basis) in the corresponding soil where the vegetable was grown.
Health risk assessment and exposure parameters
The health risk assessment model was provided by USEPA (2007) and allowed for quantitative assessment of the human health risks of Cu, Pb, As, and Cd. The mean daily dose (ADD) was used to measure the amount of daily heavy metal intake associated with local inhabitants’ vegetable consumption. The formula for calculating ADD for a single heavy metal through the diet is shown in Equation (2):31
| (2) |
where C (mg/kg on a fresh weight basis) is the food contaminant concentration, IR (kg/person/d) is the daily vegetable consumption, EF (365 days/year) is the exposure frequency, ED is the exposure duration, BW (kg) is the body weight, and AT is the mean exposure time for noncarcinogens.
The hazard index (HI) for the noncarcinogen risk of a variety of heavy metals was calculated using Equation (3):32
| (3) |
where i is the heavy metal species and n is the type of heavy metal. Cu is a noncarcinogen and As, Cd, and Pb are carcinogens. The carcinogen risk (CR) of heavy metal intake through vegetable consumption was calculated using Equation (4):
| (4) |
The IR was obtained through the questionnaire investigation and statistics presented in “China dietary and nutrition survey and tracking research.”33 The IR for children and adults was 153.84 and 373.98 g/d, respectively. The BW for children and adults was 18.77 and 61.00 kg, respectively. For the noncarcinogen risk, AT is the product of EF and ED; ED for children and adults was 4.54 and 42.61 years, respectively, which are the mean ages according to a local population survey. For the CR, AT is the whole life expectancy (74.80 years for children and 64.95 years for adults).
According to the USEPA Integrated Risk Information System statistics, the recommended reference dose values for oral intake of As, Cd, Pb, and Cu are 0.0003, 0.001, 0.004, and 0.04 mg/kg per day, respectively. The carcinogen slope factor for As, Cd, and Pb are 1.5, 0.38, and 0.0085 mg/kg per day, respectively.34,35
The HI was used to characterize inhabitants’ noncarcinogen risk of consuming vegetables. If the HI is <1, the exposed population is unlikely to experience obvious adverse effects. The acceptable risk range for CR is 10−4 according to the USEPA.
Results and Discussion
Heavy metal concentrations in farmland soil
Table 1 summarizes the heavy metal concentrations in farmland soil in the study area. The soil from the contaminated area and reference area was slightly acidic, with a pH of <7. There were significant variations of the heavy metals in the soil samples from both the contaminated and reference areas in the following order: Cu > Pb > As > Cd (p < 0.05 for all).
Table 1.
Heavy metal concentrations in soil (mg/kg)
| Heavy metal | Contaminated soils (n = 18) |
Reference soils (n = 14) |
MAL | Backgroundvalue | ||
|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | |||
| Cu | 32.35–321.99 | 128.48 ± 101.36 | 17.33–47.04 | 28.33 ± 11.31 | 50 | 25.63 |
| As | 10.16–56.42 | 20.09 ± 11.60 | 4.74–10.48 | 8.01 ± 2.14 | 30 | 16.58 |
| Cd | 0.20–2.45 | 1.38 ± 0.81 | 0.03–0.22 | 0.13 ± 0.05 | 0.3 | 0.175 |
| Pb | 19.54–87.56 | 55.16 ± 22.40 | 17.88–24.83 | 21.41 ± 2.26 | 250 | 43.35 |
Concentrations are presented in mg/kg. The mean is presented with the standard deviation.
Cu, copper; As, arsenic; Cd, cadmium; Pb, lead; MAL, maximum allowable level.
In the reference area, the maximum values of all heavy metals in soil were below the maximum allowable level (MAL) according to the Environment Quality Standard for Soils regulated by the Environmental Protecting Administration of China (EPAC).36 The concentrations of Pb, As, and Cd were all below those in the natural background soil in Daye.37 However, the concentration of Cu in soil from the reference area was slightly higher than the soil background value, which may be related to the abundance of Cu ore and the increasingly intensive mining activities. Thus, the soil from the reference area has not been contaminated but may still be affected by mining activities.
All measured heavy metal concentrations in the surface soil samples from the contaminated area were significantly higher than those in the reference area (p < 0.05) and in the background soil of Daye. The mean Cu, Pb, As, and Cd concentrations in soil from the contaminated area were 4.53, 2.57, 2.50, and 10.16 times higher than those in the reference area, respectively (p = 0.001, 0.001, 0.000, and 0.000, respectively). In addition, the mean concentration of Cu and Cd in soil from the contaminated area reached 128.48 and 55.16 mg/kg, respectively, which was found to exceed the MAL. However, the concentrations of As and Cu were within the safe limits set by the EPAC. These results suggest that the soil in the contaminated area has been greatly affected, even contaminated, by mining and smelting activities and may not be suitable for crop production. Additionally, Cd was found to be the primary contaminant.
