Abstract
The tumor microenvironment (tumor cells are located in the internal and external environment) is vital for the occurrence, growth and metastasis of tumors. An increasing number of studies have shown that exosomes are closely related to the tumor microenvironment. The mechanisms involved, however, are unclear. The focus of this review is on the exosome-related tumor microenvironment and other relevant factors, such as hypoxia, inflammation and angiogenesis. Many studies have suggested that exosomes are important mediators of metastasis, angiogenesis, and immune modulation in the tumor microenvironment. Additionally, exosomes can be isolated from bodily fluids of cancer patients, including urine, blood, saliva, milk, tumor effusion, cerebrospinal fluid, amniotic fluid and so on. Consequently, exosomes are potential biomarkers for clinical predictions and are also good drug carriers because they can cross the biofilm without triggering an immune response. Collectively, these findings illustrate that exosomes are crucial for developing potential targets for a new generation of pharmaceutical therapies that would improve the tumor microenvironment.
Keywords: exosome, tumor microenvironment, angiogenesis, hypoxia, inflammation
Introduction
It is acknowledged that cancer is the deadliest disease, responsible for disrupting human health worldwide. Cancer has long been widely studied by the scientific community, and more researchers are performing cancer research. Currently, there are many studies on cancer, but studies show that the effects of chemotherapy and radiation therapy on tumor cells themselves not only demonstrate adverse reactions but also unsatisfactory treatment effects. The main problem in current research is ignoring tumor microenvironment, the environment which tumor cells live in, which plays an important role in the evolution of cancer. The tumor microenvironment is a complex internal environment that is important for tumor survival and includes various stromal cells, extracellular matrix components and biological molecules. The important cellular components of the tumor microenvironment include fibroblasts, macrophages, inflammatory cells and important biomolecules, including various growth factors, inflammatory factors, and proteases 1-3. Factors related to the tumor microenvironment, such as hypoxia 4, inflammation, angiogenesis 5 and exosomes, play pivotal roles in tumor development, invasion and metastasis 6. Matrix cells in the tumor microenvironment communicate with cancer cells through non-cell material, such as exosomes, and play crucial roles in tumor evolution and progression 7-9. Exosomes can function as transporters in the tumor microenvironment and as biomarkers in clinical diagnoses 10. Exosomes can help miRNA to be released into the tumor microenvironment 11, and miRNA plays vital roles in many biological functions in the tumor microenvironment, such as mediation of proliferation, apoptosis, migration and invasiveness of tumor cells 12. The mRNA distribution in exosomes of tumor patients can be used to identify tumor biomarkers and provide a more specific target for the treatment and prediction of tumors.
1. Tumor microenvironment
During tumor development, the tumor microenvironment is a pivotal pathological environment and can even serve as a bridge. Tumor development, recurrence and metastases are not only determined by cancer cells but are also associated with non-tumor cells of the tumor microenvironment. It is increasingly recognized that the tumor microenvironment, which includes cells such as macrophages, dendritic cells, T cells, endothelial cells and fibroblasts, as well as extracellular matrix (ECM) components, proteases, and cytokines, plays a role in tumor evolution and metastasis 2, 8, 13. The tumor microenvironment plays a vital role in the transition from invasion growth before tumor cells invade the matrix 14. In most cases, the late-stage matrix supports the development of the tumor more than the early stage matrix 15. Stephen Paget in 1889 proposal that metastasis depends on cross-talk between selected cancer cells (the seeds) and specific tumor microenvironments (the soil) shows how cancer cells (the seeds) adapt to their environment (the soil). Therefore, this hypothesis is a good illustration of the relationships between cancer cells and the tumor microenvironment 16. Traditional tumor studies focus on the tumor cells themselves, the seeds in the “seeds and soil” hypothesis, but neglect soil research, while the microenvironment, the soil of tumor cell growth and metastasis, is quite vital 17. There is a large difference between the tumor microenvironment and normal tissue, and although the target hallmarks of the tumor microenvironment may improve cancer therapies, the tumor microenvironment is characterized by various pathologic responses, such as hypoxia, inflammation and angiogenesis 18-20.
