Abstract
Background
Health state utility values (HSUVs) are an important input to economic evaluations and the choice of HSUV can affect the estimate of relative cost-effectiveness between interventions. This systematic review identified utility scores for patients with metastatic non-small cell lung cancer (mNSCLC), as well as disutilities or utility decrements relevant to the experience of patients with mNSCLC, by treatment line and health state.
Methods
The MEDLINE®, Embase and Cochrane Library databases were systematically searched (September 2016) for publications describing HSUVs in mNSCLC in any treatment line. The EQ-5D website, the School of Health and Related Research Health Utilities Database (ScHARRHUD) and major pharmacoeconomic and clinical conferences in 2015–2016 were also queried. Studies in adults with previously treated mNSCLC were selected for further analysis. The information extracted included study design, description of treatment and health state, respondent details, instrument and tariff, HSUV or (dis) utility decrement estimates, quality of study, and appropriateness for use in economic evaluations.
Results
Of 1883 references identified, 36 publications of 34 studies were included: 19 reported EQ-5D scores; eight reported HSUVs from valuations of vignettes made by members of the public using standard gamble (SG) or time trade-off (TTO); two reported SG or TTO directly elicited from patients; two reported EQ-5D visual analogue scale scores only; one reported Assessment of Quality of Life instrument scores; one reported HSUVs for caregivers to patients with mNSCLC using the 12-item Short-Form Health Survey; and one estimated HSUVs based on expert opinion. The range of HSUVs identified for comparable health states showed how differences in study type, tariff, health state and the measures used can drive variation in HSUV estimates.
Conclusions
This systematic review provides a set of published HSUVs that are relevant to the experience of adult patients previously treated for mNSCLC. Our review begins to address the challenge of identifying reliable estimates of utility values in mNSCLC that are suitable for use in economic evaluations, and also highlights how varying estimates result from differences in methodology.
Electronic supplementary material
The online version of this article (10.1186/s12955-018-0994-8) contains supplementary material, which is available to authorized users.
Keywords: Health state utility values (HSUVs), Health-related quality of life (HRQoL), Metastatic non-small cell lung cancer (mNSCLC), Multi-attribute utility instruments (MAUIs), Standard gamble (SG), Time trade-off (TTO), Systematic literature review
Background
Non-small cell lung cancer (NSCLC) is the most common form of lung cancer, occurring in 85–90% of lung cancer cases [1], and includes adenocarcinoma (40% of all lung cancers), squamous cell carcinoma (25–30%) and large cell carcinoma (10–15%) [2]. NSCLC is staged according to the American Joint Committee on Cancer/Union for International Cancer Control system [3], and measurement of lesions follows the Response Evaluation Criteria in Solid Tumors (RECIST) [4]. Approximately 40% of patients will have metastatic NSCLC (mNSCLC) at diagnosis [5], which includes cancers found in the lung and in the lymph nodes in the middle of the chest (defined as stage IIIA and IIIB; no distant metastasis), and cancers that have spread to both lungs or to another part of the body (defined as stage IV; distant metastasis) [6, 7].
Treatment is recommended according to the stage of mNSCLC, but treatment options are limited in the later stages of disease [7, 8]. Five-year survival rates are considerably lower in later than in earlier stages of NSCLC (stage IA, 45%; stage IIIA, 14%; stage IIIB, 5%; stage IV, 1%) [9]. Moreover, symptoms such as coughing and wheezing, chest pain, hoarseness and weight loss can severely reduce functional independence in patients with mNSCLC [10, 11]. Patient-reported health-related quality of life (HRQoL) provides an overall evaluation of health, well-being and daily functioning, and is impaired in patients with mNSCLC owing both to the disease and to treatment sequelae. Maintenance or improvement of HRQoL is an important treatment goal [12].
HRQoL can be expressed as a health state utility value (HSUV) ranging from 0 (death) to 1 (full health). If the health state is considered to be worse than death, health states can be valued at less than 0. Utility values are key drivers in cost-effectiveness analyses because estimates of quality-adjusted life-years (QALYs) are obtained by multiplying HSUVs for each health state by the time spent in that health state. Estimates of cost per QALY are highly sensitive to the choice of HSUV. It is therefore important to identify specifically those HSUVs that have been derived using methods acceptable to health technology assessment (HTA) authorities [13].
HSUVs can be derived using a range of instruments and techniques [14, 15]. In brief, instruments include: generic preference-based measures such as the EQ-5D-3 L [16] or EQ-5D-5 L [17], Health Utilities Index (HUI) [18], 6-dimension Short-Form Health Survey (SF-6D) [19], Assessment of Quality of Life instrument (AQoL) [20], 15-dimensional HRQoL measure [21], Quality of Well-Being scale [22], and multi-attribute utility instrument; as well as directly elicited standard gamble (SG), time trade-off (TTO) and visual analogue scale (VAS, e.g. EuroQoL VAS [EQ-VAS]). Mapping algorithms may also be used to convert values obtained from a condition-specific questionnaire to a generic preference-based measure; or to convert data from the 12- or 36-item Short-Form Health Survey (SF-12 or SF-36) to SF-6D [23]. Techniques may vary in terms of whose health is being measured (a patient’s or a caregiver’s), who responds to the questionnaire or, if using vignettes, who considers the health-state description (the patient regarding their own health, a patient with a different disease, the patient’s closest caregiver, another caregiver, a physician or another healthcare provider). For preference-based measures, variation can stem from who values the health state (e.g. UK general population sample) and which choice-based method is used in this valuation (SG or TTO).
HTA bodies including the UK National Institute for Health and Care Excellence (NICE) [24, 25], the Scottish Medicines Consortium (SMC) [26], the Canadian Agency for Drugs and Technologies in Health (CADTH) [27], the French Haute Autorité de Santé (HAS) [28] and the Australian Pharmaceutical Benefits Advisory Committee (PBAC) [29] have stated preferences for HSUV methodology. Across these agencies, there is a preference for HSUVs estimated using generic preference-based measures. NICE has a strong preference for EQ-5D, as this reduces variability induced when different instruments are used between different disease areas. Agencies also strongly prefer patients to be the respondents, as patients can best describe their own health state. Finally, valuation estimated using a country-specific general-population tariff via a choice-based elicitation technique such as SG or TTO is preferred, as this represents societal preferences.
This systematic review had three main aims: first, to identify HSUVs for adults with previously treated mNSCLC, by treatment line and health state, and to evaluate the relevance of each health state to patients, for example, line of treatment, adverse events (AEs), response status and prognostic factors; second, to identify relevant disutilities or utility decrements associated with adverse events (irrespective of line of treatment or health state). Finally, the suitability of the HSUVs according to HTA reference case was explored and the quality of the HSUVs assessed.
Methods
Study design and search strategy
A systematic review of HSUVs in mNSCLC was undertaken to identify HSUV studies in any treatment line. Studies, published either as full papers or as conference abstracts, in patients previously treated for mNSCLC were selected for further analysis. The following databases were searched: Embase (1974 to 7 September 2016); MEDLINE® (1966 to 7 September 2016); MEDLINE In-Process and e-publications ahead of print (database inception to 7 September 2016); and the Cochrane Library (including the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, the Cochrane Central Register of Controlled Trials, the National Health Service Economic Evaluation Database and the HTA database; 1968 to 7 September 2016).
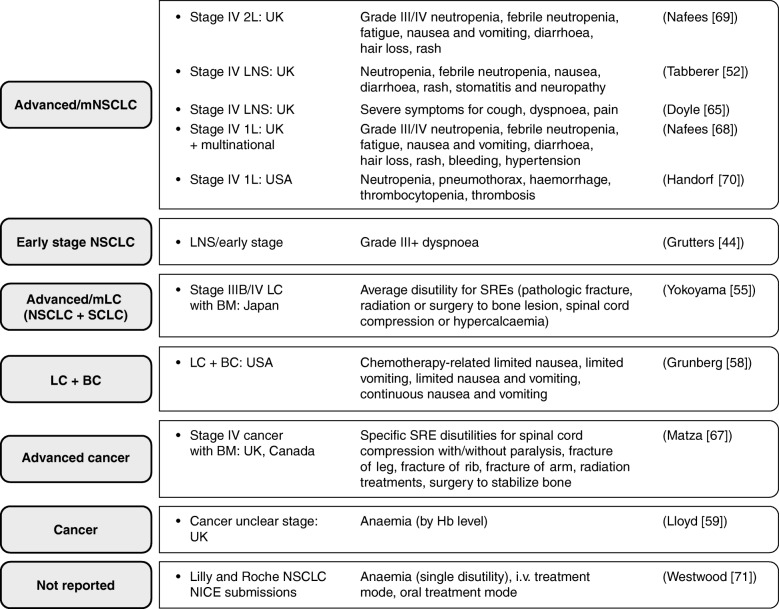
Search strings are summarized in Additional file 1: Table S1, and were constructed not only to find utilities in mNSCLC (using a wide range of NSCLC and mNSCLC terms combined with the HSUV filter adapted from Arber et al. 2015 [30]) but also to identify all relevant disutilities or utility decrements associated with AEs/comorbidities. To ensure that estimates would be available from previously treated mNSCLC populations for all AEs or comorbidity health states relevant to the experience of such patients, the strings were designed to search for disutilities or decrements from a broader group of populations, as follows: from lung cancer; for progressive disease disutilities from advanced/metastatic cancer; for disutilities associated with the most common sites of metastasis from the lung (bone, respiratory system, nervous system, adrenal gland and liver) from advanced cancer; for disutilities associated with AEs or toxicities of cancer therapy; and disutilities associated with specific grade 3–4 AEs known to occur with cancer treatments from advanced cancer populations (pneumonia, pneumonitis, increased aspartate aminotransferase, febrile neutropenia, neutropenia, infection, sepsis, fatigue, lethargy, nausea, vomiting, ulcers, stomatitis, gastrointestinal disturbance, diarrhoea, visual disturbance, hearing loss, hair loss, psychological/self-esteem changes, rash, anaemia, bleeding and hypertension). From the identified disutilities/decrements for each AE/co-morbidity health state, those from the most relevant population available could be selected following an order of decreasing population specificity from first-line mNSCLC to NSCLC, lung cancer and advanced/metastatic cancer (Fig. 1).
Fig. 1.
Studies reporting adverse event health state (dis) utilities by patient population and country. Abbreviations: 1L first line, 2L second line, BC breast cancer, BM bone metastasis, Hb haemoglobin, i.v. intravenous, LC lung cancer, LNS line of treatment not specified, mLC metastatic lung cancer, mNSCLC metastatic non-small cell lung cancer, NICE National Institute for Health and Care Excellence, NSCLC non-small cell lung cancer, SCLC small cell lung cancer, SRE skeletal-related event
Using the term “NSCLC” or “non-small cell lung cancer”, manual searching of the EQ-5D website, of the School of Health and Related Research Health Utilities Database (ScHARRHUD) and of major pharmacoeconomic and clinical conferences in 2015–2016 was conducted on 3 and 5 December 2016. Conferences included: the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) International Meetings and European Congresses; the HTA International Annual Meetings (HTAi); the Society for Medical Decision Making (SMDM) North American Meetings and European Conferences; the American Society of Clinical Oncology (ASCO) Meetings; and the European Society for Medical Oncology (ESMO) Congresses. Bibliographic reference lists of relevant systematic reviews from 2010 onwards were searched and of relevant cost-utility analyses, and HTA reports from various bodies identified in a parallel economic systematic review, including: NICE; SMC; All Wales Medicines Strategy Group (AWMSG); PBAC; CADTH; Institut National d’Excellence en Santé et en Services Sociaux; pan-Canadian Oncology Drug Review (pCODR); and HAS.
The PICOS (patient, intervention, comparator, outcome, study) statements for study inclusion and exclusion criteria are summarized in Table 1. Although, second- and later-line data were of primary interest, studies that reported utilities for patients with mNSCLC who were either treatment-naïve or in receipt of maintenance first-line treatment were included for reference at the first screening but data were not extracted. These studies are listed in Additional file 2: Table S2.
Table 1.
Inclusion criteria
| Characteristic | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Adult patients (aged ≥16 years) Locally advanced NSCLC or mNSCLC, second/subsequent line Locally advanced or metastatic NSCLC, line unspecified |
Population not of interest, e.g. • in vitro data • animal data • mixed adult/child population or child population • mixed disease populations without mNSCLC data reported separately • not disease of interest • 1 L mNSCLC data (treatment-naïve or maintenance first-line treatment) were excluded but taggeda |
| Interventions/comparators | Not relevant for QoL SR selection. Intervention-specific utility data were noted as such during data extraction | N/A |
| Outcomes | For mNSCLC patients: • individual (patient or caregiver) derived mean or median health state utilities from indirect generic HRQoL measure (EQ-5D (-3 L and -5 L), SF-6D, HUI2, HUI3, AQoL, QWB, 15D, MAUI) or direct valuation by TTO, SG or EQ-VAS • SF-36 or SF-12 • general public valuations of vignettes using TTO or SG For NSCLC or wider population: • disutilities or decrements for AEsb or progressive disease |
No outcome of interest: • expert or healthcare provider (doctor, nurse) valuations of utilities • utilities not relating to a specific health state |
| Study design | RCTs, non-RCTs, observational data | Study design not of interest: • case reports, n = 1 before-and-after studies • PK/PD study only • (Non-systematic) reviews • SRs/NMAsc |
| Date limits | Unlimited | – |
| Child citation | Citation linked to another paper but with unique data | Child citation or sub-study with no unique data, determined at first or second pass |
| Duplicate citation | Duplicate/copy | |
| Publication type | Publication type not of interest e.g. editorials, commentaries, letters, notes, press articles, unless relevant data has been published in a letter, for example, that does not appear elsewhere in the literature Confidential reports where unable to use report, or Hayes Inc. reports requiring purchase |
|
| Language | English or French Any foreign language paper with an English abstract were included if sufficient information is present in the English abstract to ensure the eligibility criteria are met |
Full text in language other than English or French with no English abstract or no abstract; or insufficient information in English language abstract of foreign language full paper to assess eligibility |
aTo enable listing in the report
bDisutilities may be included for AEs, inconvenience of treatment or progressive health states from diseases outside NSCLC (preferably from lung cancer or from advanced/metastatic cancer) where no such data are available from patients with NSCLC
cSRs were kept in until the second pass, where the full paper’s included studies were examined, after which the SR itself was excluded
Abbreviations: 1 L first line, 3 L 3-level, 5 L 5-level, 15D 15-dimensional health-related quality of life measure, AE adverse event, AQoL Assessment of Quality of Life instrument, EQ-VAS EuroQoL visual analogue scale, HRQoL health-related quality of life, HUI2/3 Health Utilities Index Mark 2/3, MAUI multi-attribute utility instrument, mNSCLC metastatic non-small cell lung cancer, N/A not available, NMA network meta-analysis, NSCLC non-small cell lung cancer, PD pharmacodynamic, PK pharmacokinetic, QoL quality of life, QWB Quality of Well-Being scale, RCT randomized controlled trial, SF-6D 6-dimension Short-Form Health Survey, SF-12/36 12/36-item Short-Form Health Survey, SG standard gamble, SR systematic review, TTO time trade-off, VAS visual analogue scale
Mapping from condition-specific to preference-based studies was not sought because it was anticipated that sufficient published utility and EQ-5D data would be available to populate the health states of an economic model, and because results based on mapping algorithms sit lower in the acceptance hierarchy used by some HTA authorities (Additional file 3: Figure S1). We have acknowledged NICE’s stated preference for EQ-5D-3 L data over EQ-5D-5 L (Additional file 3: Figure S1) and provide detailed information of the instrument used for generating data for each identified study in Table 2 [31].
