Abstract
FOXM1 (forkhead box protein M1) is a critical proliferation-associated transcription factor that is widely spatiotemporally expressed during the cell cycle. It is closely involved with the processes of cell proliferation, self-renewal, and tumorigenesis. In most human cancers, FOXM1 is overexpressed, and this indicates a poor prognosis for cancer patients. FOXM1 maintains cancer hallmarks by regulating the expression of target genes at the transcriptional level. Due to its potential role as molecular target in cancer therapy, FOXM1 was named the Molecule of the Year in 2010. However, the mechanism of FOXM1 dysregulation remains indistinct. A comprehensive understanding of FOXM1 regulation will provide novel insight for cancer and other diseases in which FOXM1 plays a major role. Here, we summarize the transcriptional regulation, post-transcriptional regulation and post-translational modifications of FOXM1, which will provide extremely important implications for novel strategies targeting FOXM1.
Keywords: FOXM1, Regulation, Transcriptional, Post-transcriptional, Post-translational
Background
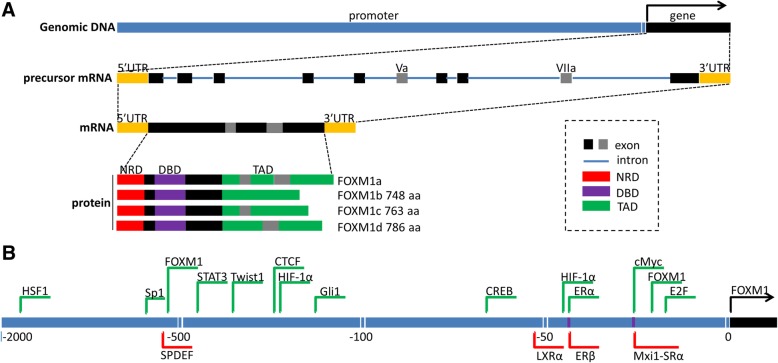
Forkhead box M1 (FOXM1), previously named HNF-3, HFH-11 or Trident, is a transcription factor of the Forkhead box (Fox) protein superfamily which is defined by a conserved winged helix DNA-binding domain1 [1]. The human FOXM1 gene consists of 10 exons, of which exons Va and VIIa can be alternatively spliced [2]. In the past, VIIa was treated as a repressor until a novel isoform (FOXM1d) that could promote the epithelial–mesenchymal transition (EMT) and metastasis by activating ROCKs in colorectal cancer was identified [3, 4]. Accordingly, there are four isoforms of human FOXM1 identified to date (Fig. 1a). FOXM1a contains both exons Va and VIIa and lacks transactivation activity, while the rest of the three, FOXM1b (which contains neither exon Va nor VIIa), FOXM1c (no VIIa) and FOXM1d (no Va) are transcriptionally active. The FOXM1 protein contains a conserved forkhead DNA-binding domain (DBD), an N-terminal repressor domain (NRD), and a C-terminal transactivation domain (TAD). The transactivation activity of TAD can be suppressed by direct interaction with the NRD [5, 6]. In addition, murine FOXM1 splice variants display the same DNA-binding specificity as human FOXM1 and bind to DNA-binding sites with the consensus sequence 5′-A-C/T-AAA-C/ T-AA-3’ [7]. The study of murine FOXM1 may also be applied to human FOXM1. For example, it has been demonstrated that Gli1 regulates FOXM1 in murine stem cells [8]. In human basal cell carcinomas and colorectal cancer cells, FOXM1 is also a direct target of Gli1 [9, 10]. This may be due to the evolutionary conservation between the DNA binding domain of both human and murine FOXM1, suggesting the FOXM1 of the two species may share target genes. As such, investigating the regulation of murine FOXM1 may provide significant implications into the dysregulation of human FOXM1. Furthermore, a mouse model is a suitable experimental model for the development of novel FOXM1 inhibitors.
Fig. 1.
Genomic structure and coding isoforms in the FOXM1 gene and transcription factor binding sites in FOXM1 promoter region. a. Genomic structure and coding isoforms of the FOXM1 gene. b. Schematic diagram of transcription factor binding sites during FOXM1 promoter region. Green: activator, red: repressor, purple: cis-acting element could be bound by both activator and repressor
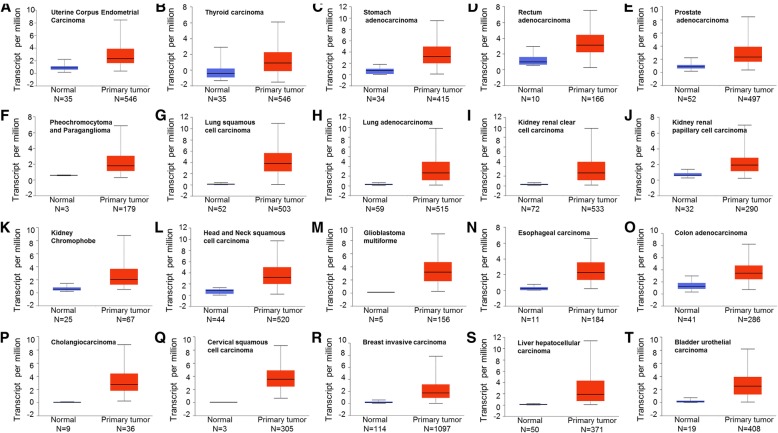
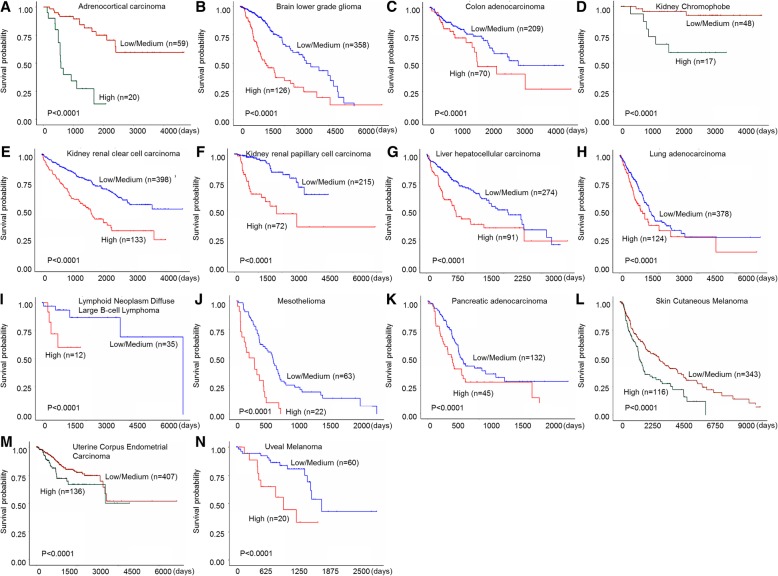
FOXM1 is detected primarily in progenitor and regenerating tissues, as well as tumor cells, which are all highly proliferative [11]. As a classic proliferation-associated transcription factor, FOXM1 directly or indirectly activates the expression of target genes at the transcriptional level and exhibits a spatiotemporal pattern whose dysregulation is involved in almost all hallmarks of tumor cells [3, 12]. Increased expression of FOXM1 is observed in a variety of human cancers, such as ovarian cancer, breast cancer, prostate cancer, hepatoma, angiosarcoma, colorectal cancer, melanoma, lung cancer, and gastric cancer [13–21], which is consistent with the results obtained from the TCGA database (Fig. 2). Inhibition of FOXM1 in cancer cells leads to decreased cell proliferation and migration, metastasis, angiogenesis, EMT, and drug resistance [22–26]. Furthermore, a recent meta-analysis revealed that elevated FOXM1 expression is related to poor prognosis in most solid tumors [27], which is also further confirmed by the TCGA database (Fig. 3) [28]. These results clearly showed the important role of FOXM1 in tumorigenesis and cancer development. Therefore, FOXM1 has been identified as a potential therapeutic target for the treatment of cancers. Although a few drugs and inhibitors have been shown to be effective at inhibiting the activity of FOXM1 in vitro, they’ve yet to pass successfully into clinical use [29]. This is likely due in major part to the poor current understanding of the regulation of FOXM1. Hence, a comprehensive review of FOXM1 regulation will thus contribute to the extensive effort and research into the gene as a therapeutic target for a number of FOXM1-dependent conditions, such as the cancers mentioned previously.
Fig. 2.
FOXM1 expression profile from the TCGA database. The FOXM1 transcript per million are presented in differernt cancers and corresponding normal tissues, including ulterine corpus endometrial carcinoma (a), thyroid carcinoma (b), stomach adenocarcinoma (c), rectum adenocarcinoma (d), prostate adenocarcinoma (e), pheochromocytoma and paraganglioma (f), lung squamous cell carcinoma (g), lung adenocarcinoma (h), kidney renal clear cell carcinoma (i),kidney renal papillary cell carcinoma (j), kidney chromophobe (k), head and neck squamous cell carcinoma (l), glioblastoma multiforme (m), esophageal carcinoma (n), colon carcinoma (o), cholangiocarcinoma (p), cervical squamous carcinoma (q), breast invasive carcinoma (r), liver hepatocellular carcinoma (s), bladder urothelial carcinoma (t)
Fig. 3.
Kaplan–Meier analyses of overall survival according to FOXM1 expression levels in different cancer suffers, including adrenocortical carcinoma (a), glioma (b), colon adencarcinoma (c), kidney chromophobe (d),kidney renal clear cell carcinoma (e),kidney renal papillary cell carcinoma (f),liver hepatocellular carcinoma (g),lung adenocarcinoma (h),ulterine corpus endometrial carcinoma (m),uveal melanoma (n)
In this review, we provide an overview of how FOXM1 is regulated and focus on the transcriptional, post-transcriptional, post-translational, and protein-protein/RNA interaction levels. Though many biomolecules regulate the expression of FOXM1, we emphasize the biomolecules that directly interact with or modify the promoter, RNA, or protein of FOXM1. At the same time, we will discuss briefly the pharmacological inhibition of FOXM1.
Transcriptional regulation of FOXM1
The core promoter regions of the FOXM1 gene contain several classic regulatory elements, such as E-boxes, and other cis-acting elements that can function as responsive elements to other transcription factors. Here, we mainly discuss the transcription factors that directly bind to the promoter regions (which are verified by electrophoretic mobility shift assays and/or chromatin immunoprecipitation assays) (Fig. 1b).