2. Exosome-related tumor microenvironment
We know that the tumor microenvironment is very important in cancer development and that exosomes are a significant component of the tumor microenvironment 10. Exosomes are thought to be between 30 and 100 nanometers in size with a classical “cup” or “dish” morphology and are released by almost all cell types, non-tumor cells or tumor cells. Exosomes carrying a broad range of cargoes, including nucleic acids, heat shock proteins and various enzymes 7, 10, 21-25. Exosomes can promote cancer metastasis and influence other cells as follows: (1) the exosomes that come from tumor cells induce epithelial mesenchymal transition and degrade the matrix; (2) tumor-secreted exosomes directly or indirectly disturb endothelial cells via activating macrophages; (3) circulating tumor cells (CSTs) and the tumor activate platelets to release exosomes, influencing immune cells; (4) exosomes that are attached to the tumor up-regulate adhesive molecules on endothelial cells; and (5) exosomes can disseminate tumor cells in a suitable niche to proliferate, forming a micro-metastasis 26.
2.1 Exosome and cell-to-cell communication
Exosomes are important components of extracellular vehicles that can mediate intercellular communication between tumor microenvironment components and play a role in the formation and development of tumors 27. Presently, there are four main forms of communication between exosomes and cells: first, exosomes act as signaling complexes to stimulate target cells; second, exosomes transmit receptors between cells; third, exosomes transport functional proteins to receptor cells; and fourth, exosomes deliver genetic information to receptor cells via mRNA and miRNA 28. Tumor cells can not only release more exosomes than normal cells but the contents of exosomes from tumors are also significantly different than those from normal cells. A large difference between exosomes from cancer cells and those from normal cells is that exosomes from cancer cells contain more miRNA than those from normal cells 29. The two-way communication between tumor cells and the stroma or tumor can significantly affect the progression of disease and sensitivity of the tumor to treatment, and extracellular vesicles mediate the communication between cancer cells and the neighboring microenvironment 30.
2.2 Exosomes and pathways
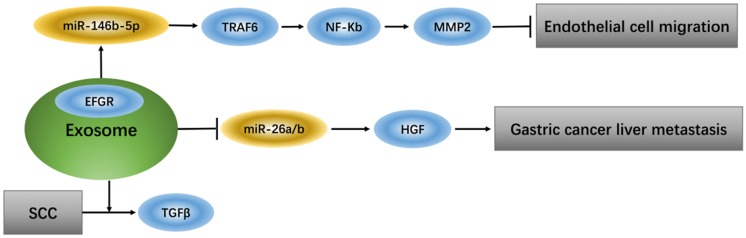
Exosomes that deliver epidermal growth factor receptor (EGFR) play a key role in regulating signaling pathways of endothelial cells because EGFR, which changes position, can effectively activate hepatocyte growth factor (HGF) by suppressing expression of its upstream miRNA (miR-26a/b). Additionally, up-regulation of liver HGF promotes gastric cancer liver metastasis, while down-regulation of liver HGF suppresses metastasis 31. TGFβ type II receptor (TβRII) is a common component in exosomes from squamous cell carcinoma (SCC) tumor cells and can stimulate TGFβ signaling in the tumor microenvironment 32. Tumor-associated macrophage (TAM)-derived exosomes from ovarian cancer can target the miR-146b-5p/TRAF6/NF-κB/MMP2 pathway to suppress endothelial cell migration 33 (Figure 1).
Figure 1.