Table 2.
Identified utility studies by line of treatment
| Author, year, country | Line of treatment | Health state | Instrument | Treatment |
|---|---|---|---|---|
| First linea | ||||
| Handorf 2012 USA [70] | 1 L | Stage IV adenocarcinoma SDis, PD, SDis+AEs (neutropenia, pneumothorax, haemorrhage, thrombocytopenia, thrombosis) | Expert opinion estimates,b published sources | NTS |
| Nafees 2016 Multinational and UK [68] | 1 L | Metastatic NSCLC common grade III/IV toxicities (neutropenia, febrile neutropenia, fatigue, nausea and vomiting, diarrhea, hair loss, rash), bleeding, hypertension | TTO (general public) | NTS |
| ≥ First linec | ||||
| Chevalier 2013 France and nine other countries [38] | 1 L, 2 L, 3/4 L and BSC | Advanced/metastatic NSCLC 1 L, 2 L, 3/4 L PF and PD | EQ-5D | NTS |
| Chouaid 2013 Multinational [39] | 1 L 55.1% 2 L 24.7% 3/4 L 17.9% BSC 2.3% |
Advanced/metastatic NSCLC 1 L, 2 L, 3/4 L, BSC and mixed line PF and PD | EQ-5D, EQ-VAS | NTS |
| Iyer 2013 France, Germany [46] | 1 L 52% 2 LL 48% |
Advanced/metastatic NSCLC | EQ-5D | NTS |
| Second line | ||||
| Blackhall 2014 Multinational [41] | 2 L after progression on platinum-based 1 L therapy | Locally advanced/metastatic ALK+ NSCLC BL and treatment-specific utilities (not PSS) | EQ-5D EQ-VAS | CRZ PEM DOC |
| Huang 2016 Worldwide [45] | 2 L after platinum-based therapy | Advanced PD-L1+ NSCLC NTS PF, PD NTS time to death |
EQ-5D | PEMB DOC |
| Langley 2013 UK, Australia [48] | 2 Ld | Treatment-specific stage IV NSCLC with BM at BL and after certain time points on treatment | EQ-5D | OSC WBRT + OSC |
| Nafees 2008 UK [69] | 2 L | Metastatic NSCLC PD, RES, SDis, common grade III/IV toxicities (neutropenia, febrile neutropenia, fatigue, nausea and vomiting, diarrhea, hair loss, rash) | SG (general public) | NTS |
| Novello 2015 Multinational [49] | 2 L | Stage III/IV recurrent NSCLC (SQ and NSQ) treatment-specific at BL and certain time points on treatment (≤ 30 weeks) | EQ-5D, EQ-VAS | NIN + DOC PLA + DOC |
| Reck 2015 Multinational [50] | 2 L | Advanced SQ NSCLC treatment-specific at BL reported. Collected also for up to 1 year but values NR in abstract | EQ-5D, EQ-VAS | NIVO DOC |
| Rudell 2016 USA, Canada, Hong Kong, Italy, Japan, Republic of Korea, Spain, Taiwan [57] | 2 L | Advanced EGFR+ NSCLC, treatment-specific at BL and 36 weeks on OSI | EQ-VAS | OSI |
| Schuette 2012 Germany, Austria [51] | 2 L | Stage III/IV NSCLC treatment-specific at BL, 6 weeks (second cycle) and sixth cycle | EQ-5D EQ-VAS |
PEM |
| Vargas 2009 Mexico [72] | 2 L after previous CHEMO | NSCLC, stage NR (assumed advanced), treatment-specific not PSS | Global QoL index | ERL Taxanes |
| ≥ Second line | ||||
| Chen 2010 UK/multinational [64] | 2 L, 3 L and BSC | Stage IIIb/IV EGFR+ NSCLC treatment-specific (not PSS) On/after DOC 2 L On/after PEM 2 L On ERL 3 L BSC |
SG (general public) | DOC PEM ERL BSC |
| Griebsch 2014 Multinational [37] | 2LLe and treatment-naïve | Stage IIIb with pleural effusion or stage IV NSCLC adenocarcinoma treatment-specific and NTS effect of progression | EQ-5D, EQ-VAS | AFA BSC CIS/PEM |
| Hirsh 2013 Multinational [40] | 2LLf | Stage IIIb/IV NSCLC BL and treatment-specific on oral AFA 50 mg q.d. + BSC or PLA + BSC | EQ-5D | AFA + BSC PLA + BSC |
| Stewart 2015 Canada [56] | Targeted therapy 84% 3LL 25% RCT 22% |
Metastatic EGFR+ NSCLC, all patients not PSS PR/SDis EGFR TKI RES CHEMO RES GEF RES ERL RES OSI PD EGFR TKI |
EQ-5D-3 L | GEF ERL OSI |
| Schwartzberg 2015 USA, Canada [60] | 2 LL | Squamous and non-squamous stage IIIb/IV NSCLC treatment-specific weeks 6–30 Treatment-specific PR, SDis and PD weeks 6–30 |
EQ-VAS | NIVO |
| Treatment line not specified | ||||
| Bradbury 2008 Canada [42] | Unclear | Advanced NSCLC Treatment-specific (not PSS) | EQ-5D | ERL BSC |
| Chang 2016 South Korea [63] | NR | Advanced NSCLC from > 360 days before death to < 30 days before death (not PSS) | TTO (general public) | NTS |
| Dansk 2016 UK [43] | NR | Synthesized advanced NSCLC PF, PD used in NICE HTAs Trial-based PF, PD Non-trial based PF, PD |
EQ-5D | NTS |
| Doyle 2008 UK [65] | NR | Metastatic NSCLC SDis, RES, severe symptoms (cough, dyspnoea, pain) | SG (general public) | NTS |
| Grunberg 2009 USA [58] | NR | Mixed cancer population chemotherapy-related nausea, vomiting, and nausea and vomiting, of different severities | SG (patient) | CHEMO |
| Grutters 2010 Netherlands [44] | NR | NSCLC with grade 3+ dyspnoea | EQ-5D | NTS |
| Jang 2010 Canada [47] | NR | Stage IV NSCLC and locally advanced NSCLC | EQ-5D | NTS |
| Linnet 2015 Denmark [62] | Unclear | Metastatic NSCLC second and third CHEMO cycles on oral VINO Patient and caregiver utilities reported |
SF-12 | VINO |
| Lloyd 2005 UK [66] | NR | Stage IV NSCLC RES, SDis i.v. treatment, SDis oral treatment, PD, end of life | SG (general public) | NTS |
| Lloyd 2008 [59] | Previous CHEMO | Anaemia by haemoglobin level | General public SG, patient TTO | NTS |
| Manser 2006 Australia [61] | NR | Stage IV NSCLC | AQoL | NTS |
| Matza 2014 UK and Canada [67] | NR | Stage IV cancer with BMs and different types of SRE (spinal cord compression with/without paralysis, fracture of leg, fracture of rib, fracture of arm), radiation treatment (2 weeks, 5 appointments/week), radiation treatment (2 appointments), surgery to stabilize bone | TTO (general public) | NTS |
| Tabberer 2006 UK [52] | NR | Advanced NSCLC RES, SDis, SDis oral treatment, SDis i.v. treatment, PD, near death, AEs (neutropenia, febrile neutropenia, nausea, diarrhoea, rash, stomatitis, neuropathy) | EQ-5D (general public) | NTS |
| Trippoli 2001 Italy [53] | NR | Metastatic NSCLC | EQ-5D, EQ-VAS | NTS |
| Westwood 2014 [71] | NR for other disutilities | Advanced NSCLC Disutility for anaemia and for i.v./oral treatment mode |
SG NR for other disutilities | NTS ERL i.v. tx |
| Yang 2014 Taiwan [54] | NR | NSCLC operable (I–IIIA) and NSCLC inoperable (IIIB/IV) | EQ-5D | NTS |
| Yokoyama 2013 Japan [55] | NR | Stage IIIB/IV mixed NSCLC/SCLC with bone metastasis and SRE (pathologic fracture, radiation or surgery to bone lesion, spinal cord compression or hypercalcaemia) | EQ-5D | NTS |
aStudies were retained, despite reporting first-line treatment only, because they reported progressive disease utility estimates similar to those seen in a second-line population, or reported AE disutility estimates from populations broader than mNSCLC
bAlthough the utilities were based on expert opinion, these were retained, as they provide disutility estimates for the adverse events pneumothorax, thrombocytopenia and thrombosis, not available elsewhere
cStudies reported data on first-line treatment and subsequent treatment lines
dPrevious treatment with systemic CHEMO or EGFR inhibitors allowed
eLux-Lung 1 trial data were in patients progressed on 1–2 lines of treatment, one of which was platinum based (could include adjuvant setting treatment line), and had PD after at least 12 wks of ERL or GEF. Lux-Lung 3 trial data were in treatment-naïve patients, so not 2 L.
fProgressed on 1–2 lines of treatment, one of which was platinum based, and had PD after at least 12 wks of ERL or GEF
Abbreviations: 1 L first line, 2 L second line, 2 LL second and subsequent line, 3LL third and subsequent line, 3/4 L third and fourth line, AE adverse event, AFA afatinib, AQoL Assessment of Quality of Life instrument, BL baseline, BM bone metastasis, BSC best supportive care, CHEMO chemotherapy, CIS cisplatin, CRZ crizotinib, DOC docetaxel, EGFR epidermal growth factor receptor, EQ-VAS EuroQol visual analogue scale, ERL erlotinib, GEF gefitinib, GEM gemcitabine, i.v. intravenous, NIN nintedanib, NIVO nivolumab, NR not reported, NSCLC non-small cell lung cancer, NSQ non-squamous, NTS not treatment-specific, OSC optimal standard care, OSI osimertinib, PD progressive disease, PEM pemetrexed, PEMB pembrolizumab, PF progression-free, PLA placebo, PR partial response, PSS progression-status-specific, q.d. once daily, QoL quality of life, RCT randomized controlled trial, RES response, SCLC small cell lung cancer, SDis stable disease, SF-12 12-item Short–Form Health Survey, SG standard gamble, SRE skeletal-related event, TKI tyrosine kinase inhibitor, TTO time trade-off, VAS visual analogue scale, VINO vinorelbine, WBRT whole-brain radiotherapy
Study selection
The screening process complied with the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [32]. Publications were de-duplicated using EndNote (Clarivate Analytics, Philadelphia, PA, USA) and using Rayyan (Qatar Computing Research Institute, Doha, Qatar) [33], an internet-based reference management system endorsed as suitable for systematic review screening by the European Network for HTA [34]. Abstracts and titles of papers were screened by one reviewer, and a 50% sample check conducted by a second reviewer; exclusion criteria are summarized in Table 1. The full texts of papers potentially meeting the selection criteria were screened by one reviewer, and a 50% sample check was conducted by a second reviewer. Discrepancies were discussed between reviewers, and any unresolved disputes were referred to a third reviewer.
Data extraction
Data were collected using a piloted data-extraction sheet. Extraction was conducted by one reviewer, and priority data elements were quality checked by a second reviewer. The information extracted included study design, whether the selection criteria yielded a population that matched the target population (i.e. previously treated adult patients with mNSCLC), health state description, instrument type, instrument scale, HSUV or (dis) utility or decrement estimates and measure of variability (median with interquartile range or mean with standard error, standard deviation or 95% confidence interval), derivation methods and if the data presented were appropriate for use in HTA submissions to NICE, SMC, CADTH, HAS and PBAC.
Quality and relevance assessment
The appropriateness of utilities reported for use in economic evaluations was determined by whether data met the requirements of the HTA body reference case; and the quality of utility estimates (based on sample size, response to the questionnaire, loss to follow-up, handling of missing data, and reporting of point and variance estimates, as discussed in NICE Decision Support Unit Technical Support Document 11 and its related publication [25, 35]; Additional file 4: Table S3). Any recommendation for, or rationale against, the use of specific utilities in a cost–utility analysis model in previously treated patients with mNSCLC was also taken into consideration in line with preliminary guidance from the ISPOR Health State Utility Good Practices Task Force [36].