Most responsive elements are located adjacent to FOXM1’s transcriptional start sites, though the furthest element is found approximately 2000 bp away. More than 75% of these binding sites act as cis-activation elements, but they do not all function through the same basic mechanisms. For example, glioma-associated oncogene homolog 1 (Gli1), CCCTC-binding factor (CTCF), cAMP responsive element-binding protein (CREB), signal transducer and activator of transcription 3 (STAT3), and E2F interact directly with their binding sites and upregulate FOXM1 expression [9, 30–34]. In human colorectal cancer (CRC), Gli1 regulates FOXM1 by directly binding to its promoter at BS4 (GCCCACCCA), which contributes to the proliferation of CRC cells [9]. The DNA-binding protein, CTCF, may regulate the motility and invasiveness of primary hepatocellular carcinoma (HCC) cells via interaction with the CTCF-binding site(s) located in the proximal promoter of FOXM1 [30]. In HBV-associated HCC, CREB directly bound to the FOXM1 promoter in response to HBx and facilitates hepatoma cell invasion and metastasis [33]. In the chronic myeloid leukemia K562 cell line, FOXM1 is transcriptionally dependent on STAT3 and crucial for its cell proliferation, cell cycle checkpoints and viability [32]. However, twist-related protein 1 (Twist1) may not be sufficient to enhance FOXM1 gene transcription unless it recruits the coactivator p300 to form a complex. In gastric cancer, Twist 1 could bind to the promoter region of FOXM1, and subsequently recruit p300 to induce its mRNA transcription [35]. Other transcription factors are stress-dependent. For example, under normoxic conditions, HIF-1α is unstable because specific proline residues are hydroxylated. Under hypoxic conditions, HIF-1α directly binds to the promoter of FOXM1 and activates its expression. Consequently, the upregulation of FOXM1 promotes the proliferation of hepatoma cells and enhances their resistance to apoptosis [36, 37]. Moreover, Dai B et al. reported that upon heat shock stress HSF1 was released from Hsp90 and translocated from cytoplasm to nucleus. Then HSF1 directly bound to FOXM1 promoter and increased FOXM1 promoter activity [38]. They also revealed that overexpression of a constitutively active HSF1 up-regulated FOXM1 expression in Hs683 cells, indicating that HSF1 may also regulate FOXM1 expression in glioma cell lines under normal conditions [38]. Taken together, the evidence suggests that HSF1 regulates the FOXM1 expression under both heat shock stress and normal conditions.
The FOXM1 promoter region also contains sites that can function as cis-suppression elements. For example, liver X receptor a (LXRa) functions as a transcriptional repressor for FOXM1 expression by binding to an inverted repeat IR2 (-52CCGTCAcgTGACCT-39) in the FOXM1 promoter region, and suppresses the proliferation of HCC cells [39]. Barsotti AM et al. reported that p53-mediated inhibition of FOXM1 is partially p21 and retinoblastoma (RB) family dependent in MCF-7 cells [40], while FOXM1 was demonstrated as direct target gene of p53 in mice hepatocytes [41]. Some responsive elements have dual roles, such as the ERE (estrogen-response element) and the E-box within the proximal promoter region of the FOXM1 gene. In ERα-positive breast cancer cells, ERα can upregulate FOXM1 expression by binding to the ERE. Conversely, ERβ binding to the ERE down-regulates FOXM1 expression [42, 43]. In a similar fashion, the E-box is a competitive binding site for c-Myc (activator) and Mxi1-SRα (repressor) [44, 45].
It is worth noting that, there exists a positive auto-regulation loop of FOXM1. In 2009, Marianna Halasi first found that siomycin A and thiostrepton, transcriptional inhibitors of FOXM1, downregulated both the transcriptional activity and expression levels of FOXM1 [46]. The phenomenon indicates the existence of an auto-regulation loop for FOXM1. Subsequently, a research study showed that disruption of FOXM1 binding site inhibited FOXM1 promoter activity, confirming that the − 745/− 738 bp region is required for the auto-regulatory activation of the FOXM1 promoter [47, 48]. Furthermore, it has been reported that nuclear AURKA can be recruited by FOXM1 as a co-factor to transactivate FOXM1 target genes in a kinase-independent manner [49]. AURKA and FOXM1 participated in a tightly coupled positive feedback loop to enhance BCSC phenotype. Moreover, AURKA can effectively transactivate the FOXM1 promoter through a Forkhead response element, whereas FOXM1 can activate AURKA expression at the transcriptional level [49]. All these findings confirm the existence of an autoregulatory loop, suggesting that FOXM1 protein can potentially bind to FOXM1 promoter region.
Herein, we have summarized all the reported transcription activators and repressors that directly bound to FOXM1 core promoter (Table 1). This will not only benefit for the discovery of novel transacting factors during the regulatory region of FOXM1 promoter, but it will also provide important implications for the design of drugs targeting FOXM1.
Table 1.
Factors reported to bind directly to FOXM1 promotor and regulated its expression
| Transcription factor | Responsive element | Act. / Rep. | Cell | Coactivator | Pos. / Neg. regulation |
|---|---|---|---|---|---|
| cMyc [109, 110] | E-Box | A | U-937 etc. | CAR [44]/− | |
| Mxi1-SRα [45] | E-Box | R | DL23 | -/FOXO3a [111] | |
| HIF-1α [36] | −271/− 267, − 47/− 42 | A | HepG2 | TNF-α [37]/− | |
| ERα [42] | −45 | A | MCF-7 ZR-75-1 | -/HDACs | |
| ERβ [43] | −45 | R | MCF-7 | ||
| E2F [34] | −24 | A | MCF-7 | MnSOD [31]/P53 [34, 112] | |
| STAT3 [32] | −440/− 432 | A | K562 | MAPK/− | |
| CREB [33] | −60/− 36 | A | HepG2 | HBx/− | |
| HSF1 [38] | − 1792/− 1767 | A | U-87MG | ||
| Twist1 [35] | − 375/− 352 | A | NCI-N87 | P300 | |
| LXRa [39] | −52/− 39 | R | HepG2 | ||
| Gli1 [9, 10] | −216/− 204 | A | HT29 | ||
| Gli1, Gli2 [8] | Not clear | A | Murine NSCs | ||
| SPDEF [47] | −670/− 660 | R | TRAMP-C2R3 | ||
| FOXM1 [46, 47] | −745/− 660, − 27/− 22 | A | TRAMP-C2R3, MCF-7 | AURKA [49]/− | |
| CTCF [30] | − 296/− 120 | A | HepG2 | ||
| Sp1 [95] | − 891 | A | HepG2 | ||
| P53 [41] | Not clear | R | mice hepatocyte |
Act. /Rep. Activator or repressor, Pos. /Neg. positive or negative regulatory factor, A activate, R repress
Post-transcriptional regulation of FOXM1
In addition to the normal processes of post-transcriptional splicing and modification, there are other mechanisms by which FOXM1 can be regulated post-transcriptionally. For example, there are a number of non-coding RNAs (ncRNAs) considered to be important in this regulation. MicroRNAs (miRNAs) are endogenous, highly conserved, non-coding RNAs of approximately 21–24 nucleotides that can guide mRNA degradation or repress translation by binding to complementary sequences in the 3′ untranslated regions (3’UTRs) of targeted mRNAs [50]. Long non-coding RNAs (lncRNAs) are ncRNAs longer than 200 nucleotides that can act as competing endogenous RNAs (ceRNAs). The ceRNAs, known as miRNA “decoys” or “sponges”, are RNA transcripts that competitively bind to the same miRNA via base pair complementarity with miRNA recognition/response elements (MREs) [51]. MicroRNAs and lncRNAs regulate each other through the binding sites of their response elements (MREs) [52, 53].
To date, dozens of miRNAs have been found to regulate the proliferation, invasion, migration, senescence, apoptosis, epithelial-mesenchymal transition (EMT), and drug sensitivity of cancer cells through binding to the 3’UTRs of FOXM1 mRNA. For example, miRNA-214 acts as a tumor repressor during the process of migration, and invasion, and is associated with sensitivity to cisplatin in cervical cancer via directly binding to the 3’UTRs of FOXM1 mRNA [54]. MirRNA-149 can inhibit EMT in non-small cell lung cancer cells (NSCLC cells) by the same mechanism (Table 2) [55]. Compared with miRNAs, few lncRNAs have been identified to regulate FOXM1 expression. In gallbladder cancer (GBC), LncRNA H19 upregulates FOXM1 expression and promotes its proliferation and invasion, through competitively ‘sponging’ miR-342-3p [56, 57]. Another lncRNA, colon cancer-associated transcript 2 (CCAT2), upregulates FOXM1 expression and promotes HCC cell growth through interaction with and suppression of miR-34a [58].
Table 2.
Non-coding RNAs interaction with FOXM1 transcript
| MicroRNA | mechanism | CeRNA | Physiological context | Cancer |
|---|---|---|---|---|
| miR-216b | 3′UTR | proliferationb, invasionb | Glioblastoma [113], melanoma [114], hepatocellular carcinoma [115] | |
| MiR-214 | 3′UTR | proliferationb, invasionb, drug sensitivitya | cervical cancer [54] | |
| miR-361-5p | 3′UTR | proliferationb, invasionb | lung cancer [116] | |
| miR-342 | 3′UTR | proliferationb, migrationb | colorectal cancer [117] | |
| miR-671-5p | 3′UTR | proliferationb,invasionb,EMTb | breast cancer [118] | |
| miR-149 | 3′UTR | drug sensitivitya, EMTb | Colorectal Cancer [55], non-small cell lung cancer [119] | |
| miR-509-5p | 3′UTR | proliferationb, migrationb, invasionb | non-small cell lung cancer [120] | |
| miR-802 | 3′UTR | proliferationb | breast cancer [121] | |
| miR-34a | 3′UTR | CCAT2 [58] | senescencea | hepatocellular carcinoma [122] |
| miR-370 | 3′UTR | proliferationb,apoptosisa | osteosarcoma [123], laryngeal squamous cell carcinoma [124], gastric carcinoma [125] | |
| miR-877 | 3′UTR | proliferationb | hepatocellular carcinoma [126] | |
| miR-320 | 3′UTR | drug sensitivitya, radiosensitivitya | colon cancer [127], Glioma [128] | |
| miR-204 | 3′UTR | invasionb, EMTb | esophageal cancer [129] | |
| miR-24-1 | 3′UTR | proliferationb | bladder cancer [130] | |
| miR-342-3p | 3′UTR | H19 [57] | proliferationb, migrationb, invasionb | cervical cancer [56] |
a: promoting
b: Inhibition
In addition, lncRNAs also regulate nascent FOXM1 transcripts. For example, lncRNA FOXM1-AS interacts with nascent FOXM1 transcripts, promoting the ALKBH5-mediated demethylation and subsequent FOXM1 up-regulation. Besides, with the emerging research of the non-coding RNAs, including the circular RNA, piwi-interacting RNA, snRNA and snoRNA, the possible regulation between these non-coding RNAs and FOXM1 require further study for determination.
Post-translational modifications of FOXM1
Post-translational modifications (PTMs) are the chemical modifications of a protein after its translation, which can have broad effects on the targets. The FOXM1 protein is modified by multiple PTMs that include phosphorylation, ubiquitination, SUMOylation, acetylation and methylation, which may have activating or inhibiting effects. These modifications determine the cellular localization, protein stabilization, and transcriptional activity of FOXM1 in normal or disease states (Table 3).
Table 3.