Exosomes regulate signaling pathway of endothelial cells
3. Other factors related to the tumor microenvironment
3.1 Hypoxia and angiogenesis (VEGF)
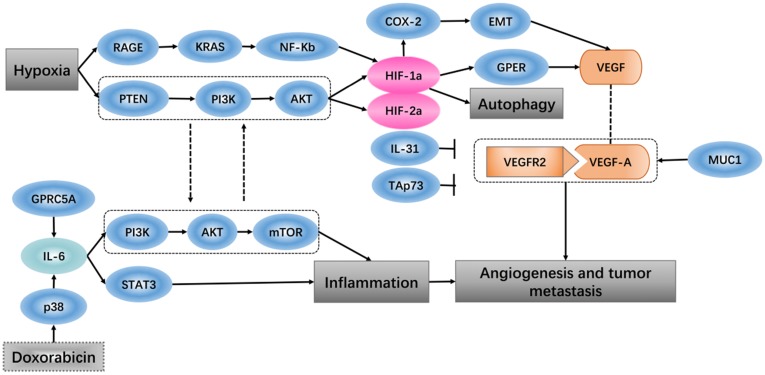
Hypoxia-inducible factor 1 (HIF-1) is thought to be the key effector of the cell response to hypoxia, which is involved in promoting angiogenesis-related transcription genes 34. When breast cancer cells and cancer-associated fibroblasts are subjected to hypoxia, HIF-1a/GPER signaling participates in the regulation of VEGF expression 35. An in vivo study showed that the tumor ascorbate levels are negatively associated with tumor growth and that HIF-1 and its target proteins can control angiogenesis 36. Expression of vascular endothelial growth factor (VEGF) is found at high levels in some hypoxic tumors. In vitro experiments have shown that soluble vascular endothelial growth factor receptor-2 (sVEGFR2) specifically binds to human vascular endothelial growth factor-A (VEGF-A), which inhibits tumor growth, and the high expression level of sVEGFR2 under hypoxic conditions plays a role in inhibition of the tumor angiogenesis 37. It has been shown that the PTEN/PI3K/AKT signaling axis, which proceeds through the proteasome, controls hypoxia-induced HIF1a to avoid hypoxic degradation in macrophages and that this signaling pathway controls tumor-induced angiogenesis 38.
3.1.1 Angiogenesis
The tumor vasculature is a key component of the microenvironment and not only influences tumor behavior but is also related to the treatment effect 39. Angiogenesis is a process that is associated with trauma, embryonic formation and solid tumors. The influence of proangiogenic factor overproduction in tumors leads to deformed vessel structures. When tumor blood vessels are abnormal, blood perfusion is low after hypoxia perfusion, leading to a microenvironment that promotes metastasis and invasion 40. In more than 80% of pancreatic ductal adenocarcinoma, the tumor-related transmembrane glycoprotein MUC1 is overexpressed, and increasing the expression levels of neuropilin-1 (NRP1, a co-receptor of VEGF) and its ligand VEGF lead to a proangiogenic tumor microenvironment 41. PI3K modulates the tumor vasculature, either directly (by inhibiting endothelial cells) or indirectly (by inhibiting angiogenesis-promoting, tumor-associated myeloid cells and VEGF production by tumor cells) 42. Identification of specific and non-redundant roles of class I PI3K isoforms in the tumor microenvironment has revealed the way that this hub regulates cancer progression 43. Pro-inflammatory cytokines in the tumor microenvironment are known for their ability to either inhibit or promote cancer progression. Interleukin-31 (IL-31), which belongs to the pro-inflammatory IL-6 cytokine family, has been characterized in autoimmune disease and tumorigenesis, and IL-31 inhibits tumor growth partly via an anti-angiogenic effect 44. In the tumor microenvironment, high expression of the extracellular matrix component tenascin-C (TNC) is related to decreased patient survival. TNC also has direct antiangiogenic effects on endothelial cells, and via tumor cells and cancer-associated fibroblasts, TNC regulates paracrine proangiogenic signal transduction 45. TAp73, a member of the p53 family, regulates tumor angiogenesis by inhibiting proangiogenic and pro-inflammatory cytokines 46.