Results
Search yields
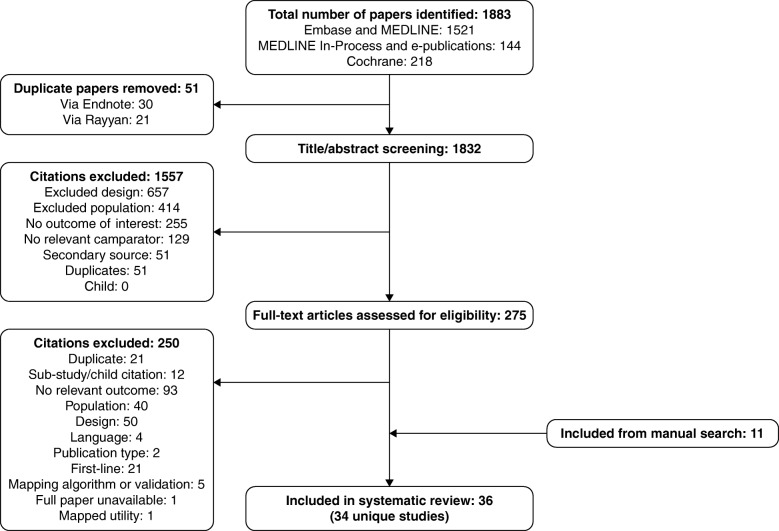
Electronic database searches identified 1883 citations (1521 from MEDLINE/Embase, 144 from MEDLINE In-Process/e-publications and 218 from the Cochrane Library databases). After de-duplication (51 citations: 30 via Endnote and 21 via Rayyan) and title/abstract screening (1557 exclusions), 275 full-text papers were reviewed. Of these, 250 were excluded (21 of which were tagged as reporting first-line treatment; Additional file 2: Table S2), yielding 25 citations that were included from electronic sources. Manual searching identified 11 citations. In total, 36 articles were included, reporting 34 studies (Table 2). Two articles [37, 38] were linked to other publications [39, 40], and were retained because they provided additional information. The study selection is summarized in a PRISMA flow chart in Fig. 2.
Fig. 2.
PRISMA flow chart for study selection. Abbreviation: PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Description of studies identified
Among the 36 articles (34 studies) identified, 19 reported EQ-5D scores [37–55] (three studies further specified the instrument as EQ-5D-3 L [39, 41, 56] and two as EQ-5D-5 L [44, 57]; Table 2), two reported SG or TTO directly elicited from patients [58, 59], two reported EQ-VAS scores only [57, 60], and one reported AQoL scores [61] (Table 2). Moreover, one study reported SF-12 scores for caregivers to patients with mNSCLC [62], eight reported HSUVs from valuations of vignettes made by members of the public using SG or TTO [59, 63–69] (one of which reported both general public-elicited SG and patient-elicited TTO) [59], and one reported disutility estimates based on expert opinion for pneumothorax, thrombocytopenia and thrombosis, adverse event health states for which disutilities were not available from other HSUV derivation methods [70]. A further two articles reported HSUVs but were unclear about how these were derived; one reported disutilities used in previous NICE submissions, for anaemia and for oral and intravenous treatment modes [71], and one reported a “global quality of life index” for second-line NSCLC [72].
Among the dataset, two studies were retained despite reporting first-line treatment only, because they reported AE disutility estimates from populations broader than mNSCLC [68, 70]; three further studies that reported first-line data also reported on subsequent treatment lines [38, 39, 46]. Eleven studies focused exclusively on HSUVs associated with second-line treatment [41, 45, 48–51, 57, 59, 69, 71, 72], and five reported HSUVs in second-line and subsequent treatment [37, 40, 56, 60, 73]. Line of treatment was unspecified in 15 studies [42–44, 47, 52–55, 58, 61–63, 65–67].
Relevant HSUVs by line of treatment
Utilities were reported for a range of health state types: treatment-specific or not, RECIST response-based or not, time-on-treatment, time-till-death, or a combination of these. Details of HSUV estimates by treatment line are given in Table 3. Among patients receiving second-line or subsequent treatment for advanced NSCLC or mNSCLC, mean HSUV estimates based on EQ-5D for stable/progression-free disease and for patients at baseline or pre-treatment were in the range 0.66–0.76 [38, 39, 41, 45, 49, 50]; in the same group, mean values for patients with progressive disease were generally lower (0.55–0.69) [38, 39, 45]. Among patients on treatment at this stage of disease and treatment line, the range of mean HSUVs based on EQ-5D was broad (0.53–0.82) [40, 41, 46, 51, 56], the highest value being associated with treatment with tyrosine kinase inhibitors [41, 56]. A similar range of HSUV values was seen among patients being treated for advanced NSCLC or mNSCLC when the treatment line was unspecified (0.53–0.77) [42, 47, 52, 53]. Only three papers specified using EQ-5D-3 L [39, 41, 56] and only two EQ-5D-5 L [44, 57].
Table 3.
Health state utility values by treatment line, health state and instrument
| Study | Health state | Utility valuea | Instrument | Tariff | Respondent details | HTA suitability |
|---|---|---|---|---|---|---|
| 1st line | ||||||
| Nafees 2016 [68] Metastatic NSCLCb |
PD vs BL state | 0.095 | TTO | N/A | Patients (but not NSCLC patients) from the general public in UK, Australia, France, China, S. Korea, Taiwan | No |
| RES no side effects vs BL | 0.773 | |||||
| SDis no side effects vs BL | 0.460 | |||||
| Chevalier 2013c [38] Advanced/metastatic NSCLC |
1 L PF | 0.69 (0.26) | EQ-5D | French (TTO) | Stage I–Stage III/IV PF and PD | Meets HAS requirements |
| 1 L PD | 0.61 (0.24) | |||||
| Chouaid 2013 [39] Advanced/metastatic NSCLC |
1 L PF | 0.71 (0.24) (95% CI, 0.67–0.76) | EQ-5D-3 L | UK | At time of advanced diagnosis, mean age 64.8 years Adenocarcinoma: 65.2% Large-cell carcinoma: 6.8% SQ cell carcinoma: 17.1% Other: 9.9% Clinical stage at time of survey: IIIb: 17.9% IV: 82.1% |
Meets NICE requirements |
| 69.31 (18.33) (95% CI, 65.9–72.8) | EQ-5D VAS | N/A | No | |||
| 1 L PD | 0.67 (0.2) (95% CI 0.59–0.75) | EQ-5D-3 L | UK | Meets NICE requirements | ||
| 58.67 (17.4) (95% CI 51.3–66.0) | EQ-5D VAS | N/A | No | |||
| Iyer 2013 [46] Advanced/metastatic NSCLC |
France, Germany 1 L | 0.63 (0.31) | EQ-5D | UK1 | Patients with: Adenocarcinoma: 56.3% Large-cell carcinoma: 11.8% SQ cell carcinoma: 29.3% Other: 2.5% Stage IIIb: 15.4% Stage: IV 84.6% |
Meets NICE requirement |
| 60.8 (19.9) | EQ-5D VAS | N/A | No | |||
| ≥ 1st line | ||||||
| Iyer 2013 [46] Advanced/metastatic NSCLC |
France, Germany 1 L/2 L | 0.58 (0.35) | EQ-5D | UK | Patients with: Adenocarcinoma: 56.3% Large-cell carcinoma: 11.8% SQ cell carcinoma: 29.3% Other: 2.5% Stage IIIb: 15.4% Stage: IV 84.6% |
Meets NICE and SMC requirements |
| France, 1 L/2 L | 0.57 (0.41) | |||||
| Germany, 1 L/2 L | 0.59 (0.31) | |||||
| France, Germany 1 L/2 L | 58.0 (19.9) | EQ-5D VAS | No | |||
| France, 1 L/2 L | 57.1 (21.1) | |||||
| Germany, 1 L/2 L | 58.6 (19.1) | |||||
| 2nd line | ||||||
| Blackhall 2014 [41] Advanced/metastatic ALK+ NSCLC |
2 L BL CRZ | 0.73 (0.24) | EQ-5D-3 L | NR | Multinational patients, locally advanced/metastatic ALK+ NSCLC, 2 L | Unclear as tariff NR |
| 2 L BL chemotherapy (PEM or DOC) | 0.70 (0.26) | |||||
| 2 L BL PEM | 0.73 (0.24) | |||||
| 2 L BL DOC | 0.67 (0.29) | |||||
| 2 L on CRZ | 0.82 (SE, 0.01) (95% CI, 0.79–0.85) |
|||||
| 2 L on Chemotherapy | 0.73 (SE, 0.02) (95% CI, 0.70–0.77) |
|||||
| 2 L on PEM | 0.74 (SE, 0.02) (95% CI, 0.70–0.79) |
|||||
| 2 L on DOC | 0.66 (SE, 0.04) (95% CI, 0.58–0.74) |
|||||
| Chevalier 2013c [38] Advanced/metastatic NSCLC |
2 L PF | 0.70 (0.22) | EQ-5D | French (TTO) | Stage I–Stage III/IV PF and PD | Meets HAS requirements |
| 2 L PD | 0.55 (0.35) | |||||
| Chouaid 2013 [39] Advanced/metastatic NSCLC |
2 L PF | 0.74 (0.18) (95% CI, 0.68–0.80) |
EQ-5D-3 L | UK | At time of advanced diagnosis, mean age 64.8 years Adenocarcinoma: 65.2% Large-cell carcinoma: 6.8% SQ cell carcinoma: 17.1% Other: 9.9% Clinical stage at time of survey: IIIb: 17.9% IV: 82.1% |
Meets NICE requirements |
| 65.0 (19.6) (95% CI, 59.2–70.8) |
EQ-5D VAS | N/A | No | |||
| 2 L PD | 0.59 (0.34) (95% CI, 0.42–0.77) |
EQ-5D-3 L | UK | Meets NICE requirements | ||
| 53.5 (23.3) (95% CI, 41.5–65.4) |
EQ-5D VAS | N/A | No | |||
| Huang 2016c [45] Advanced PD-L1+ NSCLC |
2 L PF | 0.76 (95% CI, 0.75–0.77) | EQ-5D | NR | Multinational patients with advanced NSCLC and PD-L1+ tumours in 2 L on PEMB or DOC, after platinum-based chemotherapy | Unclear as tariff NR |
| 2 L PD | 0.69 (95% CI, 0.66–0.71) | |||||
| Advanced PD-L1+ NSCLC, 2 L, > 360 days from death | 0.81 (0.79, 0.83) | Patients with advanced NSCLC and PD-L1+ tumours in 2 L on PEMB or DOC, after platinum-based chemotherapy | ||||
| Advanced PD-L1+ NSCLC, 2 L, 180–360 days from death | 0.73 (0.71, 0.75) | |||||
| Advanced PD-L1+ NSCLC, 2 L, 90–180 days from death | 0.69 (0.66, 0.72) | |||||
| Advanced PD-L1+ NSCLC, 2 L, 30–90 days from death | 0.60 (0.56, 0.64) | |||||
| Advanced PD-L1+ NSCLC, 2 L, < 30 days from death | 0.40 (0.31, 0.48) | |||||
| Iyer 2013 [46] Advanced/metastatic NSCLC |
On treatment: 2 L only | 0.53 (0.38) | EQ-5D | UK | French and German patients | Meets NICE and SMC requirements |
| 54.9 (19.3) | EQ-5D VAS | N/A | No | |||
| Langley 2013 [48] Stage IV NSCLC with brain metastases |
NSCLC with BM, previous tx allowed, OSC + WBRT 0 days | 0.63 | EQ-5D | NRd | UK and Australian NSCLC patients with brain metastases | No, as VAS tariff used |
| NSCLC with BM, previous tx allowed, OSC + WBRT 28 days | 0.49 | |||||
| NSCLC with BM, previous tx allowed, OSC + WBRT 56 days | 0.39 | |||||
| NSCLC with BM, previous tx allowed, OSC + WBRT 112 days | 0.36 | |||||
| NSCLC with BM, previous tx allowed, OSC + WBRT 168 days | 0.16 | |||||
| NSCLC with BM, previous tx allowed, OSC alone 0 days | 0.60 | |||||
| NSCLC with BM, previous tx allowed, OSC alone 28 days | 0.49 | |||||
| NSCLC with BM, previous tx allowed, OSC alone 56 days | 0.44 | |||||
| NSCLC with BM, previous tx allowed, OSC alone 112 days | 0.38 | |||||
| NSCLC with BM, previous tx allowed, OSC alone 168 days | 0.36 | |||||
| Lloyd 2008 [59] Cancer with chemotherapy-related anaemia or fatigue |
Anaemia, Hb level, ≥12.0 g/dL | 0.708 (95% CI, 0.057) | SG | N/A | General public sample from UK | No |
| 0.611 (95% CI, 0.112) | TTO | UK cancer patients who have recently experienced chemotherapy-related fatigue and anaemia completing vignette-based TTO | Meets NICE/SMC requirements but still vignette-based health state rather than patient rating own health | |||
| Nafees 2008 [59] mNSCLC |
2 L Stable diseasee | 0.65 (SE, 0.02) | SG | N/A | 100 members of general public in UK | No, but used in multiple HTA submissions |
| 2 L Responding diseasef | 0.67 | |||||
| 2 L Response gain | 0.02 (SE, 0.01) | |||||
| 2 L Progressive diseaseg | 0.47 | |||||
| Novello 2015 [49] Stage III/IV recurrent NSCLC (SQ and NSQ)h |
2 L NIN + DOC, before treatment (week 0) | 0.72 | EQ-5D | UK | Multinational patients with stage III/IV recurrent NSCLC (SQ and NSQ) in 2 L after chemotherapy Adenocarcinoma: 50.1% |
Meets NICE/SMC requirements |
| 2 L NIN + DOC, after treatment (week 30) | 0.61 | |||||
| 2 L PLA + DOC, before treatment (week 0) | 0.72 | |||||
| 2 L PLA + DOC, after treatment (week 30) | 0.62 | |||||
| 2 L NIN + DOC, before treatment (week 0) | 69.0 | EQ-5D VAS | N/A | No | ||
| 2 L NIN + DOC, after treatment (week 30) | 63.2 | |||||
| 2 L PLA + DOC, before treatment (week 0) | 69.0 | |||||
| 2 L PLA + DOC, after treatment (week 30) | 63.1 | |||||
| Reck 2015 [50] Advanced SQ NSCLC |
2 L NIVO at BL | 0.68 (0.208) | EQ-5D | NR | Multinational patients with advanced SQ NSCLC | Unclear as tariff NR |
| 2 L DOC at BL | 0.66 (0.284) | |||||
| 2 L NIVO at BL | 63.7 (18.2) | EQ-5D VAS | N/A | No | ||
| 2 L DOC at BL | 66.3 (20.5) | |||||
| Rudell 2016c [57] Advanced NSCLC, EGFR+ |
2 L OSI at BL | 65.2 (20.33) | EQ-5D-5 L VAS | N/A | Multinational patients with EGFR+ advanced NSCLC, 2 L after previous TKI | No |
| 2 L OSI at 36 weeks | 73.7 (17.33) | |||||
| Schuette 2012 [51] NSCLC Stage IIIB–IV |
2 L, PEM at BL | 0.66 (0.256) | EQ-5D | UK TTO | Austrian and German advanced/mNSCLC 2 L patients mainly after prior platinum treatment (IIIa, 6.7%; IIIb, 19.8%; IV, 73.5%) |
Meets NICE/SMC requirements |
| 2 L, PEM at 6 weeks (2nd cycle) | 0.02 (0.214) | EQ-5D gain | ||||
| 2 L, PEM at 6th cycle | 0.11 (0.228) | |||||
| 2 L, PEM at BL | 59.3 (17.8) | EQ-5D VAS | N/A | No | ||
| 2 L, PEM at 6 weeks (2nd cycle) | 3.3 (12.58) | EQ-5D VAS gain | N/A | |||
| 2 L, PEM at 6th cycle | 12.8 (17.62) | |||||
| Vargas 2009c [72] Advanced NSCLC |
2 L, on ERL | 0.81 | Global QoL index | NR | Patients with advanced NSCLC, 2 L after previous chemotherapy | No |
| 2 L, on taxanes | 0.62 | |||||
| ≥ 2nd line | ||||||
| Chen 2010c [73] Advanced NSCLCd |
2 L, DOC, during treatment | 0.45i | SG | N/A | UK general public (as algorithm based on Nafees 2008 data used to calculate utilities) | Acceptable data for SMC |
| 2 L, DOC, after treatment | 0.57 | |||||
| 2 L, PEM, during treatment | 0.54 | |||||
| 2 L, PEM, after treatment | 0.59 | |||||
| 3 L, ERL, during treatment | 0.48 | |||||
| BSC, during treatment | 0.47 | |||||
| Chevalier 2013 [38] Advanced/metastatic NSCLC |