Moreover, the level of heavy metal contamination differed in all soil sample types, which was reflected by the maximum value, minimum value, and standard deviation of each element. This shows that there are differences in the degree of soil contamination in different villages even within the same region, which may be mainly related to the specific terrain, soil types, and farming activities.
Assessment of heavy metal contamination in vegetables
The heavy metal concentrations in the edible portions of vegetables grown in contaminated soils were compared with those grown in soil from the reference area. The seven vegetables examined were cowpea, water spinach, amaranth, sweet potato leaves, tomato, eggplant, and pepper.
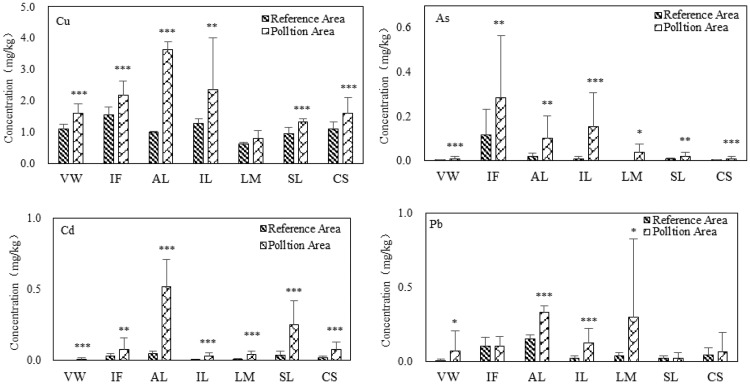
The mean heavy metal concentrations in vegetables from both the contaminated and reference areas decreased in the order of Cu > Pb > Cd > As, which is not completely consistent with the above-mentioned order of heavy metal concentrations in soil (i.e., Cu > Pb > As > Cd). The concentrations of Cu, Pb, Cd, and As in vegetables from the contaminated area ranged from 0.463 to 6.672, 0.000 to 1.474, 0.000 to 0.711, and 0.000 to 0.942 mg/kg, respectively. The concentrations of Cu, Pb, Cd, and As in vegetables from the reference area ranged from 0.618 to 1.548, 0.006 to 0.153, 0.000 to 0.048, and 0.000 to 0.095 mg/kg, respectively, which were significantly lower than those in vegetables from the contaminated area (p < 0.05 for all) (Figure 2). This result is in agreement with the findings that plants grown in topsoil contaminated with heavy metals are more likely to be contaminated.38
Figure 2.
Heavy metal concentrations in the edible parts of vegetables. The error bars indicate the standard deviation, and the asterisks indicate significant differences in heavy metal concentrations between vegetable grown in mine-affected and reference soils. *P < 0.1, **P < 0.05, and ***P < 0.001.
As shown in Figure 2, the heavy metal concentrations in different vegetables are quite different. Overall, leafy vegetables such as spinach, amaranth, and potato leaf seemed to accumulate more heavy metals in their edible parts than other types of vegetables such as cowpeas and pepper. This is consistent with the conclusions of Yang et al.39 A study by Yana et al.40 showed that heavy metals in the leaves of vegetables do not only come from the soil but are also greatly affected by contamination from smelting waste gases, whereas heavy metals in the fruits and roots of vegetables mainly come from soil. In addition, the heavy metal concentrations in vegetables are related to the different growth stages of vegetables in the same season because the ability of vegetables to enrich heavy metals varies with the growth stages.41 This may be the reason why heavy metals in the edible portions of leaf vegetables is much higher.
To evaluate the heavy metal contamination of vegetables grown in soil, the heavy metal concentrations in this study were compared with the MAL set by China, the Food and Agriculture Organization (FAO),42 and the European Communities (EC)43 (Table 2). Cu is an essential element for the human body and is therefore not regulated by Chinese food standards. However, the FAO (2011) regulates the maximum Cu level at 40 mg/kg (based on fresh weight), while the EC (2006) limits the Cu level at 20 mg/kg (based on fresh weight).
Table 2.