3.1.2 Hypoxia
Oxygen homeostasis is required by the body to generate energy. A decrease in oxygen or excess oxygen can be deleterious for cellular adaptation and growth. Hypoxia has a role not only in pathological conditions but also in physiological conditions 47. A hypoxic solid tumor microenvironment can promote tumor metastasis, epithelial mesenchymal transition and angiogenesis 48. In various tumors, hypoxia is associated with a poor response to treatment, such that hypoxia-inducible factor 1 regulates many genes that have critical cellular functions, leading cancer cells to adapt to the hypoxic environment 49. It has been reported that the expression levels of HIF-1α and tumor-infiltrating lymphocytes (TILs) are positively correlated with the tumor microenvironment of esophageal squamous cell carcinoma and that patients with high levels of HIF-1α and TILs have the worst survival rates 50. Thus, in gastric cancer cells, the hypoxic environment independently induces angiopoietin-like protein 4 and HIF-1α, which have adverse effects on the progression of the tumor 51. Recent research has shown that in the tumor microenvironment, hypoxia and HIF-1α impact heat shock protein 70 (Hsp70) and the major histocompatibility class I chain-related proteins A and B (MICA/B), which are located on the cell membrane and, as ligands, affect the sensitivity of natural killer cell-mediated cytotoxicity 52. Hypoxia-dependent and -independent alterations in immunological surveillance lead to different immune avoidance tactics 53. There is some evidence that, in tumors, hypoxic induction factor (HIF) plays an inhibitory role in the regulation of the immune response 54, 55. In hepatocellular carcinoma (HCC), transcatheter arterial chemoembolization (TACE) surgery increases the hypoxia status, and in HCC tissue, HIF-1α protein expression increases. HIF-1α stimulates the expression of COX-2 protein and positively regulates EMT, which promotes HCC invasion and metastasis, resulting in a poor prognosis 56. Regarding pancreatic cancer, in a hypoxic tumor microenvironment, expression of receptor for advanced glycation end products (RAGE) is mainly controlled by NF-κB, but not HIF-1α, and RAGE is positively regulates KRAS-driven HIF-1α signaling and hypoxia-induced autophagy via the RAGE-KRAS-HIF1α pathway 57. Pancreatic cancer cells exist in the hypoxic microenvironment and contain numerous factors that impact tumor survival, proliferation and metastasis, such as HIF-1α, which up-regulates and then induces miR-21 overexpression, allowing cells to avoid apoptosis 58. In some late glioblastoma hypoxia microenvironments, IL-1b has been found to inhibit the transactivation activity of HIF-1, inducing expression of adrenomedullin (AM), which helps glioblastoma cells fight apoptosis induced by hypoxia 59. In the tumor microenvironment, either chronic or cycling hypoxia enhances the expression or function of Livin, which promotes anti-apoptosis and resistance to ionizing radiation and temozolomide in glioblastoma 60. Cycling hypoxia contributes to the resistance to cytotoxic therapies through anti‑apoptotic effects, cycling hypoxia-induced Bcl‑xL expression via ROS‑mediated HIF‑1α and NF‑κB activation, which plays an important role in the tumor microenvironment 61.
3.2 Inflammation
In many tumors, inflammatory infiltration is a marker that results in the continued release of pro-inflammatory, anti-inflammatory, and immunosuppressive cytokines in the tumor microenvironment 62. The effect of the pro-inflammatory environment on cancer is to promote carcinogenesis by providing cytokines, growth factors and chemokines to maintain the cell proliferation rate, stimulate angiogenesis and inhibit cell apoptosis 63. Inflammation acts as a double-edged sword; on the one hand, the protective response of inflammation can eliminate harmful stimuli and restore tissue homeostasis. On the other hand, uncontrolled inflammation leads to the development of malignancies 64, 65. In many solid tumors, due to the inflammatory microenvironment, cells express a high concentration of macrophage-associated markers, which indicate a poor clinical consequence 66. A study showed that, in the tumor microenvironment, the pro-inflammatory cytokine IL-6, which is produced by endothelial cells, promotes chemical resistance, indicating that the chemotherapy drug doxorubicin induces the release of acute IL-6 in vitro through reactive oxygen-mediated p38 activation. Doxorubicin leads to endothelial senescence mainly through the senescence-related inflammatory factor IL-6, which functions in the PI3K/AKT/mTOR pathway 67. Prostate-specific IL-6 can autonomously induce prostate oncogenesis by expanding local inflammation, activating the STAT3 pathway and increasing paracrine insulin-like growth factor (IGF) signaling to reprogram gene expression68. We know that G protein-coupled receptor family C group 5 member A (GPRC5A) is a lung tumor suppressor that can be induced by retinoic acid, and overexpression of GPRC5A in head and neck squamous cells can suppress the activation of IL-6-induced STAT3 and inhibit tumor growth 69 (Figure 2).
Figure 2.