3/4 L PF | 0.61 (0.3) | EQ-5D | French (TTO) | Stage I–Stage III/IV PF and PD | Meets HAS requirements |
| 3/4 L PD | 0.42 (0.40) | |||||
| Griebsch 2014 [37] Stage IIIb (with pleural effusion)/IV NSCLC adenocarcinoma (LUX-LUNG 1)j |
Week 4, progression effect longitudinal model | −0.1 | EQ-5D | UK | Multinational advanced/metastatic NSCLC, 2 LL | Meets NICE requirements |
| Mixed effect longitudinal model IRC | −0.056 (95% CI, − 0.083 to − 0.028) |
|||||
| Mixed effect longitudinal model IN | −0.065 (95% CI, − 0.092 to − 0.039) |
|||||
| Mixed effect longitudinal model IRC, AFA | −0.06 | |||||
| Mixed effect longitudinal model IRC, BSC | −0.046 | |||||
| Mixed effect longitudinal model IINV, AFA | −0.081 | |||||
| Mixed effect longitudinal model IINV, BSC | −0.033 | |||||
| Week 4, progression effect longitudinal model | −7.3 | EQ-5D VAS | N/A | No | ||
| Mixed effect longitudinal model IRC | −3.76 (95% CI, −5.19 to −2.32) | |||||
| Mixed effect longitudinal model INV | − 3.83 (95% CI, − 5.21 to − 2.44) | |||||
| Mixed effect longitudinal model IRC, AFA | 3.63 | |||||
| Mixed effect longitudinal model IRC, BSC | −4.11 | |||||
| Mixed effect longitudinal model INV, AFA | −4.42 | |||||
| Mixed effect longitudinal model INV, BSC | −2.55 | |||||
| Hirsh 2013 [40] Stage IIIB/IV NSCLC |
3 LL on AFA + BSC | 0.71 | EQ-5D | UK | 98% adenocarcinoma PD following treatment lines 1–2, one of which was platinum based, plus PD after at least 12 weeks of ERL or GEF |
Meets NICE requirements |
| 3 LL on PLA + BSC | 0.67 | |||||
| 3 LL on AFA + BSC | 67.4 | EQ-5D VAS | N/A | No | ||
| 3 LL on PLA + BSC | 65.2 | |||||
| Schwartzbergc 2015 [60] Stage IIIb/IV NSCLC (SQ & NSQ) |
All patients wk 6 | 1.0 (21.7) | EQ-5D VAS | N/A | Patients, 2 LL, NIVO 3 mg/kg i.v. q2w | No |
| wk 12 | 5.8 (21.3) | |||||
| wk 18 | 8.2 (22.3) | |||||
| wk 24 | 8.2 (23.9) | |||||
| wk 30 | 8.4 (29.2) | |||||
| SDis wk 6 | 3.8 (19.8) | |||||
| wk 12 | 6.4 (21.9) | |||||
| wk 18 | 8.2 (20.9) | |||||
| wk 24 | 5.2(21.9) | |||||
| wk 30 | 7.2 (28.5) | |||||
| PR wk 6 | 7.3 (22.4) | |||||
| wk 12 | 6.6 (24.7) | |||||
| wk 18 | 8.1 (27.6) | |||||
| wk 24 | 18.1 (31.0) | |||||
| wk 30 | 13.7 (38.2) | |||||
| PD wk 6 | −5.8 (21.1) | |||||
| wk 12 | −3.0 (19.8) | |||||
| wk 18 | 3.9 (24.3) | |||||
| wk 24 | 6.8 (12.2) | |||||
| wk 30 | 5.5 (15.7) | |||||
| Treatment line not specified | ||||||
| Bradbury 2008c [42] Advanced NSCLC |
On ERL | 0.772 | EQ-5D | NR (possibly Canadian) | Canadian patients | Potentially relevant to CADTH |
| On BSC | 0.754 | |||||
| Chang 2016c [63] Advanced NSCLC |
> 360 days from death | 0.904 (95% CI, 0.892–0.917) |
TTO | NR | General public, South Korea | No |
| 180–360 days from death | 0.720 (95% CI, 0.692–0.748) |
|||||
| 90–180 days from death | 0.627 (95% CI, 0.598–0.655) |
|||||
| 30–90 days from death | 0.379 (95% CI, 0.349–0.409) |
|||||
| < 30 days from death | 0.195 (95% CI, 0.172–0.218) |
|||||
| Dansk 2016c [43] Advanced NSCLC |
Synthesized PF | Median, 0.706 Range, 0.620–0.815 |
Synthesized utility across > 1 instrument type | NR | Utilities synthesized included those where respondents were patients and those where they were the general public considering a hypothetical health state | No |
| Synthesized PF trial-based | Median, 0.750 Range, 0.627–0.815 |
|||||
| Synthesized PF non-trial-based | Median, 0.653 Range, 0.620–0.653 |
|||||
| Synthesized PD | Median, 0.565 Range, 0.470–0.688 |
|||||
| Synthesized PD trial-based | Median, 0.599 Range, 0.550–0.688 |
|||||
| Synthesized PD non-trial-based | Median, 0.473 Range, 0.470–0.530 |
|||||
| Doyle 2008 [65] Metastatic NSCLC |
SDis, no additional symptoms | 0.626 | SG | N/A | General public | No |
| Treatment response, no additional symptoms | 0.712 | |||||
| Grunberg 2009c [58] BC/LC |
Chemotherapy-induced nausea and vomiting of differing severity | Reported graphically | SG | N/A | Patients BC/LC | Meets NICE requirements |
| Grutters 2010c [44] NSCLC (stage unspecified) |
NSCLC with grade 3+ dyspnoea, stage unspecified | Median, 0.52 | EQ-5D-5 L | NR | Patients at an early treatment stage | No |
| Jang 2010 [47] Stage IV NSCLC |
Stage IV NSCLC | 0.75 (0.15) | EQ-5D | US | Patients with NSCLC attending a major Canadian cancer center outpatient clinic | No |
| Linnet 2015c [62] Stage IV NSCLC on oral VINO |
PCS, cycle 2 | 37.0 | SF-12 | N/A | Patients | No |
| PCS, cycle 3 | 38.6 | |||||
| MCS, cycle 2 | 47.7 | |||||
| MCS, cycle 3 | 44.2 | |||||
| PCS, cycle 2 | 52.9 | Caregivers | Potential to estimate SF-6D for caregivers to mNSCLC patients, for SMC or CADTH | |||
| PCS, cycle 3 | 53.4 | |||||
| MCS, cycle 2 | 46.2 | |||||
| MCS, cycle 3 | 44.6 | |||||
| Lloyd 2005c [66] Stage IV NSCLC |
RES | 0.70 | SGk | N/A | General public | No |
| SDis, oral treatment | 0.63 | |||||
| SDis, i.v. treatment | 0.58 | |||||
| PD | 0.42 | |||||
| End of life | 0.33 | |||||
| Manser 2006 [61] Stage IV NSCLC |
Stage IV | Median, 0.68 (IQR, 0.54–0.82) |
AQoL | Australia | Mixed stage enrolled: I, 31.5%; II, 17.4%; IIIa, 16.3%; IIIb, 7.6%; IV, 25.0% |
No |
| Matza 2014 [67] Stage IV cancer with bone metastases |
Cancer with bone metastases and no SRE | 0.47 (0.41) | TTO | N/A | General public, UK (Edinburgh and London) |
No |
| 0.47 (0.45) | General public, Canada (Montreal and Toronto) |
|||||
| 0.47 (0.42) | General public, UK and Canada | |||||
| Stewart 2015 [56] EGFR+ Stage IV NSCLC |
PR/SDis on EGFR TKIs (GEF, ERL, AZD9291) | 0.82 (SE, 0.16) | EQ-5D-3 L | NR | Patients, eligible for or on TKI tx, 55% Asian, 45% male, median age 60, 66% never smokers. Stage IV: at diagnosis, 80% when surveyed, 100% |
Unclear |
| Responded to standard chemotherapy | 0.80 (SE, 0.12) | |||||
| EGFR+, responded to GEF | 0.84 (SE, 0.14) | |||||
| EGFR+, responded to ERL | 0.82 (SE, 0.17) | |||||
| EGFR+, responded to AZD9291 | 0.83 (SE, 0.16) | |||||
| EGFR+, PD during TKI treatment (GEF, ERL, AZD9291) | 0.74 (SE, 0.08) | |||||
| EGFR+, all patients (PR/SDis/PD), 25% 3LL | 0.802 | |||||
| Tabberer 2006 [52] Advanced NSCLC |
RES | 0.49 | EQ-5D | NR | General public, UK (Cardiff, Glasgow, London and Oxford) |
No |
| SDis | 0.46 | |||||
| SDis + oral treatment | 0.45 | |||||
| SDis + i.v. treatment | 0.43 | |||||
| PD | 0.22 | |||||
| Near death | 0.15 | |||||
| Trippoli 2001 [53] Metastatic NSCLC |
Metastatic NSCLC | 0.53 (0.36) | EQ-5D | UK (TTO) | Italian patients | Meets NICE and SMC reference cases |
| 0.55 (0.22)l | EQ-5D VAS | N/A | No | |||
| Yang 2014 [54] Stage IIIB/IV NSCLC |
Stage IV inoperable, performance status 0–1 | 0.75 (0.22) | EQ-5D | Taiwan | Patients, mixed NSCLC stages: I, 0.8%; II, 0%; IIIA, 4.5%; IIIB, 16.9%; IV, 77.8% | No |
| Stage IV inoperable, performance status 0–4 | 0.75 (0.22) | |||||
| Yokoyama 2013c [55] Advanced NSCLC/SCLC |
Stage IIIB/IV NSCLC/SCLC with bone metastasis and SRE | NR | EQ-5D | NR | Patients, advanced NSCLC, 72%, SCLC, 28% NSCLC and SCLC: IIIB, 37%; IV, 63% |
No |
aMean, or mean (SD) unless stated otherwise
bVAS scores were also reported in this study but unclear whether this was EQ-VAS
cThese studies were published as abstracts or posters
dThis referenced article (https://www.ncbi.nlm.nih.gov/pubmed/10109801) is for a VAS valuation
e SDis vignette:
• You have a life-threatening illness that is stable on treatment. You are receiving cycles of treatment that require you to go to the outpatient clinic
• You have lost weight, and your appetite is reduced. You sometimes experience pain or discomfort in your chest or under your ribs, which can be treated with painkillers. You have shortness of breath, and breathing can be painful. You have a persistent nagging cough
• You are able to wash and dress yourself and do jobs around the home. Shopping and daily activities take more effort than usual
• You are able to visit family and friends but often have to cut it short because you get tired
• You sometimes feel less physically attractive than you used to. Your illness has affected your sex drive
• You worry about dying and how your loved ones will cope
f Second-line responding vignette:
• You have a life-threatening illness that is responding to treatment. You are receiving cycles of treatment which require you to go to the outpatient clinic
• You are gaining back your weight and your appetite is returning. You occasionally experience pain or discomfort in your chest or under your ribs which can be treated with painkillers. You sometimes have shortness of breath. You occasionally have a nagging cough
• You are able to wash and dress yourself and do jobs around the home. Shopping and daily activities can sometimes be tiring
• You are able to visit family and friends but sometimes have to cut it short because you get tired
• You occasionally feel less physically attractive than you used to. Your illness has somewhat affected your sex drive
• You sometimes worry about dying and how your loved ones will cope
g Second-line PD vignette:
• You have a life-threatening illness, and your condition is getting worse
• You have lost your appetite and have experienced significant weight loss. You experience pain and discomfort in your chest or under your ribs. You frequently have shortness of breath, and breathing is often painful. You have a persistent nagging cough and sometimes cough up blood. You may experience some difficulty swallowing
• You experience severe fatigue and feel too tired to go out or to see family and friends. It has affected your relationships with them
• You need assistance to wash and dress yourself. You are often unable to do jobs around the house or other daily activities. You are dependent on others to do your shopping and are unable to do your usual daily activities
• You often feel less physically attractive than you used to. You have little or no sexual drive
• You are depressed, and dying is always on your mind. You worry about how your loved ones will cope
hThis study also has utilities available every 3 weeks between week 0 and week 30 for all treatments
iAll utilities in this paper assumed to be the mean, although it is not clearly stated in the paper
j1 L data also reported from LUX-LUNG 3
kIndividual country values are also available in this publication
lThis value is reported as in the original publication
Abbreviations: 1 L first line, 2 L second line, 2 LL second and subsequent line, 3 LL third and subsequent line, AFA afatinib, ALK+ anaplastic lymphoma kinase mutation positive, AQoL Assessment of Quality of Life instrument, BC breast cancer, BL baseline, BSC best supportive care, CADTH Canadian Agency for Drugs and Technologies in Health, CI confidence interval, CRZ crizotinib, DOC docetaxel, EGFR epidermal growth factor receptor, EGFR+ epidermal growth factor receptor mutation positive, EORTC QLQ European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire, EQ-VAS EuroQol visual analogue scale, ERL erlotinib, GEF gefitinib, HAS Haute Autorité de Santé, HTA health technology assessment, IQR interquartile range, i.v. intravenous, LC lung cancer, MCS mental component summary, mNSCLC metastatic non-small cell lung cancer, N/A not applicable, NICE National Institute for Health and Care Excellence, NIN nintedanib, NIVO nivolumab, NR not reported, NSCLC non-small cell lung cancer, NSQ non-squamous, NTS not treatment-specific, OSC optimal standard care, OSI osimertinib, PCS physical component summary, PD progressive disease, PEM pemetrexed, PF progression-free, PLA placebo, PR partial response, PSS progression-status specific, q2w once every 2 weeks, QoL quality of life, RES response, SCLC small-cell lung cancer, SD standard deviation, SDis stable disease, SE standard error, SF-6D 6-dimension Short-Form Health Survey, SF-12/36 12-/36-item Short-Form Health Survey, SG standard gamble, SMC Scottish Medicines Consortium, SQ squamous, SRE skeletal-related event, TKI tyrosine kinase inhibitor, TTO time trade-off, VAS visual analogue scale, VINO vinorelbine, WBRT whole-brain radiotherapy
Disutilities for progression from a stable state were − 0.056 or − 0.065 by EQ-5D, both from Griebsch et al. [37], or − 0.1798 by general population-derived SG [69]. Overall, HSUVs varied not only by treatment line and disease state, but also by the treatment received under the same health state (potentially reflecting differences in safety profiles) and by the instrument/tariff used to derive the HSUV.