Recommended maximum levels and heavy metal concentrations for vegetables
| Standard | Cu | As | Cd | Pb |
|---|---|---|---|---|
| FAO, 2011 | 40 | 0.05 (Inorganic) | 0.2 leafy; 0.05 others | 0.3 |
| EC, 2006 | 20 | NR | 0.2 leafy; 0.05 others | 0.3 |
| CMH, 2012 | NR | 0.5 (Total) | 0.2 leafy; 0.05 others | 0.3 leafy; 0.1 others |
| Contaminated area mean | 1.930 ± 0.494 | 0.123 ± 0.087 | 0.143 ± 0.079 | 0.145 ± 0.148 |
| Reference area mean | 1.087 ± 0.151 | 0.022 ± 0.022 | 0.020 ± 0.011 | 0.055 ± 0.029 |
Levels are presented in (mg/kg, fresh weight). Data are presented as mean ± standard deviation.
Cu, copper; As, arsenic; Cd, cadmium; Pb, lead; FAO, Food and Agriculture Organization; EC, European Union Standards; CMH, Chinese Ministry of Health; NR, Not recommended.
The mean concentrations of Cu, Pb, Cd, and As in vegetables from contaminated soil in this study were lower than the MAL set by China, the FAO, and the EC. However, not all vegetables are safe to enter the food chain; the degree of contamination greatly differs among different kinds of vegetables. Contamination in amaranth species was the most severe among the sampled vegetables. Only the concentrations of Cd and Pb in amaranth species were 2.6 and 1.1 times higher, respectively, than the MAL set by China,44 the FAO, and the EC. Additionally, the concentrations of Cd in pepper and eggplant were 5.0 and 1.6 times higher, respectively, than the MAL. The concentrations of Cu, Pb, and As for both pepper and eggplant did not exceed the MAL. However, all four heavy metals in cowpeas, spinach, and sweet potato leaves were below the MAL.
Transfer of heavy metals from soil to vegetables
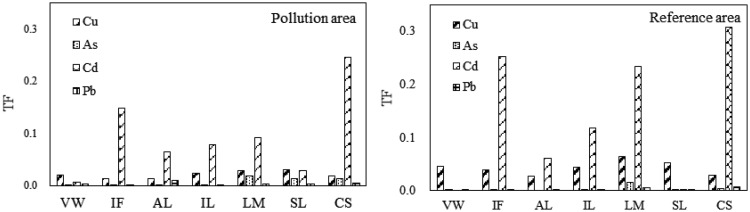
The TF can be used to assess the potential ability of heavy metals to move from soil to plant tissues. The TF of heavy metals from soil to vegetables in the contaminated and reference areas is shown in Figure 3. The mean TF value for Cu, As, Cd, and Pb from soil to vegetables in the contaminated area reached 0.021, 0.007, 0.095, and 0.003, respectively. The mean TF value for Cu, As, Cd, and Pb from soil to vegetables in the reference area reached 0.043, 0.003, 0.139, and 0.003, respectively. The TF of Cu and Cd from soil to vegetables was obviously lower in the contaminated than reference area. The TF of the four heavy metals in the two regions both showed an order of Cd > Cu > As > Pb, which is consistent with the conclusions reported by Liu et al.45 and Cai et al.46 In addition, the order was not the same as in the report by Choudhury,47 which is more likely related to the physical and chemical properties of the soil and the type of plant sampled.
Figure 3.
Transfer factor of heavy metals from soil to vegetables in contaminated area and reference area.
The TF of the different vegetables varied greatly, showing an order of amaranth > spinach > eggplant > pepper > tomato > sweet potato leaf > cowpea. The TF of leafy vegetables such as spinach and amaranth was higher than that of other vegetables such as eggplant, pepper, and tomato, which is in agreement with the findings of Xu et al.48
Additionally, the contamination of soil by Cd was the most severe, so the accumulation of Cd from soil to vegetables should be paid more attention (although the mean concentration of Cd in vegetables was lower than the MAL recommended by Chinese Ministry of Health).
Health risk of local inhabitants by contaminated vegetables
The noncarcinogen risk values of children and adults in the contaminated and reference areas were calculated according to the consumption of vegetables and the heavy metal concentrations in vegetables (Table 3). The total HI of the four heavy metals for children and adults in the contaminated area ranged from 0.72 to 7.70 and 0.54 to 5.76, respectively. The total HI of children and adults in the reference area ranged from 0.28 to 1.66 and 0.19 to 1.20, respectively. The total HI of residents in the contaminated area, including both adults and children, were higher than those the reference area. The total HI calculated in the reference area was <1 if the local residents consumed each vegetable at the same frequency, suggesting that the noncarcinogen risk of heavy metal exposure through vegetables was generally safe. Remarkably, residents in the contaminated area were exposed to a serious noncarcinogenic health risk.
Table 3.