Post-translational modifications of FOXM1
| PTM | Enzyme | Effect | Cell cycle | FOXM1 isoform |
|---|---|---|---|---|
| Phosphorylation | ||||
| T620,T627,S672 | Cyclin D-CDK4/6[6] | Transcriptional activitya stabilizationa |
G1 → S | FOXM1c |
| S331 | Raf/MEK/MAPK [60] | Nuclear translocationa | Late S | FOXM1c |
| S704 | Raf/MEK/MAPK [60] | Transcriptional activitya | Late S | FOXM1c |
| T600,T611,S638 | Cyclin A/E-Cdk2 [62, 63] | Transcriptional activitya | G2/M | FOXM1c |
| T596 | Cyclin B1-Cdk1 [64] | Transcriptional activitya | G2/M | FOXM1b |
| S678 | Cyclin B1-Cdk1 [65] | Transcriptional activitya | Late G2 | FOXM1b |
| S507,S657, T585 | Cyclin B1-Cdk1 [131] | Transcriptional activitya | G2/M | FOXM1b |
| S715,S724 | Plk1 [64] | Transcriptional activitya | G2 → M/M | FOXM1b |
| S361 | Chk2 [66] | stabilizationa | DDR | FOXM1b |
| S251 | c [59] | CDK1-dependent phosphorylationa | G2/M | FOXM1b |
| S474 | GSK3 [67] | degradationa | FOXM1b | |
| Ubiquitination | ||||
| - | APC/C-Cdh1 [71, 72] | degradationa | Late M/Early G1 | FOXM1 |
| - | CRL4-VprBP [73] | degradationa | G1/S | FOXM1 |
| - | SCF/FBXO31 [74] | degradationa | G2 → M | FOXM1 |
| - | SCF/FBXL2 [132] | degradationa | FOXM1 | |
| -(K48) | FBXW7 [67] | degradationa | FOXM1 | |
| -(K48) | RNF8,RNF168 [75] | degradationa | (DDR) | FOXM1 |
| De-ubiquitination | ||||
| - | USP5 [67] | Nuclear translocationa | FOXM1 | |
| (K48) | OTUB1 [76, 77] | degradationb | FOXM1 | |
| SUMOylation | ||||
| Lys132,144,201,218,356,368,415,440,443,460,478,495(SUMO2) | c [78] | Transcriptional activitya Nuclear translocationa |
G2/M | FOXM1c |
| Lys463(SUMO1) | PIASy [79] | Transcriptional activitya | FOXM1b | |
| Lys201,218,460,478,495(SUMO1) | Ubc9 [80] | Cytoplasmic translocationa Cdh1-mediated degradationa |
(CDR) | FOXM1c |
| -(SUMO1,2,3) | Ubc9,PIAS1 [81] | Cytoplasmic translocationa stabilizationb |
FOXM1b | |
| Lys201,218,341,445,462,480(SUMO1) | c [68] | Cytoplasmic translocationa stabilizationb |
Late M | FOXM1b |
| Acetylation | ||||
| Lys63,422,440,603,614 | CBP/p300 [82] | stabilizationa DNA-binding abilityaTranscriptional activitya |
G2/M | FOXM1c |
| Methylation | ||||
| - | SETD3 [83] | Transcriptional activityb | FOXM1 | |
K48 Lys48-linked poly-ubiquitin chains, DDR DNA-damage response, CDR cytotoxic drug response
a: Promoting
b: Inhibition
c: not clear
→ Transition
Phosphorylation
The activity of FOXM1 is of great importance to the cell cycle, and phosphorylation of FOXM1 protein plays a key role in that activity. The transcriptional activity of FOXM1 is upregulated through the cell cycle and is consistent with its phosphorylation [59]. With the progress of the cell cycle, FOXM1 phosphorylation is constantly changing. The FOXM1 protein maintains a relative hypo-phosphorylation status in the G1/S phase, exhibits increased phosphorylation from the S phase to the G2/M transition, reaches hyper-phosphorylation status in the M phase, and is subsequently dephosphorylated in the late M phase. This dynamic and tight phosphorylation change is mediated by various kinases and their positive feedback loops.
The transactivation domain (TAD) of FOXM1 can be suppressed by direct interaction with the NRD (N-terminal repression domain). To a transcription factor such as FOXM1, sufficient protein levels, nuclear localization and exposure of the TAD are indispensable for maximizing transcriptional activity. In the G1/S phase, FOXM1 mRNA reaches its peak while the FOXM1 protein exhibits low transcriptional activity due to cytoplasmic localization and NRD inhibition of the TAD [60]. In the late G1 phase, Cyclin D-CDK4/6 complexes phosphorylate multiple sites of FOXM1, including T620, T627, and S672, which then triggers the G1 to S cell cycle transition [6]. Interestingly, during this process, B55α (a subunit of protein phosphatase 2A) prevents premature activation of FOXM1 through contact with FOXM1 and repression of cyclin A-CDK [6, 61]. In the late S and G2/M phases, phosphorylation of both S331 and S704 of FOXM1 via the Raf/MEK/MAPK pathway stimulates FOXM1 nuclear translocation and thus promotes the transcriptional activity of FOXM1 [60]. During the G2 phase, cyclin A/E-CDK2 complexes phosphorylate FOXM1, including sites T600, S638, and especially T611, which relieves repression of TAD by NRD, and restores the TAD transactivation activity [62, 63]. In addition, phosphorylation at S251 is critical for cyclin-B1-Cdk1-dependent phosphorylation of FOXM1 [59]. The phosphorylation at T596 by cyclin-B1-Cdk1 on the one hand recruits Plk1 directly to phosphorylate FOXM1 at S715 and S724, which promotes the transcriptional activity of FOXM1 [64]. On the other hand, that phosphorylation recruits the transcriptional co-activator p300/CREB binding protein (CBP) to enhance its transcriptional activity [65].
In response to DNA damage, checkpoint kinase 2 (Chk2) phosphorylates FOXM1 at S361, inhibiting its degradation and increasing transcription of XRCC1 and BRCA2 genes, which are required for repair of DNA damage [66].
FOXM1 phosphorylation is also linked to ubiquitination and SUMOylation. For example, GSK3 phosphorylates FOXM1 at the S474 site, which promotes its subsequent ubiquitin-mediated degradation by FBXW7 [67]. Plk1-mediated phosphorylation of FOXM1 antagonizes its SUMOylation and facilitates cell cycle progression [68].
Ubiquitination and de-ubiquitination
Ubiquitin (Ub) is a 76-amino acid protein with seven lysine residues that can conjugate to substrate proteins and form a poly-ubiquitin chain, conferring a range of functions. For example, the K48- and K11-linked poly-ubiquitin chains lead to proteolysis of the substrate protein, while the K63-linked poly-ubiquitin chain functions in signal transduction [69]. Ubiquitination is an enzymatic PTM in which an ubiquitin protein is attached to a target protein. De-ubiquitination opposes the role of ubiquitination by removing ubiquitin from substrate proteins.
The N-terminus of the FOXM1 protein contains KEN box (K-E-N-X-X-X-N) and destruction box (R-X-X-L-X-X-X-X-N) sequences that are involved in its ubiquitin-mediated degradation. The KEN box was first found to be an anaphase promoting complex (APC) recognition signal [70], which is responsible for the Cdh1-APC-mediated ubiquitination. Both the KEN box and destruction box (D box) of FOXM1 can be recognized by some ubiquitin protein ligases. In the late M and early G1 phases,Cdh1 interacts with FOXM1 and recruits APC/C E3 ubiquitin ligases to degrade it, which inhibits cell cycle progression [71, 72]. The E3 ligase system is delicate in its regulation of FOXM1 during the cell cycle. For example, in the G1/S phases, E3 ubiquitin ligase CRL4 integrates with its receptor VprBP, which promotes FOXM1 degradation to maintain its relatively low level. In G2/M phase, when FOXM1 is indispensable, VprBP separates from CRL4, relinquishing its inhibition of FOXM1 [73]. During the G2/M transition, SCF/FBXO31 E3 ubiquitin ligases act as negative regulators of FOXM1 via ubiquitin-mediated degradation, which can maintain genomic stability [74].
FOXM1 ubiquitination is also linked to SUMOylation. For example, RNF168 can modulate DNA-damage response (DDR) by promoting protein ubiquitination. In breast cancer treated with epirubicin, FOXM1 is modified through SUMOylation, which leads to its ubiquitination and degradation by RNF168 E3 ubiquitin ligase [75].
In contrast, deubiquitinating enzymes (DUBs) can remove the poly-ubiquitin chains on FOXM1 protein. For instance, in epirubicin-resistant breast cancer and in ovarian cancer, OTUB1 catalyzes the cleavage of the K48-specific ubiquitin linkage from FOXM1, which promotes cancer progression via facilitating cell proliferation and drug resistance [76, 77].
SUMOylation
Small Ubiquitin-like Modifier (SUMO) proteins are small proteins that are covalently attached to other proteins and modify their function. In mammals, there are four SUMO isoforms, SUMO-1, SUMO-2, SUMO-3, and SUMO-4. SUMOylation is an important PTM of FOXM1 that regulates its activity, stability, and other PTMs. The functions of FOXM1 SUMOylation are diverse and may regulate its activity in an isoform-specific manner.
Multiple sites of FOXM1 are modified by SUMO-2, and this modification peaks in the M phase, which is consistent with its phosphorylation. This modification blocks the dimerization and relieves the auto-repression of FOXM1, thereby increasing its transcriptional activity [78]. Another SUMOylation of FOXM1 at K463 by SUMO-1 is also required for its transcriptional activity [79].
In contrast, SUMOlyation of FOXM1 at different sites may inhibit its activity. For instance, FOXM1c is modified by SUMO-1 at multiple sites, which promotes the cytoplasmic translocation of FOXM1c and enhances APC/Cdh1-dependent ubiquitin-mediated degradation. This modification subsequently attenuates the transcriptional activity of FOXM1c [80]. This phenomenon has been confirmed with another FOXM1 isoform-FOXM1b [81].
Other PTMs of FOXM1
FOXM1 is also regulated by acetyltransferases and methyltransferases. For instance, FOXM1 can be acetylated by p300/CBP at lysines K63, K422, K440, K603 and K614, which enhances its transcriptional activity by promoting its DNA binding affinity, protein stability, and phosphorylation sensitivity [82]. Under normoxic conditions, methyltransferase SETD3 specifically binds and methylates FOXM1, which inhibits its activity [83].
In general, we can conclude that the FOXM1 protein can be modified by multiple PTMs, including phosphorylation, ubiquitination, SUMOylation, acetylation, and so on. It has been demonstrated that these PTMs can result in the spatiotemporal control of target protein expression. This may provide new strategies for the modulation of FOXM1 expression utilizing the key enzymes involved in these PTMs.
Protein/RNA directly interacts with FOXM1 protein
The protein-protein/RNA interactions of FOXM1 are discussed in detail in a recently published review [84]. This article revisits these interactions and focuses on molecules that directly interact with FOXM1 (Table 4).