Relevant disutilities and decrements
Eleven studies identified in this systematic review reported disutilities or decrements for AE health states [44, 52, 55, 58, 59, 65, 67–71]. Only two studies reported disutilities specifically associated with second-line treatment [69, 71], and another two studies did not specify the treatment line [44, 65]; disutility and decrement data are summarized in Table 4. Utility-incorporating decrements were identified for the following AEs in the context of second-line “stable disease” or second-line “responding”: diarrhoea, fatigue, febrile neutropenia, hair loss and nausea/vomiting. Disutilities associated with second-line treatment were reported for the following events [69]: “moving from stable to progressive state” (− 0.18), neutropenia (− 0.09), febrile neutropenia (− 0.09), fatigue (− 0.07), nausea/vomiting (− 0.05), diarrhoea (− 0.05), hair loss (− 0.04) and rash (− 0.03).
Table 4.
Disutilities and decrements for adverse event health states in patients with previously treated mNSCLC
| Relevance | Author, year, country, referencea | Instrument and respondent | Utility type | Health state/disutility | Mean HSUV (SD) [SE] {95% CI} | HTA suitability | |
|---|---|---|---|---|---|---|---|
| Advanced/mNSCLC | Nafees 2008, UK [69] | SG General public |
UID | Stage IV NSCLC, 2 L, stable disease | Does not meet HTA body reference case as vignette-based utility completed by general public respondents. Has been used in multiple HTA submissions, however. Specifically 2 L and UK, good sample size (n=100) and measure of dispersion available. | ||
| + Diarrhoea | 0.61 | ||||||
| + Fatigue | 0.58 | ||||||
| + Febrile neutropenia | 0.56 | ||||||
| + Hair loss | 0.61 | ||||||
| + Nausea/vomiting | 0.61 | ||||||
| + Neutropenia | 0.56 | ||||||
| + Rash | 0.62 | ||||||
| Stage IV NSCLC, 2 L, responding disease | |||||||
| + Diarrhoea | 0.63 | ||||||
| + Fatigue | 0.60 | ||||||
| + Febrile neutropenia | 0.58 | ||||||
| + Hair loss | 0.63 | ||||||
| + Nausea/vomiting | 0.62 | ||||||
| + Neutropenia | 0.58 | ||||||
| + Rash | 0.64 | ||||||
| D | Stage IV NSCLC, 2 L, moving from stable to progressive |
−0.18 [0.022] | |||||
| Neutropenia | −0.09 [0.015] | ||||||
| Febrile neutropenia | −0.09 [0.016] | ||||||
| Fatigue | −0.07 [0.018] | ||||||
| Nausea and vomiting | −0.05 [0.016] | ||||||
| Diarrhoea | −0.05 [0.016] | ||||||
| Hair loss | −0.04 [0.015] | ||||||
| Rash | −0.03 [0.012] | ||||||
| Response gain | 0.02 [0.007] | ||||||
| Tabberer 2006, UK [52] | EQ-5D (tariff NR but likely UK TTO tariff as UK sample) General public |
D | Compared with stable disease (advanced NSCLC, line not specified) | Not suitable as general public respondents, line of treatment not specified, and no measure of dispersion reported. Good sample size, however (n=154) and Nafees et al. 2008 in 2 L does not provide disutilities for neuropathy or stomatitis so these values from Tabberer et al. may be the best available. | |||
| Febrile neutropenia | −0.27 | ||||||
| Rash | −0.06 | ||||||
| Neuropathy | −0.15 | ||||||
| Neutropenia | −0.14 | ||||||
| Nausea | −0.14 | ||||||
| Stomatitis | −0.14 | ||||||
| Diarrhoea | −0.13 | ||||||
| Doyle 2008, UK [65] | SG General public |
UID | Metastatic NSCLC, line not specified, SDis no additional symptoms | Does not meet reference case as general public respondents. However, these are the only disutilities for severe symptoms for cough, dyspnoea and pain in mNSCLC, so are best option in spite of not meeting HTA derivation method preferences (n=101) | |||
| + Cough | 0.58 | ||||||
| + Dyspnoea | 0.58 | ||||||
| + Pain | 0.56 | ||||||
| + Cough, dyspnoea and pain | 0.46 | ||||||
| D | Cough | −0.05 [0.011] | |||||
| Dyspnoea | −0.05 [0.012] | ||||||
| Pain | −0.07 [0.012] | ||||||
| Cough, dyspnoea and painc | − 0.17 b | ||||||
| Responding disease gain vs SDis | 0.09 [0.015] | As line not specified, data from Nafees et al. 2008 should be used in preference. | |||||
| Handorf 2012, USA [70] | Expert opinion | UID | Stage IV NSCLC adenocarcinoma (1 L SDis) | ||||
| + neutropenia | 0.67 | Does not meet reference case as expert opinion-derived. This AE is covered by Nafees et al. 2008, which uses a better derivation method than expert opinion. | |||||
| + pneumothorax | 0.63 | Does not meet reference case as expert opinion-derived, but these are the only estimates for these AE health states. SDis estimate was 0.670 (oral therapy) and 0.653 (i.v. chemotherapy) for disutility calculation. | |||||
| + haemorrhage | 0.63 | ||||||
| + thrombocytopenia | 0.65 | ||||||
| + thrombosis | 0.56 | ||||||
| Earlier stage NSCLC (curative intent) | Grutters 2010, country NR [44] | EQ-5D-5 L (tariff NR) Patients |
UID | NSCLC, curative intent stage, line not specified, grade III+ dyspnoea | 0.52 (median) | Patient-derived EQ-5D but tariff and measure of dispersion NR. Only source of grade III+ dyspnea. Utility for NSCLC patients without dyspnoea in this sample was 0.81, i.e. disutility − 0.29 | |
| Advanced/mLC (NSCLC+SCLC) | Yokoyama 2013, Japan [55] | EQ-5D (tariff NR) Patients |
D | Stage IIIB/IV NSCLC/SCLC with bone metastases + skeletal related event (pathologic fracture, radiation or surgery to bone lesion, spinal cord compression or hypercalcaemia) | − 0.05d | Provides NSCLC/SCLC (mixed) patient-derived EQ-5D decrement for (mixed) SREs. Data for these AEs are limited, so although this estimate is not robust (n=9 and response % low at 32%) it does provide an indication. No variability measure reported | |
| Breast cancer and lung cancer | Grunberg 2009, USA [58] | SG Patients |
UID | Base state: continuous nausea and vomiting | 0.53f | Nafees et al. 2008 provide data for nausea and vomiting but if different levels of nausea and vomiting need to be discerned then these utilities can be considered. Patient-derived SG but mixed lung/breast cancers. Good sample size (n=96) but no measure of dispersion. As the Copyright fee for Grunberg 2009 was high, these data are reported from Shabaruddin 2013 [79] (a previous SR that extracted the graphical data from Grunberg 2009) | |
| Increment | Limited nausea and limit vomiting vs continuous nausea and vomiting | + 0.53f | |||||
| Increment | Limited nausea vs continuous nausea and vomiting | + 0.55f | |||||
| Increment | Limited vomiting vs continuous nausea and vomiting | + 0.50f | |||||
| Advanced Cancer | Matza 2014, UK [67] | TTO General public |
U | Stage IV cancer with bone metastases (no skeletal-related events) | 0.47 (0.41) | Does not meet reference case as general population respondents. However, as there are no alternative utilities for bone metastases these UK utilities could be considered for NICE or SMC. Good sample size (n=126), SD available. | |
| UID | + spinal cord compression without paralysis | 0.25 (0.50) | |||||
| + spinal cord compression with paralysis | 0.13 (0.49) | ||||||
| + fracture of the leg | 0.42 (0.41) | ||||||
| + fracture of the rib | 0.44 (0.42) | ||||||
| + fracture of the arm | 0.44 (0.41) | ||||||
| + radiation treatment (2 weeks, 5 appointments/week) | 0.42 (0.42) | ||||||
| + radiation treatment (2 appointments) | 0.45 (0.41) | ||||||
| + surgery to stabilize bone | 0.40 (0.44) | ||||||
| Matza 2014, Canada [67] | TTO General public |
U | Stage IV cancer with bone metastases (no skeletal-related events) | 0.47 (0.45) | Does not meet reference case as general population respondents. However, as there are no alternative utilities for bone metastases these Canadian utilities could be considered for CADTH. Reasonable sample size (n=61), SD available. | ||
| UID | + spinal cord compression without paralysis | 0.25 (0.54) | |||||
| + spinal cord compression with paralysis | 0.19 (0.53) | ||||||
| + fracture of the leg | 0.40 (0.48) | ||||||
| + fracture of the rib | 0.43 (0.47) | ||||||
| + fracture of the arm | 0.43 (0.48) | ||||||
| + radiation treatment (2 weeks, 5 appointments/week) | 0.41 (0.50) | ||||||
| + radiation treatment (2 appointments) | 0.45 (0.45) | ||||||
| + surgery to stabilize bone | 0.39 (0.50) | ||||||
| Matza 2014, UK and Canada [67] | TTO General public |
U | Stage IV cancer with bone metastases (no skeletal-related events) | 0.47 (0.42) | Does not meet reference case as general population respondents. However, as there are no alternative utilities for bone metastases these UK+Canadian utilities could be considered for NICE, SMC or CADTH. Good sample size (n=187), SD available. | ||
| UID | + spinal cord compression without paralysis | 0.25 (0.21) | |||||
| + spinal cord compression with paralysis | 0.15 (0.50) | ||||||
| + fracture of the leg | 0.41 (0.43) | ||||||
| + fracture of the rib | 0.44 (0.43) | ||||||
| + fracture of the arm | 0.43 (0.43) | ||||||
| + radiation treatment (2 weeks, 5 appointments/week) | 0.41 (0.45) | ||||||
| + radiation treatment (2 appointments) | 0.45 (0.42) | ||||||
| + surgery to stabilize bone | 0.40 (0.46) | ||||||
| Matza 2014, UK [67] | TTO General public |
D | Stage IV cancer with bone metastases | As above | |||
| + spinal cord compression without paralysis | −0.22 (0.31) | ||||||
| + spinal cord compression with paralysis | −0.34 (0.36) | ||||||
| + fracture of the leg | −0.05 (0.09) | ||||||
| + fracture of the rib | −0.03 (0.08) | ||||||
| + fracture of the arm | −0.03 (0.07) | ||||||
| + radiation treatment (2 weeks, 5 appointments/week) | −0.05 (0.12) | ||||||
| + radiation treatment (2 appointments) | −0.02 (0.07) | ||||||
| + surgery to stabilize bone | −0.07 (0.17) | ||||||
| Matza 2014, Canada [67] | TTO General public |
D | Stage IV cancer with bone metastases | As above | |||
| + spinal cord compression without paralysis | −0.22 (0.32) | ||||||
| + spinal cord compression with paralysis | −0.28 (0.30) | ||||||
| + fracture of the leg | −0.07 (0.19) | ||||||
| + fracture of the rib | −0.04 (0.17) | ||||||
| + fracture of the arm | −0.04 (0.07) | ||||||
| + radiation treatment (2 weeks, 5 appointments/week) | −0.06 (0.21) | ||||||
| + radiation treatment (2 appointments) | −0.02 (0.11) | ||||||
| + surgery to stabilize bone | −0.08 (0.21) | ||||||
| Matza 2014, UK and Canada [67] | TTO General public |
D | Stage IV cancer with bone metastases | As above | |||
| + spinal cord compression without paralysis | −0.22 (0.31) | ||||||
| + spinal cord compression with paralysis | −0.32 (0.34) | ||||||
| + fracture of the leg | −0.06 (0.13) | ||||||
| + fracture of the rib | −0.03 (0.12) | ||||||
| + fracture of the arm | −0.04 (0.11) | ||||||
| + radiation treatment (2 weeks, 5 appointments/week) | −0.06 (0.15) | ||||||
| + radiation treatment (2 appointments) | −0.02 (0.08) | ||||||
| + surgery to stabilize bone | −0.07 (0.18) | ||||||
| Cancer, unclear stage | Lloyd 2008, UK [59] | SG General public |
UID | Anaemia associated with cancer treatment | Does not meet reference case as general population sample respondent for SG exercise. | ||
| Haemoglobin level (g/dL) | 7.0–8.0 | 0.58 {0.067} | |||||
| 8.0–9.0 | 0.61 {0.064} | ||||||
| 9.0–10.0 | 0.64 {0.060} | ||||||
| 10.0–10.5 | 0.64 {0.062} | ||||||
| 10.5–11.0 | 0.66 {0.061} | ||||||
| 11.0–12.0 | 0.70 {0.056} | ||||||
| >12.0 | 0.71 {0.057} | ||||||
| VAS General public |
UID | Haemoglobin level (g/dL) | 7.0–8.0 | 16.9 {2.6} | |||
| 8.0–9.0 | 22.3 {3.0} | ||||||
| 9.0–10.0 | 27.6 {2.9} | ||||||
| 10.0–10.5 | 32.9 {3.4} | ||||||
| 10.5–11.0 | 38.8 {3.6} | ||||||
| 11.0–12.0 | 45.9 {4.2} | ||||||
| >12.0 | 51.2 {4.3} | ||||||
| TTO Cancer patients with recent experience of chemotherapy-related anaemia or fatigue |
UID | Haemoglobin level (g/dL) | 7.0–8.0 | 0.30 {0.127} | |||
| 8.0–9.0 | 0.36 {0.126} | ||||||
| 9.0–10.0 | 0.41 {0.125} | ||||||
| 10.0–10.5 | 0.45 {0.122} | ||||||
| 10.5–11.0 | 0.45 {0.111} | ||||||
| 11.0–12.0 | 0.55 {0.105} | ||||||
| >12.0 | 0.61 {0.112} | ||||||
| U | Own current health | 0.85 {0.034} | |||||
| EQ-5D current health | 0.87 {0.076} | ||||||
| VAS Cancer patients with recent experience of chemotherapy-related anaemia or fatigue |
UID | Haemoglobin level (g/dL) | 7.0–8.0 | 21.7 {5.7} | |||
| 8.0–9.0 | 32.4 {6.6} | ||||||
| 9.0–10.0 | 34.2 {6.7} | ||||||
| 10.0–10.5 | 41.9 {6.6} | ||||||
| 10.5–11.0 | 44.7 {6.6} | ||||||
| 11.0–12.0 | 52.2 {6.8} | ||||||
| >12.0 | 62.4 {7.9} | ||||||
| U | Own current health | 87.6 {4.9} | |||||
| EQ-5D current health | 84.2 {4.57} | ||||||
| NR | Westwood 2014, NR [71] | NR | D | Anaemia | 0.073 [0.018] | Disutilities for anaemia and treatment mode have been used in previous NICE submissions. However, there is no information concerning their derivation. | |