Hazard risk index of vegetable intake
| Vegetable | Age | Contaminated area |
Reference area |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | As | Cd | Pb | Total | Cu | As | Cd | Pb | Total | ||
| VW | Children | 0.33 | 0.18 | 0.07 | 0.14 | 0.72 | 0.23 | 0.02 | 0.00 | 0.01 | 0.72 |
| Adults | 0.25 | 0.13 | 0.05 | 0.11 | 0.54 | 0.17 | 0.01 | 0.00 | 0.01 | 0.19 | |
| IF | Children | 0.45 | 2.96 | 0.63 | 0.21 | 4.26 | 0.32 | 0.88 | 0.26 | 0.21 | 1.66 |
| Adults | 0.34 | 2.22 | 0.47 | 0.16 | 3.18 | 0.24 | 0.66 | 0.19 | 0.16 | 1.24 | |
| AL | Children | 0.74 | 2.06 | 4.22 | 0.67 | 7.70 | 0.21 | 0.26 | 0.39 | 0.31 | 1.17 |
| Adults | 0.56 | 1.54 | 3.16 | 0.50 | 5.76 | 0.15 | 0.19 | 0.30 | 0.24 | 0.88 | |
| IL | Children | 0.48 | 2.12 | 0.22 | 0.26 | 3.09 | 0.26 | 0.13 | 0.00 | 0.04 | 0.44 |
| Adults | 0.36 | 1.59 | 0.17 | 0.19 | 2.31 | 0.20 | 0.10 | 0.00 | 0.03 | 0.33 | |
| LM | Children | 0.16 | 0.25 | 0.35 | 0.61 | 1.37 | 0.13 | 0.00 | 0.07 | 0.08 | 0.28 |
| Adults | 0.12 | 0.19 | 0.26 | 0.46 | 1.03 | 0.09 | 0.00 | 0.06 | 0.06 | 0.21 | |
| SL | Children | 0.27 | 0.27 | 2.07 | 0.05 | 2.66 | 0.20 | 0.10 | 0.29 | 0.04 | 0.63 |
| Adults | 0.20 | 0.20 | 1.55 | 0.04 | 1.99 | 0.15 | 0.08 | 0.22 | 0.03 | 0.47 | |
| CS | Children | 0.33 | 0.11 | 0.65 | 0.13 | 1.22 | 0.22 | 0.04 | 0.13 | 0.09 | 0.48 |
| Adults | 0.25 | 0.09 | 0.49 | 0.10 | 0.91 | 0.17 | 0.03 | 0.10 | 0.07 | 0.36 | |
| Mean | Children | 0.40 | 1.14 | 1.17 | 0.30 | 3.00 | 0.22 | 0.20 | 0.16 | 0.11 | 0.77 |
| Adults | 0.30 | 0.85 | 0.88 | 0.22 | 2.25 | 0.17 | 0.15 | 0.12 | 0.08 | 0.53 | |
Cu, copper; As, arsenic; Cd, cadmium; Pb, lead; VW, Vigna unguiculata (Linn.) Walp (cowpea); IF, Ipomoea aquatica Forsk (water spinach); AL, Amaranthus tricolor L (amaranth); IL, Ipomoea batatas Lam (sweet potato leaves); LM, Lycopersicon esculentum Miller (tomato); SL, Solanum melongena Linn (eggplant); CS, Capsicum annuum Linn. var. grossum (L.) Sendt (pepper).
In the contaminated area, the HI values for Cu and Pb in both children and adults were <1, indicating that the noncarcinogen risk caused by only oral Cu or Pb was not obvious. Notably, the total noncarcinogen risk had a mean of 77% for As and Cd in both children and adults in the contaminated area. Similarly, Lim et al.49 reported a high risk for Cd and As.
The mean noncarcinogen risk contribution rate of Cu, As, Cd, and Pb for residents in the reference area was 30%, 27%, 22%, and 15%, respectively. The situation in the reference area was greatly different from that in the contaminated area, mainly due to the different heavy metal concentrations in vegetables between the two different regions.
The CR values of adults and children in the contaminated area ranged from 5.49 × 10−5 to 1.25 × 10−3 and from 6.80 × 10−6 to 1.55 × 10−4, respectively, and those of adults and children in the reference area ranged from 3.70 × 10−6 to 2.45 × 10−4 and from 5.00 × 10−7 to 1.67 × 10−6, respectively. The CR for residents in the contaminated area was significantly higher than that in the reference region (p = 0.03). Additionally, the CR of heavy metals for children was significantly higher than that for adults (p = 0.03), which is consistent with previous studies.50 Please click here to enter your response.However, the result that the CR was higher for adults than children differed from the findings reported by Lanhua et al.,51 who may have artificially expanded the continuous exposure time of children. The exposure time was defined as the mean age of the residents, mainly taking into account the changes in the future residence and living environment of residents with different ages.