Table 4.
proteins/RNA interact with FOXM1
| Protein/RNA | Binding site | Effect | Cell cycle | FOXM1 isoform |
|---|---|---|---|---|
| B55α [61] | a | CyclinA CDK2 activity↓ | G1 | FOXM1c |
| RB [85] | 359/425 | Transcriptional activity↓ | G1 | FOXM1c |
| p19ARF [86] | 688/748 | Transcriptional activity↓ | FOXM1b | |
| NPM [87] | 195/688 | Nuclear translocation↑ | FOXM1b | |
| CDC25A [89] | C-terminal | CDK1 activity↑ | FOXM1 | |
| PHGDH [133] | N-terminal | Stabilization↑ | FOXM1 | |
| MELK [88] | N-terminal | Plk1 activity↑ | G2 → M/M | FOXM1 |
| Pin1 [134] | S331,704 | Transcriptional activity↑ | FOXM1 | |
| MTDH [90] | N-terminal | Stabilization↑ transcriptional activity↑ | FOXM1b | |
| PVT1(lncRNA) [91] | a | Stabilization↑ | FOXM1 | |
| HSP70 [135] | a | Transcriptional activity↓ | FOXM1 |
CD central domain
a not clear
↓ Decrease
↑ Increase
→ Transition
In the G1 phase, RB acts as a repressor by binding directly to the central domain of FOXM1, which may recruit NRD to inhibit TAD. Cyclin D1/Cdk4 activates FOXM1 by releasing its TAD from repression by RB, which might lead to deregulated proliferation and cancer [85]. Another tumor suppressor p19ARF interacts directly with the C-terminal (688–748) of FOXM1, and decreases FOXM1 transcriptional activity. At the same time, the interaction also diminishes FOXM1 stimulation of colony formation of U2OS cells, suggesting that p19ARF may be an effective therapeutic inhibitor of FOXM1 transformation function [86].
Several proteins can increase FOXM1 activity by promoting its stabilization, nuclear location, and phosphorylation or inhibiting its ubiquitination. For example, in cancer cells nucleophosmin (NPM) interacts with FOXM1 and their interaction is required for sustaining the level and nucleus localization of FOXM1 [87]. In glioma stem-like cell (GSC), maternal embryonic leucine-zipper kinase (MELK) increases the activity of FOXM1 by interaction with its N-terminus and promoting its phosphorylation by Plk1 [88]. As mentioned above, PLK1 binds and phosphorylates FOXM1 leading to its activation and increased gene expression, which are required for mitotic progression. In addition, cell division cycle 25A (CDC25A) interacts with the C-terminus and enhances CDK1-dependent phosphorylation of FOXM1 [89]. This phosphorylation is required to release the inhibitory function of the NRD during G2 phase (as mentioned above). Another protein, metadherin/astrocyte elevated gene-1 (MTDH), directly interacts with FOXM1 via the N-terminal inhibitory domain of MTDH, and this interaction disrupts the binding of Cdh-1 (mentioned above) to FOXM1, thus protecting FOXM1 from subsequent proteasomal degradation. At the same time, MTDH also binds to FOXM1 target gene promoters and enhances its transcriptional activity. All these interactions promote cell cycle progression, angiogenesis, and cancer cell invasion in vitro and in vivo [90].
As mentioned above, lncRNA can act as an oncogenic factor through the regulation of the transcriptional level of FOXM1. Interestingly, the lncRNA PVT1 can bind to FOXM1 protein and elevate its levels by reducing its degradation and enhancing its stability, subsequently promoting the proliferation and invasion of gastric cancer cells [91]. This implies that noncoding RNA can not only function at the transcriptional level, but also play a role in other processes. The clarification of the interaction binding sites between FOXM1 and other proteins will provide implication for the design of short peptides and small molecular targeting FOXM1.
Pharmacological inhibition of FOXM1
Significant progress has been accomplished over the last few years in terms of the pharmacological inhibition of FOXM1 in cancer [92–94]. It is apparent, as outlined in the above discussion, that the inhibition of FOXM1 expression (at the levels of transcription, translation, and post-translation) and/or its interactions with target sites (block DBD, nuclear localization, protein-protein interaction) may be an effective way to inhibit FOXM1-mediated biological effects (Table 5). For example, Sp1 directly binds to the promotor of FOXM1 and activates its transcription. Thiazolidinedione (TZD) inhibits FOXM1 expression through downregulation of Sp1, which may negatively regulate tumor cell growth and promote apoptosis [95]. Diarylheptanoids, from medicinal plants, can also suppress FOXM1 and expression of its target genes by suppressing Gli1 in pancreatic cancer cells [96].
Table 5.
Inhibitors of FOXM1 whose mechanism has been elucidated
| Inhibitors | Mechanism | Effect | Cancer cells |
|---|---|---|---|
| Thiazolidinediones | Inhibit Sp1 | mRNA exprssionb | hepatoma [95] |
| Diarylheptanoids | Inhibit Gli1 | mRNA and protein exprssionb | pancreatic cancer [96] |
| RCM-1 | increase ubiquitination | protein degradationa | osteosarcoma [99] |
| Thiostrepton | Interact with FOXM1 | binding of FOXM1 to target sitesb | breast cancer [98] |
| honokiol | Interact with FOXM1 | binding of FOXM1 to target sitesb | osteosarcoma [100] |
| FDI-6 | Block FOXM1 DBD | binding of FOXM1 to target sitesb | MCF-7[128] |
| FOXM1 Apt | target FOXM1 DBD | binding of FOXM1 to target sitesb | HEK 293 T [136] |
| Peptide 9R-P201 | target FOXM1 DBD | FOXM1 and garget gene expressionb | HepG2 [137] |
a: Promoting;
b: Inhibition
That said, the biological effects of targeting transcription factors are diversified, and it may not be the best therapeutic solution. The siRNA and ARF peptide target FOXM1 is relatively specific and effective, but the drug-targeted delivery and immune responses may be a major obstacle to be clinical use. Another classical FOXM1 inhibitor thiostrepton, a thiazole antibiotic, inhibits FOXM1 through interacting directly with FOXM1 protein and acting as a proteasome inhibitor [97, 98]. Several recent studies have found that small molecule inhibitors work well to inhibit FOXM1. Sun L et al. identified a small molecule RCM-1 by high-throughput screen, which blocks the nuclear localization and increases the proteasomal degradation of FOXM1 with less effect on other FOX family transcription factors [99]. Another small molecule, honokiol, inhibits FOXM1 by specific binding in a way that is structurally strict [100]. Although much work has been done, there is much more to accomplish to identify specific FOXM1 inhibitors and to validate them in clinical trials. These FOXM1 inhibitors may be used as single agents or in combination with low-dose chemotherapy for cancer treatment.
FOXM1 in the tumor microenvironment
The tumor microenvironment is created by the tumor and dominated by tumor-induced interactions. Although various immune effector cells are recruited to the tumor site, their anti-tumor functions are suppressed. Infiltrates of inflammatory cells present in human tumors are chronic in nature and are enriched in regulatory T cells (Treg) as well as myeloid suppressor cells (MSC) [101]. Immunotherapeutic strategies, including cancer vaccines, oncolytic viruses, adoptive transfer of ex vivo activated T and natural killer cells, and administration of antibodies or recombinant proteins, are now being described at a breathtaking pace, especially after the clinical application of the monoclonal antibody blocking of cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD1) [102]. Few research have revealed the role of FOXM1 in immune cells: FOXM1 may be involved in the determination of induced Tregs (iTreg) versus Teff development during T cell differentiation [103]. Furthermore, latest reports revealed that FOXM1 modulates atherosclerosis by inducing macrophage proliferation [104]. However, little is known about the possible role of FOXM1 in tumor microenvironment. Interestingly, FOXM1 (362–370) (YLVPIQFPV), FOXM1(373–382) (SLVLQPSVKV), and FOXM1(640–649) (GLMDLSTTPL) peptides primed HLA-A2-restricted cytotoxic T lymphocytes (CTLs) in the HLA-A2 transgenic mice, suggesting that FOXM1 may be a suitable target for immunotherapy against cancers. However, the HLAA2-restricted epitopes of FOXM1 identified need to be further clinically tested [105]. As the important role of FOXM1 in cell proliferation and determination of cell fate, more study is needed to reveal the possible role of FOXM1 in the tumor infiltrating immune cells.
Conclusions and future perspectives
FOXM1 is a crucial regulator of many biological processes and tissues, and dysregulation of FOXM1 can significantly contribute to tumorigenesis and cancer progression. For its potential as a target for cancer therapies, FOXM1 was named the Molecule of the Year in 2010 [106]. Over the past few decades, understanding of the regulation and function of FOXM1 has rapidly increased, providing new insights into the roles of this transcription factor in cancer and other diseases. At the same time, some small molecule inhibitors that target FOXM1 have promising potential as drugs for cancer treatment [107, 108]. However, there are important challenges that limit the translation of promising drugs into clinical practice. Before the entry of FOXM1 inhibitors into clinical trials, more thorough preclinical studies on their anti-tumor efficacy are still needed. In addition, the toxicity of the above drugs should also be fully evaluated. It is not quite clear how the interaction and isoforms switch between FOXM1a and FOXM1b or FOXM1c or FOXM1d. It also remains unclear how the isoforms of FOXM1 interact and what role they may play in the regulation of FOXM1, in disease progression, or in response to relevant therapeutic strategies. Importantly, although the crystal structure of the FOXM1 DNA-recognition domain has been fully identified [7], it is vital that the complete structure of the FOXM1 protein be elucidated. This will be of utmost importance for the discovery of novel FOXM1 inhibitors.
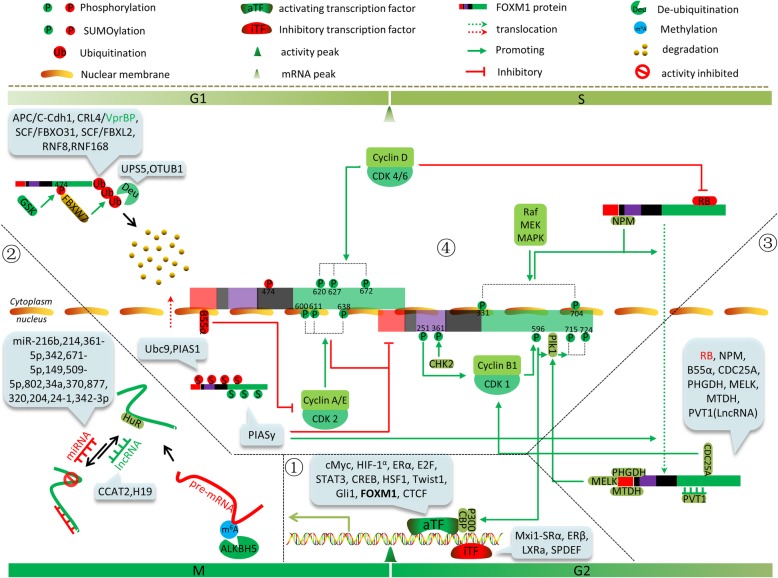
In this review, we have summarized many of the activators and repressors that directly interact with or modify FOXM1 at multiple levels and drew the FOXM1-interaction network diagram (Fig. 4). The comprehensive understanding of the regulation of FOXM1 will provide a basis for further investigation, which may provide new potential therapeutic strategies.
Fig. 4.