| 2 L NSCLC | Oral therapy (ERL) | 0.014 [0.012] | |||||
| 2 L NSCLC | i.v. therapy | 0.043 [0.020] | |||||
| Patients without NSCLC 1 L setting |
Nafees 2016, Multinational and UK [68]g | TTO Patients (but not NSCLC patients) from the general public in UK, Australia, France, China, S. Korea, Taiwan |
D | Bleeding vs BL (stable no side effects) | −0.25 | No | |
| Hypertension vs BL (stable no side effects) | −0.03 | ||||||
| UID | Responding + bleeding vs BL | 0.534 | |||||
| Responding + hypertension vs BL | 0.749 | ||||||
| Stable + bleeding vs BL | 0.508 | ||||||
| Stable + hypertension vs BL | 0.729 | ||||||
aSome studies identified in this systematic review were not included in this table for the following reasons: Grunberg 2009 [58] and Grutters 2010 [44] did not report values
bThe italics indicate a calculated utility. This is calculated from the values reported in Doyle 2008 [65] Table 3 (difference between stable disease no other symptoms and stable disease with cough, dyspnoea and pain, to obtain the disutility). It is not calculated by adding the disutilities, as this would not be valid
cValues were calculated from ‘SDis + cough, dyspnea and pain’ utility minus ‘SDis no additional symptoms’ utility
dThe study did not indicate if mean or median
eSome studies identified in this systematic review were not included in this table for the following reasons: Grunberg 2009 [58] and Grutters 2010 [44] did not report values, and Nafees 2016 [68] reported variation in two decrements (bleeding, hypertension) based on the different populations in which valuation was undertaken
fAs reported in Shabarruddin 2013 [79], base state and utility increments were presented on different scales: base state was based on standard gamble scale between perfect health (arbitrary score of 100) or immediate death (arbitrary score of 0) while the utility increments were based on a scale between perfect health (arbitrary score of 100) and the surrogate negative anchor of continuous nausea/vomiting (re-set to an arbitrary score of 0)
gValues are presented for global population (United Kingdom, Australia, France, China, Taiwan, Korea). Note that country-specific data are also available
Abbreviations: 1 L first line, BL baseline, CI confidence interval, D decrement, ERL erlotinib, HSUV health state utility value, HTA health technology assessment, i.v. intravenous, LC lung cancer, mLC metastatic lung cancer, mNSCLC metastatic non-small cell lung cancer, NICE National Institute for Health and Care Excellence, NR not reported, NSCLC non-small cell lung cancer, SCLC small cell lung cancer, SD standard deviation, SE standard error, SG standard gamble, TTO time trade-off, U utility, UID utility incorporating decrement for adverse events, VAS visual analogue scale
Further recommended sources of AE health state (dis) utilities were as follows (Fig. 1): in 2 L from general population SG in Nafees et al. 2008 [69]; in metastatic NSCLC (line unspecified) from general population SG in Doyle et al. 2008 [65]; in 1 L from patients without NSCLC using directly elicited TTO in Nafees et al. 2016 [68]; in 2 L in NSCLC as reported in Westwood et al. 2014 [71]; in cancer with bone metastases for skeletal-related events from general population TTO in Matza et al. 2014 [67]; stage IV NSCLC in 1 L from expert opinion estimates in Handorf et al. 2012 (expert-opinion-derived utilities from this study were included, as they are the only source of estimates for pneumothorax, thrombocytopenia and thrombosis disutilities) [70]; and anaemia from general population SG or from patient-derived TTO in Lloyd et al. 2008 [59].
Description of HTA-relevant HSUVs and disutilities
Of the 36 publications, 13 provided HSUVs that meet the NICE reference case or are considered acceptable to the HTA agencies of interest [37–40, 42, 45, 46, 49, 53, 56, 58, 64, 69], based on the measurement technique for generation of HSUVs, as outlined in Additional file 3: Figure S1. The main characteristics of these studies are presented in Table 3. These 13 publications reported data from multinational studies [37–40, 45, 49, 64], and from Canada [42, 56], France/Germany [46], USA [58], Italy [53] and the UK [69]. In these studies, HRQoL was measured using EQ-5D [37–40, 42, 45, 46, 49, 53, 56], EQ-VAS [37, 39, 40, 49] and SG [58, 64, 69]. The HTA suitability of disutilities and decrements for AE health states in previously treated patients are reported in Table 4.
Discussion
Economic evaluation, particularly cost–utility analysis, provides important information for guiding decision-making in health care, and its use in HTA is increasing globally. Such evaluation includes examination of the time spent in different disease states and uses an HSUV for each disease state to calculate QALYs; HSUVs therefore play a key role in economic evaluation. As summarized in Additional file 3: Figure S1, NICE, SMC, CADTH, HAS and PBAC prefer utilities to be estimated using a generic preference-based instrument, with health states described by patients through use of a questionnaire, and with the health state valued using a country-specific tariff that reflects societal preferences. As the aim of this systematic review was to evaluate the experience of adults with previously treated mNSCLC, the synthesis of health state utility estimates was outside its scope. However, the findings presented here may provide a basis for generation of an accurate estimate of the mean HSUV for use in economic evaluations [74, 75].
This systematic review identified HSUVs relevant to the experience of previously treated adult patients with mNSCLC. Search strings were designed to allow (dis) utilities from a broader population (including lung cancer, advanced/metastatic cancer and specific metastases common in patients with lung cancer). In the absence of second-line mNSCLC (dis) utilities, alternatives were selected with decreasing population specificity and relevance from first-line mNSCLC, NSCLC, lung cancer or advanced/metastatic cancer, as outlined in Fig. 1. Ordering the HSUVs by line of treatment reflects the practice of switching treatment at progression. However, for the newer immunotherapies, patients may remain on treatment post-progression, and their HRQoL may remain at pre-progression levels. Thus, HSUVs estimated for progression status-specific health states from patients receiving chemotherapy may not be suitable to apply to the equivalent health states when patients receive immunotherapy.
In total, the 36 identified articles reported 591 HSUVs relevant to the experience of previously treated adult patients with mNSCLC, and 11 of these studies reported a total of 195 disutilities or decrements for AE health states that are relevant to the experience of patients with mNSCLC. The range of HSUVs identified for comparable health states, such as progression-free/stable disease among patients treated second-line for advanced/metastatic NSCLC [39, 45], highlights how differences in study type, tariff, health state and the measures used can drive variation in HSUV estimates. For instance, disutilities for progression from a stable state were − 0.056 or − 0.065 using EQ-5D, [37] or − 0.1798 by general-population-derived SG. [69] To overcome such variations, where possible, HSUV studies should seek to use instruments, respondents and valuation populations that are most acceptable to HTA bodies. However, there are instances where variation in methods can be justified. For example, disutility values derived from vignettes and a general public sample were used by Nafees et al. [69], because asking patients suffering such toxicities to complete HRQoL questionnaires was considered to be too burdensome for patients and potentially unethical. Moreover, although the variation may be large, it helps decision makers to identify where variability exists and informs the design of sensitivity analyses.
In the 36 publications identified, 13 provided HSUVs that meet the NICE reference case or are considered acceptable to the HTA agencies of interest [37–40, 42, 45, 46, 49, 53, 56, 58, 64, 69]. These were deemed suitable because HRQoL was measured using the EQ-5D [37–40, 42, 45, 46, 49, 53, 56] or SG [58, 64, 69], both measures preferred or accepted by several HTA authorities. This endeavour fills an important gap in the field because hitherto, only two reports had described HSUVs in mNSCLC [68, 69]; neither was a systematic review of the literature, nor did they assess their appropriateness for use in economic evaluations.
This systematic review did not identify an HSUV report based on data from the OAK trial (NCT02008227), because it was published as a congress abstract after the cut-off date for literature searching [76]. However, the HSUVs are relevant to the aims of this systematic review, and a brief description is provided below for completeness. Patients with locally advanced NSCLC or mNSCLC after failure of platinum-containing chemotherapy were randomized in a phase 3 trial to receive atezolizumab or docetaxel [76, 77]. As part of the trial, patients completed the EQ-5D, and the resultant HSUVs were presented by time point before death. This study is similar to Huang et al. 2016, which also presented time-to-death EQ-5D utilities for a similar patient group receiving immunotherapy, except comparing pembrolizumab and docetaxel [45]. Overall, HSUVs were very similar between studies at approximately equivalent time points. In the OAK study, the following HSUVs were reported by time point before death: 0.77 (> 210 days), 0.71 (105–210 days), 0.61 (35–105 days) and 0.39 (< 35 days). For comparison, HSUVs published by Huang et al. 2016 were 0.73 (180–360 days), 0.69 (90–180 days), 0.60 (30–90 days) and 0.40 (< 30 days). A further study evaluating the efficacy of immunotherapy in patients with NSCLC showed that baseline mean EQ-VAS and EQ-5D index scores were similar for nivolumab (63.7 and 0.68, respectively) and docetaxel (66.3 and 0.66, respectively) [50].
Strengths of this systematic review include the wide range of data sources searched and the search string design, which enabled identification of disutilities and utility decrements for a wide range of AEs and progressive disease states (e.g. common sites of metastasis from lung cancer) of relevance to the experience of patients previously treated for mNSCLC. We have presented HSUVs by line of treatment, allowing use in economic modelling, and have discussed HSUVs likely to be accepted by the HTA bodies of interest. Inadequate or inconsistent reporting is common, and low sample sizes and response rates considerably impact on the reported confidence intervals of the reported results. However, among the studies identified here, most reported sample size (over 100 respondents in most cases), many provided a measure of variability for the values reported, and several were based on response rates greater than 80% (although response rates were unreported in more than half of the studies). Moreover, the use of only published HSUVs can be a limitation, as HTA submissions may use HSUVs that have not been previously published. As part of this systematic review, we have therefore searched HTA submissions for any relevant utilities; most HTAs use data reported by Nafees et al. [69]
Limitations of this review include that the label for the upper bound of the utility scale (e.g. “full health” or “perfect health”) was not recorded. This has been shown to be a significant predictor of utility in lung cancer [78], so variation in utilities due to a different upper bound label cannot be explored. A further limitation concerns data extraction from some studies presented as congress abstracts or posters. Owing to the word restrictions placed on conference proceedings they may not be considered a robust data source in comparison with full publications. Furthermore, both screening and data extraction were conducted primarily by a single reviewer, and only 50% of studies were checked by a second reviewer. The exclusion of studies that used mapping to derive EQ-5D and utility values is a further limitation of this study; however, sufficient data obtained through direct measurement were identified to be informative.
Conclusions
This systematic review begins to address the challenge of identifying reliable estimates of utility values in mNSCLC that are suitable for use in economic evaluations. Our work has also highlighted that these estimates are vulnerable to variations in study type, tariff, health state and the measures used, and that shortcomings in reporting are common.