The CR contribution rate of different heavy metals for residents in both the contaminated and reference areas showed the order of As > Cd > Pb (Table 4). The CR for As was close to that of Cd, and both were the main source of the CR. As had a low contamination level in vegetables but a high CR for residents; this was mainly due to the high carcinogen slope factor of As determined by the biological toxicity and carcinogenicity.
Table 4.
Carcinogen risk value of vegetable intake (10−4)
| Vegetable | Age | Contaminated area |
Reference area |
||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Pb | Total | As | Cd | Pb | Total | ||
| VW | Children | 0.049 | 0.016 | 0.003 | 0.068 | 0.004 | 0.000 | 0.000 | 0.005 |
| Adults | 0.396 | 0.129 | 0.024 | 0.549 | 0.035 | 0.000 | 0.002 | 0.037 | |
| IF | Children | 0.809 | 0.146 | 0.004 | 0.960 | 0.239 | 0.060 | 0.004 | 0.304 |
| Adults | 6.542 | 1.183 | 0.035 | 7.761 | 1.936 | 0.484 | 0.035 | 2.455 | |
| AL | Children | 0.563 | 0.974 | 0.014 | 1.551 | 0.070 | 0.091 | 0.006 | 0.167 |
| Adults | 4.553 | 7.875 | 0.112 | 12.54 | 0.563 | 0.736 | 0.052 | 1.351 | |
| IL | Children | 0.580 | 0.051 | 0.005 | 0.636 | 0.035 | 0.000 | 0.001 | 0.037 |
| Adults | 4.688 | 0.413 | 0.043 | 5.144 | 0.287 | 0.002 | 0.007 | 0.296 | |
| LM | Children | 0.069 | 0.080 | 0.013 | 0.162 | 0.000 | 0.017 | 0.002 | 0.019 |
| Adults | 0.561 | 0.644 | 0.102 | 1.307 | 0.000 | 0.139 | 0.014 | 0.152 | |
| SL | Children | 0.073 | 0.477 | 0.001 | 0.551 | 0.028 | 0.067 | 0.001 | 0.096 |
| Adults | 0.593 | 3.856 | 0.008 | 4.457 | 0.229 | 0.541 | 0.007 | 0.776 | |
| CS | Children | 0.031 | 0.150 | 0.003 | 0.183 | 0.010 | 0.030 | 0.002 | 0.042 |
| Adults | 0.251 | 1.209 | 0.022 | 1.482 | 0.082 | 0.240 | 0.015 | 0.337 | |
| Mean | Children | 0.311 | 0.271 | 0.006 | 0.587 | 0.055 | 0.038 | 0.002 | 0.096 |
| Adults | 2.512 | 2.187 | 0.049 | 4.749 | 0.447 | 0.306 | 0.019 | 0.772 | |
Cu, copper; As, arsenic; Cd, cadmium; Pb, lead; VW, Vigna unguiculata (Linn.) Walp (cowpea); IF, Ipomoea aquatica Forsk (water spinach); AL, Amaranthus tricolor L (amaranth); IL, Ipomoea batatas Lam (sweet potato leaves); LM, Lycopersicon esculentum Miller (tomato); SL, Solanum melongena Linn (eggplant); CS, Capsicum annuum Linn. var. grossum (L.) Sendt (pepper).
Conclusion
The concentrations of four heavy metals near the mine were significantly higher than those in the reference area, indicating that the soil in the contaminated area is greatly affected by mining and smelting activities. Contamination of soil with Cd was the most serious, with a mean concentration of 4.6 times higher than the maximum permitted level set by the China Ministry of Environmental Protection. The mean concentrations of Cu, Pb, Cd, and As in different parts of vegetables in contaminated soils were lower than the MAL set by China, the FAO, and the EC, but the Cd and Pb concentrations in some vegetables exceeded the MAL. The heavy metal concentrations in vegetables are largely dependent on the ability to accumulate heavy metals. The TF of the four heavy metals showed the order of Cd > Cu > As > Pb. From the viewpoint of the soil concentration and enrichment ability, Cd should be paid more attention. Residents in the reference area seem to be safe. However, residents in the contaminated area are exposed to a serious health risk with an mean of a 77% contribution from As and Cd. The total HI of the four heavy metals in children and adults in the contaminated area ranged from 0.72 to 7.70 and 0.54 to 5.76, respectively. The CR values for adults and children in the contaminated area ranged from 5.49 × 10−5 to 1.25 × 10−3 and from 6.80 × 10−6 to 1.55 × 10−4, respectively. Similarly, both Cd and As were the main source of CR.