Schematic diagram of FOXM1 regulation network. This schematic diagram attempts to address the regulation of FOXM1 in a spherical way. Green: promotion of FXOM1 expression or transcriptional activity; Red: repression of FOXM1 expression or transcriptional activity. ①.Transcriptional regulation of FOXM1: iTF (inhibitory transcription factor) binds to the promoter region of FOXM1 and inhibits its transcription. aTF (activating transcription factor) binds to the promoter region of FOXM1 and enhances its transcription. ②.Post-transcriptional regulation of FOXM1: m6A methylation of FOXM1 pre-mRNA and the miRNAs binding to 3′UTRs of FOXM1 mRNA and guiding FOXM1 are listed. lncRNAs can act as ceRNAs and block the suppression of FOXM1 by miRNAs. ③. Protein/RNA directly interacts with FOXM1 protein: The interaction of protein/RNA with FOXM1 protein alter the cellular localization, stabilization, or transcriptional activity of FOXM1. ④. Post-translational modifications of FOXM1 protein
Acknowledgements
We apologize to those colleagues whose work has not been cited due to space limitation.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81773141, to C. Hu) and Basic and Frontier Research Project of Chongqing, Science and Technology Commission (No. cstc2016jcyjA1930, to C. Hu).
Abbreviations
- APC/C
Anaphase-promoting complex-cyclosome
- AURKA
Aurora kinase A
- CCAT2
Colon cancer-associated transcript 2
- CDC25A
Cell division cycle 25A
- ceRNAs
Competing endogenous RNAs
- Chk2
Checkpoint kinase 2
- CRC
Colorectal cancer
- CREB
cAMP responsive element-binding protein
- CTCF
CCCTC-binding factor
- DBD
Forkhead DNA-binding domain
- EMT
Epithelial-mesenchymal transition
- ERE
Estrogen-response element
- FBXW7
F-box/WD repeat-containing protein 7
- FDI-6
A small molecule compound
- FOXM1 Apt
FOXM1-specific single stranded DNA aptamer
- FOXM1
Human Forkhead Box M1
- GBC
Gallbladder cancer
- Gli1
Glioma-associated oncogene homolog 1
- GSC
Glioma stem-like cell
- HCC
Hepatocellular carcinoma
- HDAC
Histone deacetylases
- HIF-1
Hypoxia-inducible factor 1
- HSF1
Heat shock factor 1
- lncRNAs
Long non-coding RNAs
- LXRa
Liver X receptor a
- MAPK
Mitogen-activated Protein Kinase
- MELK
Maternal embryonic leucine-zipper kinase
- miRNAs
MicroRNAs
- MnSOD
Manganese-dependent superoxide dismutase
- MREs
Response elements
- MTDH
Metadherin/astrocyte elevated gene-1
- ncRNA
Non-coding RNA
- NPM
Nucleophosmin
- NRD
N-terminal repressor domain
- p19ARF
19-kD alternative reading frame (ARF) protein
- Peptide 9R-P201
A peptidomimetics from the phage random library
- RCM-1
Robert Costa Memorial drug–1
- SETD3
SET domain-containing protein
- STAT3
Signal transducer and activator of transcription 3
- SUMO
Small Ubiquitin-like Modifier
- TAD
C-terminal transactivation domain
- TNF-α
Tumor necrosis factor alpha
- Twist1
Twist-related protein 1
Authors’ contributions
G-BL, X-ZL, SZ, CL, S-MY, LY, C-JH, and J-YB were involved in the conception, design and drafting of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guo-Bin Liao, Email: 393724944@qq.com.
Xin-Zhe Li, Email: lixinzhe73@126.com.
Shuo Zeng, Email: 742721586@qq.com.
Cheng Liu, Email: chenghistory@163.com.
Shi-Ming Yang, Email: shimingyang@yahoo.com.
Li Yang, Email: yl_101@163.com.
Chang-Jiang Hu, Email: hcj6699@yahoo.com.
Jian-Ying Bai, Email: drbaijy2015@163.com.
References
- 1.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 2.Korver W, Roose J, Heinen K, Weghuis DO, de Bruijn D, van Kessel AG, Clevers H. The human TRIDENT/HFH-11/FKHL16 gene: structure, localization, and promoter characterization. Genomics. 1997;46:435–442. doi: 10.1006/geno.1997.5065. [DOI] [PubMed] [Google Scholar]
- 3.Zona S, Bella L, Burton MJ, Nestal de Moraes G, Lam EW-F. FOXM1: an emerging master regulator of DNA damage response and genotoxic agent resistance. Biochim Biophys Acta. 2014;1839:1316–1322. doi: 10.1016/j.bbagrm.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zhang L, Du Y, Zheng H, Zhang P, Sun Y, Wang Y, Chen J, Ding P, Wang N, Yang C, Huang T, Yao X, Qiao Q, Gu H, Cai G, Cai S, Zhou X, Hu W. A novel FOXM1 isoform, FOXM1D, promotes epithelial-mesenchymal transition and metastasis through ROCKs activation in colorectal cancer. Oncogene. 2017;36:807–819. doi: 10.1038/onc.2016.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park HJ, Wang Z, Costa RH, Tyner A, Lau LF, Raychaudhuri P. An N-terminal inhibitory domain modulates activity of FoxM1 during cell cycle. Oncogene. 2008;27:1696–1704. doi: 10.1038/sj.onc.1210814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, Vidal M, Gygi SP, Braun P, Sicinski P. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littler DR, Alvarezfernández M, Stein A, Hibbert RG, Heidebrecht T, Aloy P, Medema RH, Perrakis A. Structure of the FoxM1 DNA-recognition domain bound to a promoter sequence. Nucleic Acids Res. 2010;38:4527–4538. doi: 10.1093/nar/gkq194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besharat ZM, Abballe L, Cicconardi F, Bhutkar A, Grassi L, Le Pera L, Moretti M, Chinappi M, D’Andrea D, Mastronuzzi A. Foxm1 controls a pro-stemness microRNA network in neural stem cells. Sci Rep. 2018;8 10.1038/s41598-018-21876-y. [DOI] [PMC free article] [PubMed]
- 9.Wang D, Hu G, Du Y, Zhang C, Lu Q, Lv N, Luo S. Aberrant activation of hedgehog signaling promotes cell proliferation via the transcriptional activation of forkhead box M1 in colorectal cancer cells. J Exp Clin Cancer Res. 2017;36:23. doi: 10.1186/s13046-017-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teh M-T, Wong S-T, Neill GW, Ghali LR, Philpott MP, Quinn AG. FOXM1 is a downstream target of Gli1 in basal cell carcinomas. Cancer Res. 2002;62:4773–4780. [PubMed] [Google Scholar]
- 11.Nandi D, Cheema PS, Jaiswal N, Nag A. FoxM1: repurposing an oncogene as a biomarker. Semin Cancer Biol. 2017; 10.1016/j.semcancer.2017.08.009. [DOI] [PubMed]
- 12.Wierstra I. FOXM1 (Forkhead box M1) in tumorigenesis: overexpression in human cancer, implication in tumorigenesis, oncogenic functions, tumor-suppressive properties, and target of anticancer therapy. Adv Cancer Res. 2013;119:191–419. doi: 10.1016/B978-0-12-407190-2.00016-2. [DOI] [PubMed] [Google Scholar]
- 13.Tassi RA, Todeschini P, Siegel ER, Calza S, Cappella P, Ardighieri L, Cadei M, Bugatti M, Romani C, Bandiera E, Zanotti L, Tassone L, Guarino D, Santonocito C, Capoluongo ED, Beltrame L, Erba E, Marchini S, D’Incalci M, Donzelli C, Santin AD, Pecorelli S, Sartori E, Bignotti E, Odicino F, Ravaggi A. FOXM1 expression is significantly associated with chemotherapy resistance and adverse prognosis in non-serous epithelial ovarian cancer patients. J Exp Clin Cancer Res. 2017;36:63. doi: 10.1186/s13046-017-0536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdeljaoued S, Bettaieb I, Nasri M, Adouni O, Goucha A, El Amine O, Boussen H, Rahal K, Gamoudi A. Overexpression of FOXM1 is a potential prognostic marker in male breast Cancer. Oncol Res Treat. 2017;40:167–172. doi: 10.1159/000458156. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Liu Y, Yuan B, Yin L, Peng Y, Yu X, Zhou W, Gong Z, Liu J, He L, Li X. FOXM1 promotes the progression of prostate cancer by regulating PSA gene transcription. Oncotarget. 2017;8:17027–17037. doi: 10.18632/oncotarget.15224.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egawa M, Yoshida Y, Ogura S, Kurahashi T, Kizu T, Furuta K, Kamada Y, Chatani N, Hamano M, Kiso S, Hikita H, Tatsumi T, Eguchi H, Nagano H, Doki Y, Mori M, Takehara T. Increased expression of Forkhead box M1 transcription factor is associated with clinicopathological features and confers a poor prognosis in human hepatocellular carcinoma. Hepatol Res. 2017;47:1196–1205. doi: 10.1111/hepr.12854. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Kohashi K, Yamada Y, Iwasaki T, Maekawa A, Kuda M, Hoshina D, Abe R, Furue M, Oda Y. Prognostic significance of Forkhead box M1 (FOXM1) expression and antitumor effect of FOXM1 inhibition in Angiosarcoma. J Cancer. 2016;7:823–830. doi: 10.7150/jca.14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Zhong H, Li L, Ji W, Zhang X. Overexpressed transcription factor FOXM1 contributes to the progression of colorectal cancer. Mol Med Rep. 2016;13:2696–2700. doi: 10.3892/mmr.2016.4875. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Kohashi K, Yamada Y, Maekawa A, Kuda M, Furue M, Oda Y. Prognostic significance of forkhead box M1 (FoxM1) expression and antitumour effect of FoxM1 inhibition in melanoma. Histopathology. 2016;69:63–71. doi: 10.1111/his.12909. [DOI] [PubMed] [Google Scholar]
- 20.Kong F-F, Qu Z-Q, Yuan H-H, Wang J-Y, Zhao M, Guo Y-H, Shi J, Gong X-D, Zhu Y-L, Liu F, Zhang W-Y, Jiang B. Overexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancer. Oncol Rep. 2014;31:2660–2668. doi: 10.3892/or.2014.3129. [DOI] [PubMed] [Google Scholar]
- 21.Okada K, Fujiwara Y, Takahashi T, Nakamura Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori M, Doki Y. Overexpression of forkhead box M1 transcription factor (FOXM1) is a potential prognostic marker and enhances chemoresistance for docetaxel in gastric cancer. Ann Surg Oncol. 2013;20:1035–1043. doi: 10.1245/s10434-012-2680-0. [DOI] [PubMed] [Google Scholar]
- 22.Zhong S, Zhou A, Qi F, Li Z, Yu Z, Lu Y, Liu X. Downregulating forkhead box M1 inhibits proliferation by inhibiting autophagy in the sw480 cell line. Biomed Rep. 2017;7:47–50. doi: 10.3892/br.2017.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Niu Y, Huang C. Role of FoxM1 in the progression and epithelial to mesenchymal transition of gastrointestinal Cancer. Recent Pat Anticancer Drug Discov. 2017;12:247–259. doi: 10.2174/1574892812666170424144352. [DOI] [PubMed] [Google Scholar]
- 24.Wang K, Zhu X, Zhang K, Zhu L, Zhou F. FoxM1 inhibition enhances chemosensitivity of docetaxel-resistant A549 cells to docetaxel via activation of JNK/mitochondrial pathway. Acta Biochim Biophys Sin Shanghai. 2016;48:804–809. doi: 10.1093/abbs/gmw072. [DOI] [PubMed] [Google Scholar]
- 25.Yu C, Chen L, Yie L, Wei L, Wen T, Liu Y, Chen H. Targeting FoxM1 inhibits proliferation, invasion and migration of nasopharyngeal carcinoma through the epithelialto-mesenchymal transition pathway. Oncol Rep. 2015;33:2402–2410. doi: 10.3892/or.2015.3834. [DOI] [PubMed] [Google Scholar]