Additional files
Table S1. Search strings. (DOCX 90 kb)
Table S2. Listing of first-line mNSCLC studies with utility data excluded at second pass [80–100]. (DOCX 84 kb)
Figure S1. Hierarchy of preferred methodology for generation of HSUVs for different HTA agencies. (PDF 1172 kb)
Table S3. Quality assessment of identified studies. (DOCX 111 kb)
Acknowledgements
Medical writing support was provided by Christian Eichinger of PharmaGenesis London, London, UK and was funded by Roche.
Availability of data and materials
Not applicable. The data presented in this systematic review are published and can be found in the cited original references.
Disclosures
NP is an employee of F. Hoffmann-La Roche. AA was an employee of F. Hoffman-La Roche at the time the study was conducted. KSM is an employee of Epidemica Ltd.
Abbreviations
- AE
Adverse event
- AQoL
Assessment of Quality of Life instrument
- CADTH
Canadian Agency for Drugs and Technologies in Health
- HAS
Haute Autorité de Santé
- HRQoL
Health-related quality of life
- HSUV
Health state utility value
- HTA
Health technology assessment
- mNSCLC
Metastatic non-small cell lung cancer
- NICE
National Institute for Health and Care Excellence
- NSCLC
Non-small cell lung cancer
- PBAC
Australian Pharmaceutical Benefits Advisory Committee
- PICOS
Patient, intervention, comparator, outcome, study
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
Quality-adjusted life-year
- RECIST
Response Evaluation Criteria in Solid Tumors
- SF-12/SF-36
12-/36-item Short-Form Health Survey
- SF-6D
6-dimension Short-Form Health Survey
- SG
Standard gamble
- SMC
Scottish Medicines Consortium
- TTO
Time trade-off
- VAS
Visual analogue scale
Authors’ contributions
NP, AA and KSM carried out the conception and design, participated in the review and critique process, drafted the manuscript, and revised it critically for intellectual content. The systematic review was carried out by KSM from Epidemica Ltd., UK and was funded by Roche. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Noman Paracha, Phone: +41 61 688 2661, Email: noman.paracha@roche.com.
Ahmed Abdulla, Email: ahmed.abdulla@digipharm.ch.
Katherine S. MacGilchrist, Email: katherine@epidemica.eu
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. What is non-small cell lung cancer? Available at https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Accessed 24 Aug 2017.
- 3.Edge SB, Byrd DR, Compton CC. AJCC cancer staging handbook. 7. New York, NY: Springer; 2010. [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Tsao AS. Lung carcinoma. Available at http://www.msdmanuals.com/professional/pulmonary-disorders/tumors-of-the-lungs/lung-carcinoma. Accessed 8 Nov 2017.
- 6.CancerCare®. Types and staging of lung cancer. Available at https://www.lungcancer.org/find_information/publications/163-lung_cancer_101/268-types_and_staging. Accessed 24 Aug. 2017.
- 7.Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:1–27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 8.Lemjabbar-Alaoui H, Hassan OU, Yang YW, Buchanan P. Lung cancer: biology and treatment options. Biochim Biophys Acta. 1856;2015:189–210. doi: 10.1016/j.bbcan.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. Non-small cell lung cancer survival rates, by stage. Available at https://www.cancer.org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer.html. Accessed 24 Aug 2017.
- 10.Camps C, del Pozo N, Blasco A, Blasco P, Sirera R. Importance of quality of life in patients with non-small-cell lung cancer. Clin Lung Cancer. 2009;10:83–90. doi: 10.3816/CLC.2009.n.010. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society. Non-small cell lung cancer signs and symptoms. Available at https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/signs-symptoms.html. Accessed 24 Aug 2017.
- 12.Cella DF, Patel JD. Improving health-related quality of life in non-small-cell lung cancer with current treatment options. Clin Lung Cancer. 2008;9:206–212. doi: 10.3816/CLC.2008.n.030. [DOI] [PubMed] [Google Scholar]
- 13.Brazier JE, Papaioannou D, Cantrell A. Identifying and reviewing health state utility values for populating decision models. In: Shemilt I, Mugford M, Vale L, Marsh K, Donaldson C, editors. Evidence-based decisions and economics: health care, social welfare, education and criminal justice. 2. Oxford: Wiley-Blackwell; 2010. [Google Scholar]
- 14.Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96:5–21. doi: 10.1093/bmb/ldq033. [DOI] [PubMed] [Google Scholar]
- 15.Paracha N, Thuresson PO, Moreno SG, MacGilchrist KS. Health state utility values in locally advanced and metastatic breast cancer by treatment line: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16:549–559. doi: 10.1080/14737167.2016.1222907. [DOI] [PubMed] [Google Scholar]
- 16.EQ-5D: EQ-5D-3L user guide. Basic information on how to use the EQ-5D-3L instrument. Version 5.1.2015; Available at https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-3L_UserGuide_2015.pdf. Accessed 22 May 2018.
- 17.EQ-5D: EQ-5D-5L user guide. Basic information on how to use the EQ-5D-5L instrument. Version 2.1.2015; Available at https://euroqol.org/wp-content/uploads/2016/09/EQ-5D-5L_UserGuide_2015.pdf. Accessed 22 May 2018.
- 18.Torrance GW, Feeny DH, Furlong WJ, Barr RD, Zhang Y, Wang Q. Multiattribute utility function for a comprehensive health status classification system. Health utilities index mark 2. Med Care. 1996;34:702–722. doi: 10.1097/00005650-199607000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21:271–292. doi: 10.1016/S0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 20.Assessment of Quality of Life. What is AQoL? 2014; Available at: https://www.aqol.com.au/index.php/aqoldevelopment. Accessed 22 May 2018.
- 21.15D instrument: Use of 15D. Available from http://15d-instrument.net/use-of-the-15d/. Accessed 22 May 2018).
- 22.Seiber WJ, Grossel EJ, David KM, Ganiats TG, Kaplan RM. Quality of well being Self-Administered (QWB-SA) scale. User's Manual. 2008; Available at https://hoap.ucsd.edu/qwb-info/QWB-Manual.pdf. Accessed 22 May 2018.
- 23.Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care. 2004;42:851–859. doi: 10.1097/01.mlr.0000135827.18610.0d. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013; Available at https://www.nice.org.uk/process/pmg9/chapter/foreword. Accessed 8 Nov 2017. [PubMed]
- 25.National Institute for Health and Care Excellence. Decision Support Unit. NICE DSU Technical Support Document 11. Alternatives to EQ-5D for generating health state utility values. 2011; Available at http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2016/03/TSD11-Alternatives-to-EQ-5D_final.pdf. Accessed 8 Nov 2017]. [PubMed]
- 26.Scottish Medicine Consortium. Guidance to manufacturers for completion of new product assessment form (NPAF). 2014; Available at https://www.scottishmedicines.org.uk/Submission_Process/Submission_guidance_and_forms/Templates-Guidance-for-Submission. Accessed 8 Nov 2017.
- 27.Canadian Agency for Drugs and Technologies. Guidelines for the economic evaluation of health technologies: Canada 3rd Edition. 2016; Available at https://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf. Accessed 8 Nov. 2017.
- 28.Haute Autorité de Santé (HAS). Choices in methods for economic evaluation. 2012; Available at https://www.has-sante.fr/portail/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf. Accessed 8 Nov 2017.
- 29.Pharmaceutical Benefits Advisory Committee. Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (Version 5.0). 2016; Available at https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf. Accessed 8 Nov 2017.
- 30.Arber M, Garcia S, Veale T, Edwards M, Shaw A, Glanville J. Sensitivity of a search filter designed to identify studies reporting health state utility values. In HTA International Conference, Oslo, Norway. 2015.
- 31.National Institute for Health and Care Excellence: Position statement on use of the EQ-5D-5L valuation set. 2017; Available at: https://www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/NICE-technology-appraisal-guidance/eq5d5l_nice_position_statement.pdf. Accessed 3 of July 2018
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta–analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 33.Qatar Computing Research Institute. Rayyan. Available at http://rayyan.qcri.org/. Accessed 20 Nov 2017.
- 34.European Network for Health Technology Assessment. Process of information retrieval for systematic reviews and health technology assessments on clinical effectiveness. 2017; Available at https://www.eunethta.eu/process-of-information-retrieval-for-systematic-reviews-and-health-technology-assessments-on-clinical-effectiveness/. Accessed 3 July 2018.
- 35.Papaioannou D, Brazier J, Paisley S. Systematic searching and selection of health state utility values from the literature. Value Health. 2013;16:686–695. doi: 10.1016/j.jval.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 36.International Society of Pharmacoeconomics and Outcomes Research: Identification, review and use of health state utility data in cost-effectiveness models: good practices for outcomes research. 2018. Available from https://www.ispor.org/member-groups/task-forces/health-state-utility-estimates-in-cost-effectiveness-models. Accessed 24 May 2018.
- 37.Griebsch I, Palmer M, Fayers PM, Ellis S. Is progression-free survival associated with a better health-related quality of life in patients with lung cancer? Evidence from two randomised trials with afatinib. BMJ Open. 2014;4:e005762. doi: 10.1136/bmjopen-2014-005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chevalier J, Le Lay K, De Pouvourville G. Health state utility values in advanced non-small cell lung cancer patients. Value Health. 2013;16:A419. doi: 10.1016/j.jval.2013.08.550. [DOI] [Google Scholar]
- 39.Chouaid C, Agulnik J, Goker E, Herder GJM, Lester JF, Vansteenkiste J, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8:997–1003. doi: 10.1097/JTO.0b013e318299243b. [DOI] [PubMed] [Google Scholar]
- 40.Hirsh V, Cadranel J, Cong XJ, Fairclough D, Finnern HW, Lorence RM, et al. Symptom and quality of life benefit of afatinib in advanced non-small-cell lung cancer patients previously treated with erlotinib or gefitinib: results of a randomized phase iib/iii trial (lux-lung 1) J Thorac Oncol. 2013;8:229–237. doi: 10.1097/JTO.0b013e3182773fce. [DOI] [PubMed] [Google Scholar]
- 41.Blackhall F, Kim DW, Besse B, Nokihara H, Han JY, Wilner KD, et al. Patient-reported outcomes and quality of life in PROFILE 1007: a randomized trial of crizotinib compared with chemotherapy in previously treated patients with ALK-positive advanced non-small-cell lung cancer. J Thorac Oncol. 2014;9:1625–1633. doi: 10.1097/JTO.0000000000000318. [DOI] [PubMed] [Google Scholar]
- 42.Bradbury PA, Jang R, Isogai P, Ng R, Mittmann N, Evans W, et al. A cost utility analysis of erlotinib in patients with previously treated advanced non-small-cell lung cancer (NSCLC) Value Health. 2008;11:A72. doi: 10.1016/S1098-3015(10)70236-9. [DOI] [Google Scholar]
- 43.Dansk V, Large S, Bertranou E, Bodnar C, Dyer MT, Ryan J. A review of health state utility values used in UK NICE appraisals in advanced NSCLC. Value Health. 2016;19:A745. doi: 10.1016/j.jval.2016.09.2278. [DOI] [Google Scholar]
- 44.Grutters J, Joore M, Wiegman E, Langendijk H, De Ruysscher D, Hochstenbag M, et al. Health-related quality of life in patients surviving non-small cell lung cancer. Radiother Oncol. 2010;96:S344–S345. doi: 10.1136/thx.2010.136390. [DOI] [PubMed] [Google Scholar]
- 45.Huang M, Pellissier J, Liao J. A trial-based EuroQol EQ-5D health utility analysis in patients with previously treated advanced NSCLC. Value Health. 2016;7:PCN198. [Google Scholar]
- 46.Iyer S, Taylor-Stokes G, Roughley A. Symptom burden and quality of life in advanced non-small cell lung cancer patients in France and Germany. Lung Cancer. 2013;81:288–293. doi: 10.1016/j.lungcan.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Jang RW, Isogai PK, Mittmann N, Bradbury PA, Shepherd FA, Feld R, et al. Derivation of utility values from European Organization for Research and Treatment of Cancer Quality of Life-Core 30 questionnaire values in lung cancer. J Thorac Oncol. 2010;5:1953–7. [DOI] [PubMed]
- 48.Langley RE, Stephens RJ, Nankivell M, Pugh C, Moore B, Navani N, et al. Interim data from the Medical Research Council QUARTZ trial: does whole brain radiotherapy affect the survival and quality of life of patients with brain metastases from non-small cell lung cancer? Clin Oncol (R Coll Radiol) 2015;25:e23–e30. doi: 10.1016/j.clon.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Novello S, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Analysis of patient-reported outcomes from the LUME-lung 1 trial: a randomised, double-blind, placebo-controlled, phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer. Eur J Cancer. 2015;51:317–326. doi: 10.1016/j.ejca.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 50.Reck M, Coon C, Taylor F, DeRosa M, Penrod JR, Dastani H, et al. Evaluation of overall health status in patients with advanced squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 017. Eur J Cancer. 2015;51:S599–S600. doi: 10.1016/S0959-8049(16)31656-2. [DOI] [Google Scholar]
- 51.Schuette W, Tesch H, Buttner H, Krause T, Soldatenkova V, Stoffregen C. Second-line treatment of stage III/IV non-small-cell lung cancer (NSCLC) with pemetrexed in routine clinical practice: evaluation of performance status and health-related quality of life. BMC Cancer. 2012;12:14. doi: 10.1186/1471-2407-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabberer M, Stamuli E, Walker M, Summerhayes M, Lees M. Utilities associated with non-small cell lung cancer (NSCLC): a community study. Value Health. 2006;9:A298. doi: 10.1016/S1098-3015(10)63502-4. [DOI] [Google Scholar]
- 53.Trippoli S, Vaiani M, Lucioni C, Messori A. Quality of life and utility in patients with non-small cell lung cancer. PharmacoEconomics. 2001;19:855–863. doi: 10.2165/00019053-200119080-00007. [DOI] [PubMed] [Google Scholar]
- 54.Yang SC, Lai WW, Chang HY, Su WC, Chen HHW, Wang JD. Estimation of loss of quality-adjusted life expectancy (QALE) for patients with operable versus inoperable lung cancer: adjusting quality-of-life and lead-time bias for utility of surgery. Lung Cancer. 2014;86:96–101. doi: 10.1016/j.lungcan.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama T, Kunikane H, Katakami N, Yokota I, Saio Y, Shimozuma K, et al. A prospective analysis of the association between skeletal-related events and quality of life in patients with advanced lung cancer (CSP-HOR13) Eur J Cancer. 2013;49:S284. doi: 10.3892/ol.2018.9680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart EL, Labbe C, Brown C, Perez-Cosio A, Vennettilli A, Patel D, Cheng N, Liang M, Gill G, Leung Y, et al. Patient-reported health utility scores (HUS) in non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations by drug therapy. Pharmacoepidemiol Drug Saf. 2015;24:52. doi: 10.1002/pds.3710. [DOI] [PubMed] [Google Scholar]
- 57.Rudell K, Papadakis K, Bodnar C, Hoyle C, Ghlorghlu S, Ryden A. The impact of osimertinib on function and health status for patients with EGFR mutation-positive advanced non-small cell lung cancer. J Clin Oncol. 2016;34:e20548. doi: 10.1200/JCO.2016.34.15_suppl.e20548. [DOI] [Google Scholar]
- 58.Grunberg SM, Weeks J, Magnan WF, Herndon J, Naughton ML, Blackwell KLC, et al. Determination of utility scores for control of chemotherapy-induced nausea or vomiting – CALGB 309801. J Support Oncol. 2009;7:W17–W22. [Google Scholar]
- 59.Lloyd A, van Hanswijck de Jonge P, Doyle S, Cornes P. Health state utility scores for cancer-related anemia through societal and patient valuations. Value Health. 2008;11:1178–1185. doi: 10.1111/j.1524-4733.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 60.Schwartzberg L, Chandler J, Reynold C, Garon EB, Stepanski EJ, Keogh GP, et al. Symptom analysis and quality of life (QoL) in patients treated with nivolumab (NIVO) as ≥2nd line therapy for advanced non-small cell lung cancer (aNSCLC) Eur J Cancer. 2015;51:S628–S629. doi: 10.1016/S0959-8049(16)31730-0. [DOI] [Google Scholar]
- 61.Manser RL, Wright G, Byrnes G, Hart D, Conron M, Carter R, et al. Validity of the assessment of quality of life (AQoL) utility instrument in patients with operable and inoperable lung cancer. Lung Cancer. 2006;53:217–229. doi: 10.1016/j.lungcan.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 62.Linnet H, Hansen O, Meldgaard P, Berdeaux G, Mercier F. Health related quality of life of caregivers and patients treated for metastatic non-small cell lung cancer (NSCLC) with oral vinorelbine. Value Health. 2015;18:A473. doi: 10.1016/j.jval.2015.09.1262. [DOI] [Google Scholar]
- 63.Chang C, Park S, Choi YR, Tan SC, Kang SH, Back HJ, et al. Measurement of utilities by time to death related to advanced non-small cell lung cancer in South Korea. Value Health. 2016;19:A744. doi: 10.1016/j.jval.2016.09.2276. [DOI] [Google Scholar]
- 64.Chen W. Cost-effectiveness of epidermal growth factor receptor gene mutation testing for patients with advanced non-small cell lung cancer living in Ontario. Value Health. 2011;14:A82. doi: 10.1016/j.jval.2011.02.459. [DOI] [Google Scholar]
- 65.Doyle S, Lloyd A, Walker M. Health state utility scores in advanced non-small cell lung cancer. Lung Cancer. 2008;62:374–380. doi: 10.1016/j.lungcan.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Lloyd AJ, Van Hanswijck De Jonge P, Doyle S, Walker M, Farina C. Development and elicitation of health state utilities in metastatic non small cell lung cancer (NSCLC) in the UK. 27th North American Meeting of the Society for Medical Decision Making. 2005; Available at https://smdm.confex.com/smdm/2005ca/techprogram/P2165.HTM. Accessed 20 Nov. 2017.