From a health risk perspective, the environmental quality standards and food safety standards of some heavy metals, such as As, may need to be improved. The concentration of As meets the MAL in vegetables but appears to largely contribute to the human health risk because of the higher toxicity to cells or organisms. The sources and transformation of heavy metal contamination require further study to provide a basis for health risk management from the perspective of an entire mining area.
Acknowledgments
The authors thank the Research Center for Environment and Health, Zhongnan University of Economics and Law in Wuhan, China. We are also very grateful for the opinions of the peer reviewers, who have helped to improve the quality of our manuscript and inspired us to continue our research in this field of study.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research was supported by the Humanities and Social Sciences Foundation of the Ministry of Education of China (17YJAZH105, 17YJCZH081) and the 58th China Postdoctoral Science Foundation (2015M582316).
References
- 1.Itoh H, Iwasaki M, Sawada N, et al. Dietary cadmium intake and breast cancer risk in Japanese women: A case-control study. Int J Hyg Envir Heal 2014; 217: 70–77. DOI:10.1016/j.ijheh.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 2.Luo CL, Liu CP, Wang Y, et al. Heavy metal contamination in soils and vegetables near an e-waste processing site, south China. J Hazard Mater 2011; 186: 481–490. DOI:10.1016/j.jhazmat.2010.11.024 [DOI] [PubMed] [Google Scholar]
- 3.Li F, Qiu ZZ, Zhang JD, et al. Investigation, pollution mapping and simulative leakage health risk assessment for heavy metals and metalloids in groundwater from a typical brownfield, middle China. Int J Env Res Pub He 2017; 14. DOI:10.3390/ijerph14070768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez-Andreotta MD, Brusseau ML, Artiola JF, Maier RM. A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils. Sci Total Environ 2013; 443: 299–306. DOI:10.1016/j.scitotenv.2012.10.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji K, Kim J, Lee M, et al. Assessment of exposure to heavy metals and health risks among residents near abandoned metal mines in Goseong, Korea. Environ Pollut 2013; 178: 322–328. DOI:10.1016/j.envpol.2013.03.031 [DOI] [PubMed] [Google Scholar]
- 6.Harrison RM. and Chirgawi MB. The assessment of air and soil as contributors of some trace metals to vegetable plants I. Use of a filtered air growth cabinet. Sci Total Environ 1989; 83: 13–34; DOI:10.1016/0048-9697(89)90003-X [DOI] [PubMed] [Google Scholar]
- 7.Li F, Zhang JD, Jiang W, et al. Spatial health risk assessment and hierarchical risk management for mercury in soils from a typical contaminated site, China. Environ Geochem Hlth 2017; 39: 923–934. DOI:10.1007/s10653-016-9864-7 [DOI] [PubMed] [Google Scholar]
- 8.Duruibe JO, Ogwuegbu MOC, Egwurugwu JN. Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2007; 2: 112–118. DOI: 10.1016/j.proenv.2011.09.146 [Google Scholar]
- 9.Hough RL, Breward N, Young SD, et al. Assessing potential risk of heavy metal exposure from consumption of home-produced vegetables by urban populations. Environ Health Persp 2004; 112: 215–221. DOI: 10.1289/ehp.5589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeganeh M, Afyuni M, Khoshgoftarmanesh AH, et al. Health risks of metals in soil, water, and major food crops in Hamedan Province, Iran. Hum Ecol Risk Assess 2012; 18: 547–568. DOI:10.1080/10807039.2012.672886 [Google Scholar]
- 11.Ha H, Olson JR, Bian L, et al. Analysis of heavy metal sources in soil using kriging interpolation on principal components. Environ Sci & Technol 2014; 48: 4999–5007. DOI: 10.1021/es405083f [DOI] [PubMed] [Google Scholar]
- 12.Li F, Huang JH, Zeng GM, et al. Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J Geochem Explor 2013; 132: 75–83. DOI: 10.1016/j.gexplo.2013.05.007 [Google Scholar]
- 13.Huang JH, Li F, Zeng GM, et al. Integrating hierarchical bioavailability and population distribution into potential eco-risk assessment of heavy metals in road dust: A case study in Xiandao District, Changsha city, China. Sci Total Environ 2016; 541: 969–976. DOI:10.1016/j.scitotenv.2015.09.139. [DOI] [PubMed] [Google Scholar]