Hypoxia and angiogenesis, inflammation
4. Exosomes in cancer
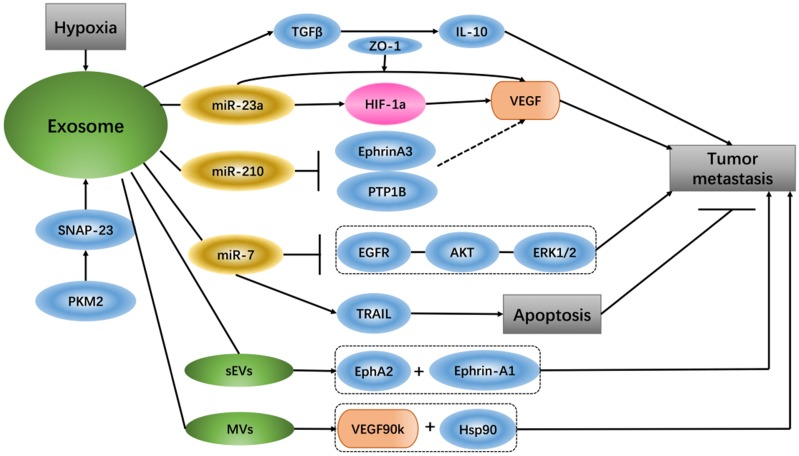
It has been reported that glioma-derived exosomes that contain linc-POU3F3 can regulate the tumor microenvironment and influence angiogenesis. HBMECs treated with exosomes that have increased amounts of linc-POU3F3 can upregulate Angio, bFGFR, VEGF and bFGF expression, and these gene and protein expression levels are mainly pro-angiogenesis factors in angiogenesis regulation 70. Exosomes contain high levels of miR-210 in the hypoxic tumor environment, and exosome-released miR-210 from hypoxic cells can inhibit Ephrin-A3 and PTP1B, which are the target genes of miR-210 and have an intimate connection with VEGF expression and endothelial cell recruitment 71. Under hypoxic conditions, lung cancer cells produce more exosomes than under normoxic conditions, and the expression levels of miR-23a are up-regulated in these exosomes. Hypoxia lung cancer-derived exosomal miR-23a induces prolyl hydroxylase 1/2 and tight junction protein ZO-1 to activate vascular endothelial cells; in simple terms, hypoxia tumor cells increase angiogenesis and permeability by regulating the exosomal miR-23a →PHD1/2 →HIF-1α and →ZO-1 regulatory pathway 72. Studies have shown that small extracellular vesicles (sEVs) can be used as important media by senescent cells to promote tumor progression. The sEVs released by senescent cells carry EphA2, which can combine with ephrin-A1 and EphA2/ephrin-A1 to reverse signaling, eventually promoting cancer cell proliferation 73. A special extracellular vesicle subgroup, microvesicles (MVs), from breast cancer cells activates VEGF receptors and promotes tumor angiogenesis, mainly via 90-kDa VEGF (VEGF90k), which interacts with Hsp90 and then combines with the vesicles. MV-associated VEGF90k is weakly expressed following exposure to bevacizumab, which is ineffective at blocking the activation of related VEGF receptors in MVs, and using an Hsp90 inhibitor that affects the release of VEGF90K from MVs can restore the sensitivity of VEGF90K to bevacizumab 74.