- 26.Jiang L, Wang P, Chen L, Chen H. Down-regulation of FoxM1 by thiostrepton or small interfering RNA inhibits proliferation, transformation ability and angiogenesis, and induces apoptosis of nasopharyngeal carcinoma cells. Int J Clin Exp Pathol. 2014;7:5450–5460. [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Wu D, Yu Q, Li L, Wu P. Prognostic value of FOXM1 in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:32298–32308. doi: 10.18632/oncotarget.15764.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandrashekar DS, Bashel B, Sah B, Creighton CJ, Poncerodriguez I, Bvsk C, Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19:649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo C-Y, Muir KW, Lam EW-F. FOXM1: from cancer initiation to progression and treatment. Biochim Biophys Acta. 2012;1819:28–37. doi: 10.1016/j.bbagrm.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Zhang Y, Zou X, Chan AWH, Zhang R, Lee TK-W, Liu H, Lau EY-T, Ho NP-Y, Lai PBS, Cheung Y-S, K.-F. To, Wong HK, Choy KW, Keng VW, Chow LMC, Chan KKY, Cheng AS, Ko BCB. CTCF-FOXM1 axis regulates tumor growth and metastasis in hepatocellular carcinoma. J Pathol. 2017; 10.1002/path.4976. [DOI] [PMC free article] [PubMed]
- 31.Chen P-M, Wu T-C, Shieh S-H, Wu Y-H, Li M-C, Sheu G-T, Cheng Y-W, Chen C-Y, Lee H. MnSOD promotes tumor invasion via upregulation of FoxM1-MMP2 axis and related with poor survival and relapse in lung adenocarcinomas. Mol Cancer Res. 2013;11:261–271. doi: 10.1158/1541-7786.MCR-12-0527. [DOI] [PubMed] [Google Scholar]
- 32.Mencalha AL, Binato R, Ferreira GM, Du Rocher B, Abdelhay E. Forkhead box M1 (FoxM1) gene is a new STAT3 transcriptional factor target and is essential for proliferation, survival and DNA repair of K562 cell line. PLoS One. 2012;7:e48160. doi: 10.1371/journal.pone.0048160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia L, Huang W, Tian D, Zhu H, Zhang Y, Hu H, Fan D, Nie Y, Wu K. Upregulated FoxM1 expression induced by hepatitis B virus X protein promotes tumor metastasis and indicates poor prognosis in hepatitis B virus-related hepatocellular carcinoma. J Hepatol. 2012;57:600–612. doi: 10.1016/j.jhep.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Millour J, de Olano N, Horimoto Y, Monteiro LJ, Langer JK, Aligue R, Hajji N, Lam EWF. ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Mol Cancer Ther. 2011;10:1046–1058. doi: 10.1158/1535-7163.MCT-11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian J, Luo Y, Gu X, Zhan W, Wang X. Twist1 promotes gastric cancer cell proliferation through up-regulation of FoxM1. PLoS One. 2013;8:e77625. doi: 10.1371/journal.pone.0077625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia L-M, Huang W-J, Wang B, Liu M, Zhang Q, Yan W, Zhu Q, Luo M, Zhou Z-Z, Tian D-A. Transcriptional up-regulation of FoxM1 in response to hypoxia is mediated by HIF-1. J Cell Biochem. 2009;106:247–256. doi: 10.1002/jcb.21996. [DOI] [PubMed] [Google Scholar]
- 37.Xia L, Mo P, Huang W, Zhang L, Wang Y, Zhu H, Tian D, Liu J, Chen Z, Zhang Y, Chen Z, Hu H, Fan D, Nie Y, Wu K. The TNF-alpha/ROS/HIF-1-induced upregulation of FoxMI expression promotes HCC proliferation and resistance to apoptosis. Carcinogenesis. 2012;33:2250–2259. doi: 10.1093/carcin/bgs249. [DOI] [PubMed] [Google Scholar]
- 38.Dai B, Gong A, Jing Z, Aldape KD, Kang S-H, Sawaya R, Huang S. Forkhead box M1 is regulated by heat shock factor 1 and promotes glioma cells survival under heat shock stress. J Biol Chem. 2013;288:1634–1642. doi: 10.1074/jbc.M112.379362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu C, Liu D, Zhang Y, Lou G, Huang G, Chen B, Shen X, Gao M, Gong W, Zhou P, Dai S, Zeng Y, He F. LXRalpha-mediated downregulation of FOXM1 suppresses the proliferation of hepatocellular carcinoma cells. Oncogene. 2014;33:2888–2897. doi: 10.1038/onc.2013.250. [DOI] [PubMed] [Google Scholar]
- 40.Barsotti AM, Prives C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene. 2009;28:4295–4305. doi: 10.1038/onc.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurinna S, Stratton SA, Coban Z, Schumacher JM, Grompe M, Duncan AW, Barton MC. p53 regulates a mitotic transcription program and determines ploidy in normal mouse liver. Hepatology. 2013;57(2013):2004. doi: 10.1002/hep.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Millour J, Constantinidou D, Stavropoulou AV, Wilson MSC, Myatt SS, Kwok JM-M, Sivanandan K, Coombes RC, Medema RH, Hartman J, Lykkesfeldt AE, Lam EW-F. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance. Oncogene. 2010;29:2983–2995. doi: 10.1038/onc.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horimoto Y, Hartman J, Millour J, Pollock S, Olmos Y, Ho K-K, Coombes RC, Poutanen M, Makela SI, El-Bahrawy M, Speirs V, Lam EW-F. ERbeta1 represses FOXM1 expression through targeting ERalpha to control cell proliferation in breast cancer. Am J Pathol. 2011;179:1148–1156. doi: 10.1016/j.ajpath.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, Moore DD, Trumpp A. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology. 2008;48:1302–1311. doi: 10.1002/hep.22475. [DOI] [PubMed] [Google Scholar]
- 45.Delpuech O, Griffiths B, East P, Essafi A, Lam EW-F, Burgering B, Downward J, Schulze A. Induction of Mxi1-SR alpha by FOXO3a contributes to repression of Myc-dependent gene expression. Mol Cell Biol. 2007;27:4917–4930. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halasi M, Gartel AL. A novel mode of FoxM1 regulation: positive auto-regulatory loop. Cell Cycle. 2009;8:1966–1967. doi: 10.4161/cc.8.12.8708. [DOI] [PubMed] [Google Scholar]
- 47.Cheng X-H, Black M, Ustiyan V, Le T, Fulford L, Sridharan A, Medvedovic M, Kalinichenko VV, Whitsett JA, Kalin TV. SPDEF inhibits prostate carcinogenesis by disrupting a positive feedback loop in regulation of the Foxm1 oncogene. PLoS Genet. 2014;10:e1004656. doi: 10.1371/journal.pgen.1004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gartel AL. Targeting FOXM1 auto-regulation in cancer. Cancer Biol Ther. 2015;16:185–186. doi: 10.4161/15384047.2014.987566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang N, Wang C, Wang Z, Zona S, Lin S-X, Wang X, Yan M, Zheng F-M, Li S-S, Xu B, Bella L, Yong J-S, Lam EW-F, Liu Q. FOXM1 recruits nuclear aurora kinase a to participate in a positive feedback loop essential for the self-renewal of breast cancer stem cells. Oncogene. 2017;36:3428–3440. doi: 10.1038/onc.2016.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 3’ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Z, Yan Y, Qian L, Gong Z. Long non-coding RNAs act as regulators of cell autophagy in diseases (Review) Oncol Rep. 2017;37:1359–1366. doi: 10.3892/or.2017.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152:1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamura S, Imai-Sumida M, Tanaka Y, Dahiya R. Interaction and cross-talk between non-coding RNAs. Cell Mol Life Sci. 2017; 10.1007/s00018-017-2626-6. [DOI] [PMC free article] [PubMed]
- 54.Wang J-M, Ju B-H, Pan C-J, Gu Y, Li M-Q, Sun L, Xu Y-Y, Yin L-R. MiR-214 inhibits cell migration, invasion and promotes the drug sensitivity in human cervical cancer by targeting FOXM1. Am J Transl Res. 2017;9:3541–3557. [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Xie T, Mao X, Xue L, Chu X, Chen L. MicroRNA-149 increases the sensitivity of colorectal Cancer cells to 5-fluorouracil by targeting Forkhead box transcription factor FOXM1. Cell Physiol Biochem. 2016;39:617–629. doi: 10.1159/000445653. [DOI] [PubMed] [Google Scholar]
- 56.Li X-R, Chu H-J, Lv T, Wang L, Kong S-F, Dai S-Z. miR-342-3p suppresses proliferation, migration and invasion by targeting FOXM1 in human cervical cancer. FEBS Lett. 2014;588:3298–3307. doi: 10.1016/j.febslet.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 57.Wang S-H, Ma F, Tang Z-H, Wu X-C, Cai Q, Zhang M-D, Weng M-Z, Zhou D, Wang J-D, Quan Z-W. Long non-coding RNA H19 regulates FOXM1 expression by competitively binding endogenous miR-342-3p in gallbladder cancer. J Exp Clin Cancer Res. 2016;35:160. doi: 10.1186/s13046-016-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen F, Bai G, Li Y, Feng Y, Wang L. A positive feedback loop of long noncoding RNA CCAT2 and FOXM1 promotes hepatocellular carcinoma growth. Am J Cancer Res. 2017;7:1423–1434. [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y-J, Dominguez-Brauer C, Wang Z, Asara JM, Costa RH, Tyner AL, Lau LF, Raychaudhuri P. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. J Biol Chem. 2009;284:30695–30707. doi: 10.1074/jbc.M109.007997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma RYM, Tong THK, Cheung AMS, Tsang ACC, Leung WY, Yao K-M. Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J Cell Sci. 2005;118:795–806. doi: 10.1242/jcs.01657. [DOI] [PubMed] [Google Scholar]