- 14.Krishna AK, Mohan KR. Distribution, correlation, ecological and health risk assessment of heavy metal contamination in surface soils around an industrial area, Hyderabad, India. Environ Earth Sci 2016; 75. DOI:10.1007/s12665-015-5151-7 [Google Scholar]
- 15.Noli F, Tsamos P. Concentration of heavy metals and trace elements in soils, waters and vegetables and assessment of health risk in the vicinity of a lignite-fired power plant. Sci Total Environ 2016; 563: 377–385. DOI:10.1016/j.scitotenv.2016.04.098 [DOI] [PubMed] [Google Scholar]
- 16.Izhar S, Goel A, Chakraborty A, et al. Annual trends in occurrence of submicron particles in ambient air and health risk posed by particle bound metals. Chemosphere 2016; 146: 582–590. DOI:10.1016/j.chemosphere.2015.12.039 [DOI] [PubMed] [Google Scholar]
- 17.Cao SZ, Duan XL, Zhao XG, et al. Health risks of children's cumulative and aggregative exposure to metals and metalloids in a typical urban environment in China. Chemosphere 2016; 147: 404–411. DOI:10.1016/j [DOI] [PubMed] [Google Scholar]
- 18.Järup L. Hazards of heavy metal contamination. Brit Med Bull 2003; 68: 167. DOI:10.1093/bmb/ldg032 [DOI] [PubMed] [Google Scholar]
- 19.Kabatapendias A. Trace Elements in Soils and Plants, 3rd ed. Crc Press, London: Washington, D.C, 2000, p.114.
- 20.MEPPRC (Ministry of Environmental Protection of the People’s Republic of China), 2006. Report on the state of the environment in China, MEPPRC, Beijing, China.
- 21.Zhuang P, McBride MB, Xia HP, et al. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci Total Environ 2009; 407: 1551–1561. DOI:10.1016/j.scitotenv.2008.10.061 [DOI] [PubMed] [Google Scholar]
- 22.Huang K, Xie S, Bao Z. Environmental geochemistry of heavy metal and trace elements in tailings of Tongliishan Copper and Iron Mine, Daye, Hubei Province. Geochimica 2008; 37: 213–222. http://en.cnki.com.cn/Article_en/CJFDTOTAL-DQHX200803005.htm [Google Scholar]
- 23.Yaping W, Zhengyu B, Suming W. Advances in environmental effects of mine solid waste and environmental effects of the tailings in Daye. Bulletin of mineralogy, petrol and geochem 1998; 17: 97–101. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=kydh802.005&dbname=CJFD&dbcode=CJFQ [Google Scholar]
- 24.Lihua X, Jun Y, Zhenzhen Q, et al. Survey of occupation exposure to PM10 and health risk assessment for tunnel workers. Environ Sci 2015; 8: 2768–74. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hjkz201508009&dbname=CJFD&dbcode=CJFQ [Google Scholar]
- 25.Ministry of Environmental Protection of the People’s Republic of China, HJ/166-2004. Technical Specification for Soil Environmental Monitoring; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2004.
- 26.Evans PS. Plant root distribution and water use patterns of some pasture and crop species. New Zealand Journal of Agricultural Research 1978; 21: 261–265. DOI:10.1080/00288233.1978.10427408 [Google Scholar]
- 27.United States Environmental Protection Agency, Method 3050B. Acid digestion of sediments, sludges, and soils; United States Environmental Protection Agency: United States, 1996.
- 28.Yunnan Bureau of quality and technical supervision, DB53/T 288-2009. Determination of Lead in food, Arsenic, Iron, Calcium, Zinc, Aluminium, Sodium, Magnesium, Boron, Manganese, Copper, Barium, Strontium, Titanium, Tin, Cadmium, Chromium and Vanadium content by inductively coupled plasma atomic emission spectrometry (ICP-AES) method; Yunnan Bureau of quality and technical supervision: Yunnan, China, 2009. [Google Scholar]
- 29.United States Environmental Protection Agency. Exposure Factors Handbook: 2011 Edition. Washington, DC, USA: United States Environmental Protection Agency, 2011. [Google Scholar]
- 30.Hu WY, Huang B, Tian K, et al. Heavy metals in intensive greenhouse vegetable production systems along Yellow Sea of China: Levels, transfer and health risk. Chemosphere 2017; 167: 82–90. DOI:10.1016/j.chemosphere.2016.09.122 [DOI] [PubMed] [Google Scholar]
- 31.USEPA. Risk Assessment Guidance for Superfund Volume 1. Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); EPA/540/R/99/005 Office of Superfund Remediation and Technology Innovation; Environmental Protection Agency: Washington, DC, USA, 2004.