In in vivo and in vitro experiments of hepatocellular carcinoma (HCC), exosomes from HCC can induce sorafenib resistance, mainly via the HGF/c-Met/Akt signaling pathway, and inhibit sorafenib-induced apoptosis 75. Compared with un-activated macrophages, exosomes from M2 macrophages express a higher level of miR-21. In gastric cancer cells, exosomal transfer of miR-21 from M2 macrophages mediates the resistance to DDP as well as the down-regulation of PTEN and up-regulation AKT. The apoptosis-associated gene Bcl-2 is also increased along with miR-21 overexpression 76. miR-21 delivered by exosomes, comes from neighboring stromal cells in the omental tumor microenvironment, is transferred to ovarian cancer cells can conferring chemo-resistance. In ovarian cancer, APAF1 is the downstream target of miR-21, and their mRNA expression is inversely correlated. Additionally, APAF1 can mediate miR-21-induced paclitaxel resistance in ovarian cancer 77. It has been reported that exosomes from breast cancer cells treated with paclitaxel (PTX) promote cell survival and chemo-resistance. PTX-treated cells generate exosomes enriched with survivin, and survivin from exosomes promotes cell survival 78. It was discovered that shikonin inhibits the proliferation of MCF-7 cells by reducing tumor-derived exosomal miR-128, while miR-128 in exosomes negatively regulates the gene level of Bax in MCF-7 cells and promotes cell proliferation 79. Exosomes involved in miR-7 can mediate apoptosis and inhibit tumor growth because miR-7 is a key sensitizer for TRAIL (TNF-related apoptosis inducing ligand)-induced apoptosis, and XIAP is the direct downstream of miR-7 80. It has been reported that miRNAs from exosomes can be used as intercommunication media between tumor cells and macrophages. TWEAK stimulation increases the level of miR-7 in both macrophages and macrophage-secreted exosomes. The level of miR-7 in recipient epithelial ovarian cancer (EOC) cells is increased due to EOC cell ingestion of the corresponding exosomes, and miR-7 eventually weakens EGFR/AKT/ERK1/2 pathway signal conduction related to EOC transfer 81. Gemcitabine increases cancer-associated fibroblast (CAF)-derived exosome release, and these exosomes play a role in the up-regulation of Snail and miR-146a expression. Snail is a chemo-resistance-inducing factor in recipient epithelial cells that promotes proliferation and drug resistance 82. Tumor cell-derived extracellular vesicles (EVs) can cause apoptosis of human mesenchymal cells, which leads to the destruction of the peritoneal cortex and promotion of metastasis of tumor cells. EVs from ascites of ovarian cancer patients carry MMP1 mRNA, which can induce apoptosis of interstitial cells 83. In cancer, exosomes act as communicators between tumors and their microenvironment, and exosome secretion due to lung cancer cell regulation causes cancer cell migration via TGF-β and IL-10 84. Pyruvate kinase type M2 (PKM2) is an important enzyme of anaerobic glycolysis in tumor cells that plays a pivotal role in exosome release from tumor cells. Phosphorylated synaptosome-associated protein 23 (SNAP-23) is the phosphorylated substrate of PKM2 in tumor cells, which regulates the retention and release of exosomes, PKM2 phosphorylation and dimers not only transforms tumor cell metabolism from oxidative phosphorylation to anaerobic glycolysis but also promotes the secretion of exosomes in tumor cells by direct phosphorylation of SNAP23 85 (Figure 3).
Figure 3.
Exosomes in cancer
5. Tumor-derived exosomal miRNA as biomarkers
An early diagnosis is important for the prognosis of cancer. miRNAs in tumor-derived exosomes are potential diagnostic biomarkers for early-stage, non-small cell lung cancer (NSCLC) for next-generation sequencing compared with normal healthy cells. In NSCLC, miR-181-5p, miR-30a-3p, miR-30e-3p and miR-361-5p in exosomes are specific biomarkers for adenocarcinoma, and miR-10b-5p, miR-15b-5p and miR-320b in exosomes are specific biomarkers for squamous cell carcinoma 86. miR-96 in exosomes is a serum biomarker for lung cancer, and its expression is positively correlated with high-grade, metastatic lung cancer. Expression of LIM-domain-only protein 7 (LMO7) is inversely related to the grade in lung cancer, and LMO7 overexpression has an inhibitory effect on miR-96 87. In the tumor microenvironment, miR-25-3p and miR-92a-3p are released into the serum by liposarcoma (LPS) cells through extracellular vehicles, and these miRNAs can be used as potential diagnostic biomarkers. miR-25-3p and miR-92a-3p, in a TLR7/8-dependent manner, activate tumor-associated macrophages to induce IL-6 secretion. In turn, IL-6 promotes LPS cell proliferation, invasion and transfer through interactions with the surrounding environment 88.