- 61.Alvarez-Fernandez M, Halim VA, Aprelia M, Laoukili J, Mohammed S, Medema RH. Protein phosphatase 2A (B55alpha) prevents premature activation of forkhead transcription factor FoxM1 by antagonizing cyclin a/cyclin-dependent kinase-mediated phosphorylation. J Biol Chem. 2011;286:33029–33036. doi: 10.1074/jbc.M111.253724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luscher-Firzlaff JM, Lilischkis R, Luscher B. Regulation of the transcription factor FOXM1c by cyclin E/CDK2. FEBS Lett. 2006;580:1716–1722. doi: 10.1016/j.febslet.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 63.Laoukili J, Alvarez M, Meijer LAT, Stahl M, Mohammed S, Kleij L, Heck AJR, Medema RH. Activation of FoxM1 during G2 requires cyclin a/Cdk-dependent relief of autorepression by the FoxM1 N-terminal domain. Mol Cell Biol. 2008;28:3076–3087. doi: 10.1128/MCB.01710-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu Z, Malureanu L, Huang J, Wang W, Li H, van Deursen JM, Tindall DJ, Chen J. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol. 2008;10:1076–1082. doi: 10.1038/ncb1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Major ML, Lepe R, Costa RH. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol Cell Biol. 2004;24:2649–2661. doi: 10.1128/MCB.24.7.2649-2661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan Y, Raychaudhuri P, Costa RH. Chk2 mediates stabilization of the FoxM1 transcription factor to stimulate expression of DNA repair genes. Mol Cell Biol. 2007;27:1007–1016. doi: 10.1128/MCB.01068-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Li Y, Xue J, Gong A, Yu G, Zhou A, Lin K, Zhang S, Zhang N, Gottardi CJ, Huang S. Wnt-induced deubiquitination FoxM1 ensures nucleus beta-catenin transactivation. EMBO J. 2016;35:668–684. doi: 10.15252/embj.201592810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang J, Yuan C, Wu J, Elsayed Z, Fu Z. Polo-like kinase 1-mediated phosphorylation of Forkhead box protein M1b antagonizes its SUMOylation and facilitates its mitotic function. J Biol Chem. 2015;290:3708–3719. doi: 10.1074/jbc.M114.634386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, Komuves L, French DM, Ferrando RE, Lam C, Compaan D, Yu C, Bosanac I, Hymowitz SG, Kelley RF, Dixit VM. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 70.Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. doi: 10.1101/gad.14.6.655.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park HJ, Costa RH, Lau LF, Tyner AL, Raychaudhuri P. Anaphase-promoting complex/cyclosome-CDH1-mediated proteolysis of the forkhead box M1 transcription factor is critical for regulated entry into S phase. Mol Cell Biol. 2008;28:5162–5171. doi: 10.1128/MCB.00387-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laoukili J, Alvarez-Fernandez M, Stahl M, Medema RH. FoxM1 is degraded at mitotic exit in a Cdh1-dependent manner. Cell Cycle. 2008;7:2720–2726. doi: 10.4161/cc.7.17.6580. [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Arceci A, Bird K, Mills CA, Choudhury R, Kernan JL, Zhou C, Bae-Jump V, Bowers A, Emanuele MJ. VprBP/DCAF1 regulates the degradation and nonproteolytic activation of the cell cycle transcription factor FoxM1. Mol Cell Biol. 2017;37 10.1128/MCB.00609-16. [DOI] [PMC free article] [PubMed]
- 74.Jeffery JM, Kalimutho M, Johansson P, Cardenas DG, Kumar R, Khanna KK. FBXO31 protects against genomic instability by capping FOXM1 levels at the G2/M transition. Oncogene. 2017;36:1012–1022. doi: 10.1038/onc.2016.268. [DOI] [PubMed] [Google Scholar]
- 75.Kongsema M, Zona S, Karunarathna U, Cabrera E, Man EPS, Yao S, Shibakawa A, Khoo U-S, Medema RH, Freire R, Lam EW-F. RNF168 cooperates with RNF8 to mediate FOXM1 ubiquitination and degradation in breast cancer epirubicin treatment. Oncogene. 2016;5:e252. doi: 10.1038/oncsis.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karunarathna U, Kongsema M, Zona S, Gong C, Cabrera E, Gomes AR, Man EPS, Khongkow P, Tsang JW-H, Khoo U-S, Medema RH, Freire R, Lam EW-F. OTUB1 inhibits the ubiquitination and degradation of FOXM1 in breast cancer and epirubicin resistance. Oncogene. 2016;35:1433–1444. doi: 10.1038/onc.2015.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Zhou X, Xu M, Weng W, Zhang Q, Yang Y, Wei P, Du X. OTUB1-catalyzed deubiquitination of FOXM1 facilitates tumor progression and predicts a poor prognosis in ovarian cancer. Oncotarget. 2016;7:36681–36697. doi: 10.18632/oncotarget.9160.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schimmel J, Eifler K, Sigurethsson JO, Cuijpers SAG, Hendriks IA, Verlaan-de Vries M, Kelstrup CD, Francavilla C, Medema RH, Olsen JV, Vertegaal ACO. Uncovering SUMOylation dynamics during cell-cycle progression reveals FoxM1 as a key mitotic SUMO target protein. Mol Cell. 2014;53:1053–1066. doi: 10.1016/j.molcel.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Wang C-M, Liu R, Wang L, Nascimento L, Brennan VC, Yang W-H. SUMOylation of FOXM1B alters its transcriptional activity on regulation of MiR-200 family and JNK1 in MCF7 human breast cancer cells. Int J Mol Sci. 2014;15:10233–10251. doi: 10.3390/ijms150610233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Myatt SS, Kongsema M, Man CW-Y, Kelly DJ, Gomes AR, Khongkow P, Karunarathna U, Zona S, Langer JK, Dunsby CW, Coombes RC, French PM, Brosens JJ, Lam EW-F. SUMOylation inhibits FOXM1 activity and delays mitotic transition. Oncogene. 2014;33:4316–4329. doi: 10.1038/onc.2013.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jaiswal N, John R, Chand V, Nag A. Oncogenic human papillomavirus 16E7 modulates SUMOylation of FoxM1b. Int J Biochem Cell Biol. 2015;58:28–36. doi: 10.1016/j.biocel.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Lv C, Zhao G, Sun X, Wang P, Xie N, Luo J, Tong T. Acetylation of FOXM1 is essential for its transactivation and tumor growth stimulation. Oncotarget. 2016;7 10.18632/oncotarget.11332. [DOI] [PMC free article] [PubMed]
- 83.Cohn O, Feldman M, Weil L, Kublanovsky M, Levy D. Chromatin associated SETD3 negatively regulates VEGF expression. Nat Publ Gr. 2016:1–10. 10.1038/srep37115. [DOI] [PMC free article] [PubMed]
- 84.Gartel AL. FOXM1 in Cancer: interactions and vulnerabilities. Cancer Res. 2017;77:3135–3139. doi: 10.1158/0008-5472.CAN-16-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wierstra I, Alves J. Transcription factor FOXM1c is repressed by RB and activated by cyclin D1/Cdk4. Biol Chem. 2006;387:949–962. doi: 10.1515/BC.2006.119.. [DOI] [PubMed] [Google Scholar]
- 86.Kalinichenko VV, Major ML, Wang X, Petrovic V, Kuechle J, Yoder HM, Dennewitz MB, Shin B, Datta A, Raychaudhuri P, Costa RH. Foxm1b transcription factor is essential for development of hepatocellular carcinomas and is negatively regulated by the p19 ARF tumor suppressor. Genes Dev. 2004;18:830–850. doi: 10.1101/gad.1200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bhat UG, Jagadeeswaran R, Halasi M, Gartel AL. Nucleophosmin interacts with FOXM1 and modulates the level and localization of FOXM1 in human cancer cells. J Biol Chem. 2011;286:41425–41433. doi: 10.1074/jbc.M111.270843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joshi K, Banasavadi-Siddegowda Y, Mo X, Kim S-H, Mao P, Kig C, Nardini D, Sobol RW, Chow LML, Kornblum HI, Waclaw R, Beullens M, Nakano I. MELK-dependent FOXM1 phosphorylation is essential for proliferation of glioma stem cells. Stem Cells. 2013;31:1051–1063. doi: 10.1002/stem.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan C, Liu Y, Shen J, Curtis A, Newman C, Hock JM, Li X. Novel interactions between FOXM1 and CDC25A regulate the cell cycle. PLoS One. 2012;7:e51277. doi: 10.1371/journal.pone.0051277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang L, He K, Yan S, Yang Y, Gao X, Zhang M, Xia Z, Huang Z, Huang S, Zhang N. Metadherin/astrocyte elevated gene-1 positively regulates the stability and function of forkhead box M1 during tumorigenesis. Neuro-Oncology. 2017;19:352–363. doi: 10.1093/neuonc/now229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu M-D, Wang Y, Weng W, Wei P, Qi P, Zhang Q, Tan C, Ni S-J, Dong L, Yang Y, Lin W, Xu Q, Huang D, Huang Z, Ma Y, Zhang W, Sheng W, Du X. A positive feedback loop of lncRNA-PVT1 and FOXM1 facilitates gastric Cancer growth and invasion. Clin Cancer Res. 2017;23:2071–2080. doi: 10.1158/1078-0432.CCR-16-0742. [DOI] [PubMed] [Google Scholar]
- 92.Mónica AF, Medema RH. Novel functions of FoxM1: from molecular mechanisms to cancer therapy. Front Oncol. 2013;3:30. doi: 10.3389/fonc.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Halasi M, Gartel AL. Targeting FOXM1 in cancer. Biochem Pharmacol. 2013;85:644–652. doi: 10.1016/j.bcp.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 94.Wierstra I. FOXM1 (Forkhead box M1) in tumorigenesis. Overexpression in human cancer, implication in tumorigenesis, oncogenic functions, tumor-suppressive properties, and target of anticancer therapy. 1st ed: Elsevier Inc; 2013. 10.1016/B978-0-12-407190-2.00016-2. [DOI] [PubMed]
- 95.Petrovic V, Costa RH, Lau LF, Raychaudhuri P, Tyner AL. Negative regulation of the oncogenic transcription factor FoxM1 by thiazolidinediones and mithramycin. Cancer Biol Ther. 2010;9:1008–1016. doi: 10.4161/cbt.9.12.11710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dong GZ, Ji HJ, Lee YI, Lee SY, Zhao HY, Jeon R, Lee HJ, Ryu JH. Diarylheptanoids suppress proliferation of pancreatic cancer PANC-1 cells through modulating shh-Gli-FoxM1 pathway. Arch Pharm Res. 2017;40:509–517. doi: 10.1007/s12272-017-0905-2. [DOI] [PubMed] [Google Scholar]
- 97.Gartel AL. Thiazole antibiotics Siomycin a and Thiostrepton inhibit the transcriptional activity of FOXM1. Front Oncol. 2013;3:150. doi: 10.3389/fonc.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hegde NS, Sanders DA, Rodriguez R, Balasubramanian S. The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat Chem. 2011;3:829. doi: 10.1038/nchem.1162. [DOI] [PubMed] [Google Scholar]
- 99.Sun L, Ren X, Wang IC, Pradhan A, Zhang Y, Flood HM, Han B, Whitsett JA, Kalin TV, Kalinichenko VV. The FOXM1 inhibitor RCM-1 suppresses goblet cell metaplasia and prevents IL-13 and STAT6 signaling in allergen-exposed mice. Sci Signal. 2017;10:eaai8583. doi: 10.1126/scisignal.aai8583. [DOI] [PubMed] [Google Scholar]
- 100.Halasi M, Hitchinson B, Shah BN, Váraljai R, Khan I, Benevolenskaya EV, Gaponenko V, Arbiser JL, Gartel AL. Honokiol is a FOXM1 antagonist. Cell Death Dis. 2018;9:84. doi: 10.1038/s41419-017-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73. doi: 10.1186/s12916-016-0623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prots I, Skapenko A, Lipsky PE, Schulzekoops H. Analysis of the transcriptional Program of developing induced regulatory T cells. PLoS One. 2011;6:e16913. doi: 10.1371/journal.pone.0016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.M.C. Gage, N. Bécares, R. Louie, K.E. Waddington, Y. Zhang, T.H. Tittanegro, S. Rodr’\iguez-Lorenzo, A. Jathanna, B. Pourcet, O.M. Pello, J. V la Rosa, A. Castrillo, I. Pineda-Torra, Disrupting LXRα phosphorylation promotes FoxM1 expression and modulates atherosclerosis by inducing macrophage proliferation, Proc Natl Acad Sci (2018). doi:10.1073/pnas.1721245115. [DOI] [PMC free article] [PubMed]
- 105.Yokomine K, Senju S, Nakatsura T, Irie A, Hayashida Y, Ikuta Y, Harao M, Imai K, Baba H, Iwase H. The forkhead box M1 transcription factor as a candidate of target for anti-cancer immunotherapy, Int. J Cancer J Int Du Cancer. 2010;126:2153–2163. doi: 10.1002/ijc.24836.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Teh MT. Wins Molecule of the Year 2010. 2010. http://ismcbbpr.synthasite.com/molyearnews/foxm1-wins-molecule-of-the-year-2010.