- 32.USEPA. Risk Assessment Guidance for Superfund Volume 1. Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); EPA/540/R/99/005 Office of Superfund Remediation and Technology Innovation; Environmental Protection Agency: Washington, DC, USA, 2004.
- 33.Fengying Zhai, China dietary and nutrition survey and tracking research (in Chinese), Science Press, Beijing, China: 2008, pp.1–90. ISBN: 9787030210951. http://cpfd.cnki.com.cn/Article/CPFDTOTAL-EGYN200507001004.htm
- 34.USEPA. Integrated Risk Information System [M] 2000.
- 35.Budroe JD, Fowles JR. Technical Supper Document for Describing Available Cancer Potency Factors [M]. Califonia Environmental Protention Agency Office of Environmental Health Hazard Assessment Air Toxicology and Epidemiology Section 2002.
- 36.Environmental Protecting Administration of China, EPAC, GB 15618-1995. Environment Quality Standard for Soils; Environmental Protecting Administration of China, EPAC, Beijing, China, 1995.
- 37.Wang D, Zhu Y, Zeng L, et al. Study on background value of soil heavy metals in Daye. J Huazhong Normal Univers 1982; 1: 96–107. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=hzsz198201012&dbname=CJFD&dbcode=CJFQ [Google Scholar]
- 38.Muchuweti A, Birkett JW, Chinyanga E, et al. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: Implications for human health. Agr Ecosyst Environ 2006; 112: 41–8. DOI:10.1016/j.agee.2005.04.028 [Google Scholar]
- 39.Yang Y, Zhang FS, Li HF, et al. Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J Environ Manage 2009; 90: 1117–1122. DOI:10.1016/j.jenvman.2008.05.004 [DOI] [PubMed] [Google Scholar]
- 40.Yana C, Kelin L, Jian Z, et al. Analysis of heavy metal pollution in soil-vegetables at mining area in Hunan. Chinese Agricult Sci bulletin 2012; 28: 226–232. http://en.cnki.com.cn/Article_en/CJFDTOTAL-ZNTB201235047.htm [Google Scholar]
- 41.Tani FH, Barrington S. Zinc and copper uptake by plants under two transpiration ratios Part I. Wheat (Triticumaestivum L.). Environ Pollut 2005; 138: 538–547. DOI:10.1016/j.envpol.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 42.FAO/WHO, 2011. FAO/WHO. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods, Fifth Session, pp 64–89.
- 43.European Union. Commission regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union L 2006; 364, 5–24. [Google Scholar]
- 44.Chinese Ministry of Health, CMH, GB 2762-2012. Maximum Levels of Contaminants in Foods. Chinese Ministry of Health, CMH: Beijing, China, 2012.
- 45.Liu HY, Probst A, Liao BH. Metal contamination of soils and crops affected by the Chenzhou lead/zinc mine spill (Hunan, China). Sci Total Environ 2005; 339: 153–166. DOI:10.1016/j.scitotenv.2004.07.030 [DOI] [PubMed] [Google Scholar]
- 46.Cai LM, Xu ZC, Qi JY, et al. Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 2015; 127: 127–135. DOI:10.1016/j.chemosphere.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 47.Choudhury TR. Heavy metals contamination in vegetables and its growing soil. Int J Environ An Ch 2015; 2: 6. DOI:/10.4172/jreac.1000142 [Google Scholar]
- 48.Xu L, Lu AX, Wang JH, et al. Accumulation status, sources and phytoavailability of metals in greenhouse vegetable production systems in Beijing, China. Ecotox Environ Safe 2015; 122: 214–220. DOI:10.1016/j.ecoenv.2015.07.025 [DOI] [PubMed] [Google Scholar]
- 49.Lim HS, Lee JS, Chon HT, et al. Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au-Ag mine in Korea. J Geochem Explor 2008; 96: 223–230. DOI:10.1016/j.gexplo.2007.04.008 [Google Scholar]
- 50.Sun Q, Ying C, Deng J, et al. Heavy metal pollution characteristics and health risk assessment of soil-vegetables in Daye mining area. Chemic Environ 2013; 32: 671–677. http://en.cnki.com.cn/Article_en/CJFDTOTAL-HJHX201304023.htm [Google Scholar]
- 51.Lanhua W, Mingming L, Ying Z, et al. Characteristics and health risk assessment of heavy metal pollution in soil of a vegetable base in North China. J Earth 2014; 35: 191–196. http://en.cnki.com.cn/Article_en/CJFDTOTAL-DQXB201402012.htm [Google Scholar]