- 67.Matza LS, Chung K, Van Brunt K, Brazier JE, Braun A, Currie B, et al. Health state utilities for skeletal-related events secondary to bone metastases. Eur J Health Econ. 2014;15:7–18. doi: 10.1007/s10198-012-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13:e195–e203. doi: 10.1111/ajco.12477. [DOI] [PubMed] [Google Scholar]
- 69.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84. doi: 10.1186/1477-7525-6-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Handorf EA, McElligott S, Vachani A, Langer CJ, Demeter MB, Armstrong K, et al. Cost effectiveness of personalized therapy for first-line treatment of stage IV and recurrent incurable adenocarcinoma of the lung. J Oncology Pract. 2012;8:267–274. doi: 10.1200/JOP.2011.000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westwood M, Joore M, Whiting P, van Asselt T, Ramaekers B, Armstrong N, et al. Epidermal growth factor receptor tyrosine kinase (EGFR-TK) mutation testing in adults with locally advanced or metastatic non-small cell lung cancer: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2014;18:1–165. doi: 10.3310/hta18620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vargas J, Gomez Rangel JD, Martínez-Barrera L, Mendoza D, Paladio Á. Evaluation of the quality of life in patients with non-small cell lung cancer who failed to first line chemotherapy in treatment with erlotinib vs taxanes. Value Health. 2009;12:A284. doi: 10.1016/S1098-3015(10)74392-8. [DOI] [Google Scholar]
- 73.Chen E, Buerki C, Saad F, Laouri M. Initial evaluation of the health economic impact of a prognostic 15-gene expression-based signature in early-stage NSCLC patient management. J Thorac Oncol. 2010;5:S241. [Google Scholar]
- 74.Ara R: Synthesising heath state utility values. Presented at the International Society of Pharmacoeconomics Outcomes Research 20th Annual European Congress, 4–8 November 2017, Glasgow, UK.
- 75.Petrou S, Kwon J, Madan J. A practical guide to conducting a systematic review and meta-analysis of health state utility values. Pharmacoeconomics. 2018. 10.1007/s40273-018-0670-1. [DOI] [PubMed]
- 76.Purchase JP, Paracha N, Abdulla A. Health-related quality of life in cancer immunotherapy: second line non-small-cell lung cancer. Value Health. 2017;20:A450. doi: 10.1016/j.jval.2017.08.294. [DOI] [Google Scholar]
- 77.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sturza J. A review and meta-analysis of utility values for lung cancer. Med Decis Mak. 2010;30:685–693. doi: 10.1177/0272989X10369004. [DOI] [PubMed] [Google Scholar]
- 79.Shabaruddin FH, Chen L-C, Elliott RA, Payne K. A systematic review of utility values for chemotherapy-related adverse events. PharmacoEconomics. 2013;31:277–288. doi: 10.1007/s40273-013-0033-x. [DOI] [PubMed] [Google Scholar]
- 80.Balçik Y, Şahin B. Cost-effectiveness analysis of pemetrexed and gemcitabine treatment for advanced nonsmall cell lung cancer in Turkey. Turk J Med Sci. 2016;46:152–158. doi: 10.3906/sag-1408-4. [DOI] [PubMed] [Google Scholar]
- 81.Barney BJ, Wang XS, Lu C, Liao Z, Johnson VE, Cleeland CS, et al. Prognostic value of patient-reported symptom interference in patients with late-stage lung cancer. Qual Life Res. 2013;22:2143–2150. doi: 10.1007/s11136-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belani CP, Pereira JR, Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Effect of chemotherapy for advanced non-small cell lung cancer on patients’ quality of life. A randomized controlled trial. Lung Cancer. 2006;53:231–239. doi: 10.1016/j.lungcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Billingham L, Gaunt P, Jarrett H, Dunlop D, Thompson J, O'Byrne KJ, et al. Quality of life in advanced non-small cell lung cancer, effects of cisplatin dose and carboplatin in combination with gemcitabine: results from BTOG2, a British thoracic oncology group phase III trial in 1363 patients. J Thorac Oncol. 2011;6:S319–S320. doi: 10.1097/JTO.0b013e3182011f70. [DOI] [Google Scholar]
- 84.Chouaid C, Bischoff H, Vergnenegre A, Heigener D, Taylor- Stokes G, Roughley A, et al. Real life outcomes and health-related quality of life (HRQOL) in 1st line non-squamous non-small cell lung cancer (NSCLC): a European pilot study analysing bevacizumab-based versus non-bevacizumab-based treatments. J Thorac Oncol. 2011;6:S1284–S1285. [Google Scholar]
- 85.Chouaid C, Bischoff HG, Vergnenegre A, Heigener DF, Taylor-Stokes G, Roughley A, et al. Health-related quality of life (HRQOL) in 1st line non-squamous non-small cell lung cancer (NSCLC) patients in a real life setting: bevacizumab-based versus non-bevacizumab based therapy in a European pilot study. Value Health. 2011;14:A171. doi: 10.1016/j.jval.2011.02.949. [DOI] [Google Scholar]
- 86.Dranitsaris G, Cottrell W, Evans WK. Cost-effectiveness of chemotherapy for non small-cell lung cancer. Curr Opin Oncol. 2002;14:375–383. doi: 10.1097/00001622-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 87.Felip E, Blackhall FH, Mok T, Cappuzzo F, Wilner KD, Reisman A, et al. Impact of crizotinib on patient-reported general health status compared with chemotherapy in patients with no prior systemic treatment for advanced non-squamous ALK-positive non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33:8101. [Google Scholar]
- 88.Fleeman N, Bagust A, McLeod C, Greenhalgh J, Boland A, Dundar Y, et al. Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer. Health Technol Assessment. 2010;14:47–53. doi: 10.3310/hta14Suppl1/07. [DOI] [PubMed] [Google Scholar]
- 89.Galetta D, Cinieri S, Pisconti S, Gebbia V, Morabito A, Borsellino N, et al. Cisplatin/pemetrexed followed by maintenance pemetrexed versus carboplatin/paclitaxel/bevacizumab followed by maintenance bevacizumab in advanced nonsquamous lung cancer: the GOIM (Gruppo Oncologico Italia Meridionale) ERACLE phase III randomized trial. Clin Lung Cancer. 2015;16:262–273. doi: 10.1016/j.cllc.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 90.Galetta D, Pisconti S, Cinieri S, Gebbia V, Morabito A, Borsellino N, et al. First-line pemetrexed plus cisplatin followed by maintenance pemetrexed vs carboplatin-paclitaxel plus bevacizumab followed by maintenance bevacizumab (ERACLE) in advanced non squamous non-small cell lung cancer: a quality of life-oriented, multicenter randomized phase III trial of the GOIM (Gruppo Oncologico Italia Meridionale) J Thorac Oncol. 2013;8:S1002. [Google Scholar]
- 91.Gridelli C, De Marinis F, Pujol JL, Reck M, Ramlau R, Parente B, et al. Safety, resource use, and quality of life in paramount: a phase III study of maintenance pemetrexed versus placebo after induction pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Thorac Oncol. 2012;7:1713–1721. doi: 10.1097/JTO.0b013e318267cf84. [DOI] [PubMed] [Google Scholar]
- 92.Hirsh V, Yang JCH, Tan EH, O'Byrne K, Zhang L, Boyer MJ, et al. First-line afatinib (a) vs gefitinib (G) for patients (pts) with EGFR mutation positive (EGFRm+) NSCLC (LUX-lung 7): patient-reported outcomes (PROs) and impact of dose modifications on efficacy and adverse events (AEs) J Clin Oncol. 2016;34:S9046. doi: 10.1200/JCO.2016.34.15_suppl.9046. [DOI] [Google Scholar]
- 93.Khan I, Morris S, Hackshaw A, Lee SM. Cost-effectiveness of first-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy. BMJ Open. 2015;5:e006733. doi: 10.1136/bmjopen-2014-006733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lal R, Hillerdal GN, Shah RNH, Crosse B, Thompson J, Nicolson M, et al. Feasibility of home delivery of pemetrexed in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer. 2015;89:154–160. doi: 10.1016/j.lungcan.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 95.Langer CJ, Hirsh V, Amiri K, Ko A, Knoble JL, Johnson ML, et al. Quality of life (QoL) by response: an interim analysis of patients (pts) with squamous (SCC) NSCLC treated with nab-paclitaxel/carboplatin (nab-P/C) induction therapy in the phase III ABOUND.sqm study. J Clin Oncol. 2016;34:S64. doi: 10.1200/jco.2016.34.26_suppl.64. [DOI] [Google Scholar]
- 96.Langer CJ, Hirsh V, Okamoto I, Lin FJ, Wan Y, Whiting S, et al. Survival, quality-adjusted survival, and other clinical end points in older advanced non-small-cell lung cancer patients treated with albumin-bound paclitaxel. Br J Cancer. 2015;113:20–29. doi: 10.1038/bjc.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reck M, Socinski MA, Luft A, Szczesna A, Dediu M, Ramlau R, et al. The effect of necitumumab in combination with gemcitabine plus cisplatin on tolerability and on quality of life: results from the phase 3 SQUIRE trial. J Thorac Oncol. 2016;11:808–818. doi: 10.1016/j.jtho.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 98.Shallwani SM, Simmonds MJ, Kasymjanova G, Spahija J. Quality of life, symptom status and physical performance in patients with advanced non-small cell lung cancer undergoing chemotherapy: an exploratory analysis of secondary data. Lung Cancer. 2016;99:69–75. doi: 10.1016/j.lungcan.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 99.Tongpak P, Thongprasert S, Permsuwan U. Utility of advanced non-small cell lung cancer patients in Thailand: preliminary study. Value Health. 2012;15:A657. doi: 10.1016/j.jval.2012.08.322. [DOI] [Google Scholar]
- 100.Yalçin Balçik P, Şahin B. Cost-effectiveness analysis of pemetrexed and gemcitabine treatment for advanced nonsmall cell lung cancer in Turkey. Turk J Med Sci. 2016;46:152–158. doi: 10.3906/sag-1408-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strings. (DOCX 90 kb)
Table S2. Listing of first-line mNSCLC studies with utility data excluded at second pass [80–100]. (DOCX 84 kb)
Figure S1. Hierarchy of preferred methodology for generation of HSUVs for different HTA agencies. (PDF 1172 kb)
Table S3. Quality assessment of identified studies. (DOCX 111 kb)
Data Availability Statement
Not applicable. The data presented in this systematic review are published and can be found in the cited original references.