- 107.Gormally MV, Dexheimer TS, Marsico G, Sanders DA, Lowe C, Matak-Vinkovic D, Michael S, Jadhav A, Rai G, Maloney DJ, Simeonov A, Balasubramanian S. Suppression of the FOXM1 transcriptional programme via novel small molecule inhibition. Nat Commun. 2014;5:5165. doi: 10.1038/ncomms6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gartel AL. FoxM1 inhibitors as potential anticancer drugs. Expert Opin Ther Targets. 2008;12:663–665. doi: 10.1517/14728222.12.6.663. [DOI] [PubMed] [Google Scholar]
- 109.Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, Cocito A, Amati B. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17:1115–1129. doi: 10.1101/gad.1067003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan H, Zhu Y, Wei W, Shao S, Rui X. Transcription factor FoxM1 is the downstream target of c-Myc and contributes to the development of prostate cancer. World J Surg Oncol. 2018;16:59. doi: 10.1186/s12957-018-1352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McGovern UB, Francis RE, Peck B, Guest SK, Wang J, Myatt SS, Krol J, Kwok JM-M, Polychronis A, Coombes RC, Lam EW-F. Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol Cancer Ther. 2009;8:582–591. doi: 10.1158/1535-7163.MCT-08-0805. [DOI] [PubMed] [Google Scholar]
- 112.Pandit B, Halasi M, Gartel AL. p53 negatively regulates expression of FoxM1. Cell Cycle. 2009;8:3425–3427. doi: 10.4161/cc.8.20.9628. [DOI] [PubMed] [Google Scholar]
- 113.Zhang T, Ma G, Zhang Y, Huo H, Zhao Y. miR-216b inhibits glioma cell migration and invasion through suppression of FoxM1. Oncol Rep. 2017;38:1751–1759. doi: 10.3892/or.2017.5824. [DOI] [PubMed] [Google Scholar]
- 114.Sun M, Wang X, Tu C, Wang S, Qu J, Xiao S. microRNA-216b inhibits cell proliferation and migration in human melanoma by targeting FOXM1 in vitro and in vivo. Cell Biol Int. 2017; 10.1002/cbin.10754. [DOI] [PubMed]
- 115.Zheng W-W, Zhou J, Zhang C-H, Liu X-S. MicroRNA-216b is downregulated in hepatocellular carcinoma and inhibits HepG2 cell growth by targeting Forkhead box protein M1. Eur Rev Med Pharmacol Sci. 2016;20:2541–2550. [PubMed] [Google Scholar]
- 116.Hou XW, Sun X, Yu Y, Zhao HM, Yang ZJ, Wang X, Cao XC. miR-361-5p suppresses lung cancer cell lines progression by targeting FOXM1. Neoplasma. 2017;64:526–534. doi: 10.4149/neo_2017_406. [DOI] [PubMed] [Google Scholar]
- 117.Weng W, Okugawa Y, Toden S, Toiyama Y, Kusunoki M, Goel A. FOXM1 and FOXQ1 are promising prognostic biomarkers and novel targets of tumor-suppressive miR-342 in human colorectal Cancer. Clin Cancer Res. 2016;22:4947–4957. doi: 10.1158/1078-0432.CCR-16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tan X, Fu Y, Chen L, Lee W, Lai Y, Rezaei K, Tabbara S, Latham P, Teal CB, Man Y-G, Siegel RS, Brem RF, Fu SW. miR-671-5p inhibits epithelial-to-mesenchymal transition by downregulating FOXM1 expression in breast cancer. Oncotarget. 2016;7:293–307. doi: 10.18632/oncotarget.6344.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Y. Ke, W. Zhao, J. Xiong, R. Cao, miR-149 inhibits non-small-cell lung Cancer cells EMT by targeting FOXM1., Biochem Res Int 2013 (2013) 506731. doi:10.1155/2013/506731. [DOI] [PMC free article] [PubMed]
- 120.Ma N, Zhang W, Qiao C, Luo H, Zhang X, Liu D, Zang S, Zhang L, Bai J. The tumor suppressive role of MiRNA-509-5p by targeting FOXM1 in non-small cell lung Cancer. Cell Physiol Biochem. 2016;38:1435–1446. doi: 10.1159/000443086. [DOI] [PubMed] [Google Scholar]
- 121.Yuan F, Wang W. MicroRNA-802 suppresses breast cancer proliferation through downregulation of FoxM1. Mol Med Rep. 2015;12:4647–4651. doi: 10.3892/mmr.2015.3921. [DOI] [PubMed] [Google Scholar]
- 122.Xu X, Chen W, Miao R, Zhou Y, Wang Z, Zhang L, Wan Y, Dong Y, Qu K, Liu C. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget. 2015;6:3988–4004. doi: 10.18632/oncotarget.2905.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Duan N, Hu X, Yang X, Cheng H, Zhang W. MicroRNA-370 directly targets FOXM1 to inhibit cell growth and metastasis in osteosarcoma cells. Int J Clin Exp Pathol. 2015;8:10250–10260. [PMC free article] [PubMed] [Google Scholar]
- 124.Yungang W, Xiaoyu L, Pang T, Wenming L, Pan X. miR-370 targeted FoxM1 functions as a tumor suppressor in laryngeal squamous cell carcinoma (LSCC) Biomed Pharmacother. 2014;68:149–154. doi: 10.1016/j.biopha.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 125.Feng Y, Wang L, Zeng J, Shen L, Liang X, Yu H, Liu S, Liu Z, Sun Y, Li W, Chen C, Jia J. FoxM1 is overexpressed in helicobacter pylori-induced gastric carcinogenesis and is negatively regulated by miR-370. Mol Cancer Res. 2013;11:834–844. doi: 10.1158/1541-7786.MCR-13-0007. [DOI] [PubMed] [Google Scholar]
- 126.Huang X, Qin J, Lu S. Up-regulation of miR-877 induced by paclitaxel inhibits hepatocellular carcinoma cell proliferation though targeting FOXM1. Int J Clin Exp Pathol. 2015;8:1515–1524. [PMC free article] [PubMed] [Google Scholar]
- 127.Wan L-Y, Deng J, Xiang X-J, Zhang L, Yu F, Chen J, Sun Z, Feng M, Xiong J-P. miR-320 enhances the sensitivity of human colon cancer cells to chemoradiotherapy in vitro by targeting FOXM1. Biochem Biophys Res Commun. 2015;457:125–132. doi: 10.1016/j.bbrc.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 128.Li T, Ma J, Han X, Jia Y, Yuan H, Shui S, Guo D. MicroRNA-320 enhances Radiosensitivity of glioma through down-regulation of Sirtuin type 1 by directly targeting Forkhead box protein M1. Transl Oncol. 2018;11:205–212. doi: 10.1016/j.tranon.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun Y, Yu X, Bai Q. miR-204 inhibits invasion and epithelial-mesenchymal transition by targeting FOXM1 in esophageal cancer. Int J Clin Exp Pathol. 2015;8:12775–12783. [PMC free article] [PubMed] [Google Scholar]
- 130.Inoguchi S, Seki N, Chiyomaru T, Ishihara T, Matsushita R, Mataki H, Itesako T, Tatarano S, Yoshino H, Goto Y, Nishikawa R, Nakagawa M, Enokida H. Tumour-suppressive microRNA-24-1 inhibits cancer cell proliferation through targeting FOXM1 in bladder cancer. FEBS Lett. 2014;588:3170–3179. doi: 10.1016/j.febslet.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 131.Park TJ, Kim JY, Oh SP, Kang SY, Kim BW, Wang HJ, Song KY, Kim HC, Lim IK. TIS21 negatively regulates hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1 regulation loop. Hepatology. 2008;47:1533–1543. doi: 10.1002/hep.22212. [DOI] [PubMed] [Google Scholar]
- 132.Li L, Pan D, Chen H, Zhang L, Xie W. F-box protein FBXL2 inhibits gastric cancer proliferation by ubiquitin-mediated degradation of forkhead box M1. FEBS Lett. 2016;590:445–452. doi: 10.1002/1873-3468.12071. [DOI] [PubMed] [Google Scholar]
- 133.Liu J, Guo S, Li Q, Yang L, Xia Z, Zhang L, Huang Z, Zhang N. Phosphoglycerate dehydrogenase induces glioma cells proliferation and invasion by stabilizing forkhead box M1. J Neuro-Oncol. 2013;111:245–255. doi: 10.1007/s11060-012-1018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kruiswijk F, Hasenfuss SC, Sivapatham R, Baar MP, Putavet D, Naipal KAT, van den Broek NJF, Kruit W, van der Spek PJ, van Gent DC, Brenkman AB, Campisi J, Burgering BMT, Hoeijmakers JHJ, de Keizer PLJ. Targeted inhibition of metastatic melanoma through interference with Pin1-FOXM1 signaling. Oncogene. 2016;35:2166–2177. doi: 10.1038/onc.2015.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Halasi M, Varaljai R, Benevolenskaya E, Gartel AL. A novel function of molecular chaperone HSP70: SUPPRESSION OF ONCOGENIC FOXM1 AFTER PROTEOTOXIC STRESS. J Biol Chem. 2016;291:142–148. doi: 10.1074/jbc.M115.678227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xiang Q, Tan G, Jiang X, Wu K, Tan W, Tan Y. Suppression of FOXM1 transcriptional activities via a single-stranded DNA aptamer generated by SELEX. Sci Rep. 2017;7:45377. doi: 10.1038/srep45377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bi Z, Liu W, Ding R, Wu Y, Dou R, Zhang W, Xue Y, Liu X, Xiong L, Guo Z. A novel peptide, 9R-P201, strongly inhibits the viability, proliferation and migration of liver cancer HepG2 cells and induces apoptosis by down-regulation of FoxM1 expression. Eur J Pharmacol. 2016;796:175–189. doi: 10.1016/j.ejphar.2016.12.029. [DOI] [PubMed] [Google Scholar]