Conclusions
Studies on tumors have mainly focused on the tumor cells, ignoring the microenvironment of tumor survival. The tumor microenvironment is a good soil for tumor growth, proliferation, invasion and metastasis 89. Exosome are widely distributed in different body fluids and have a long half-life. Exosomes can influence the occurrence and development of tumors by interacting with various factors in the tumor microenvironment, such as hypoxia-, inflammation- and angiogenesis-associated factors. Exosomes are nano-scale vesicles that are secreted by cells. As carriers, exosomes from tumor cells carry genetic material and proteins to other cells, leading to important information transmission between cells 90. Intercellular communication is an indispensable cornerstone for the maintenance and development of multicellular tissue. The tumor microenvironment is a local environment composed of tumor cells with local infiltrating immune cells and stromal cells as well as the active media secreted by them.
Additionally, tumor-derived exosomes, through interactions with other cells of the tumor microenvironment, can regulate angiogenesis, tumor progression, metastasis and immune escape. The pathological processes mediated by exosomes give exosomes large potential as biomarkers 91. Understanding the mechanism of exosomes is of great value for early screening, accurate diagnosis and prognosis assessment. Exosomes play an important role in tumor metastasis. They locate the position of transfer and activate specific cells to secrete specific cytokines to recruit cells to form the microenvironment before the tumor metastasizes. Exosomes induce transformation of epithelial mesenchymal cells and have the ability to migrate as well as promote the migration of tumor cells. Thus, miRNA can be transported to tumor cells to regulate the expression of corresponding genes that affect tumor cell growth. Through different proteins, miRNA can promote angiogenesis and induce immune escape, affecting the occurrence of metastatic tumor development.
Exosomes that are secreted can be used as the medium of signal transduction in tumor sensitivity to chemotherapy drugs and participate in the tumor process. Studying the mechanism of drug resistance by exosomes is helpful to better understand and treat neoplasms. Exosomes can be stable in urine, blood, saliva, milk, tumor effusion, cerebrospinal fluid, amniotic fluid and other fluids, especially in the tumor microenvironment. Exosomes can move back and forth in fluid, allowing them to cross the biofilm and deliver drugs without triggering an immune response 92. However, it is difficult to purify exosomes, which is a difficult problem to overcome for current research and application.
Acknowledgments
This work was supported by grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the Research Foundation of Education Bureau of Jiangsu Province (16KJA360001) (to X.Z.), the National Natural Science Foundation of China (81503374) (to M.C.) and the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007-2013/ under REA grant agreement No. PIRSES-GA-2013-612589: CHETCH (China and Europe taking care of healthcare solutions). The authors thank the Editor and Reviewers for their significant contributions during the revision period.
Author Contributions
Dr Xu Zhang designed the review. Cheng Hu, Meijuan Chen, Rilei Jiang and Yuanyuan Guo collected the data from publications. Cheng Hu developed the database, wrote the manuscript, and edited the final text. Dr Xu Zhang and Dr Mianhua Wu provided financial support. All authors read and approved the final manuscript.
References
- 1.Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y. et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC medicine. 2015;13:45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou J, Shahi P, Werb Z. microRNA-mediated regulation of the tumor microenvironment. Cell cycle. 2013;12:3262–71. doi: 10.4161/cc.26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Q, Yan X, Chen K, Huang Q, Melancon MP, Lopez G. et al. Macrophages as a potential tumor-microenvironment target for noninvasive imaging of early response to anticancer therapy. Biomaterials. 2018;152:63–76. doi: 10.1016/j.biomaterials.2017.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochimica et biophysica acta. 2016;1863:382–91. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee E, Pandey NB, Popel AS. Crosstalk between cancer cells and blood endothelial and lymphatic endothelial cells in tumour and organ microenvironment. Expert reviews in molecular medicine. 2015;17:e3. doi: 10.1017/erm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y. et al. Role of tumor microenvironment in tumorigenesis. Journal of Cancer. 2017;8:761–73. doi: 10.7150/jca.17648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Yuan X, Shi H, Wu L, Qian H, Xu W. Exosomes in cancer: small particle, big player. Journal of hematology & oncology. 2015;8:83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteside TL. Tumor-Derived Exosomes and Their Role in Cancer Progression. Advances in clinical chemistry. 2016;74:103–41. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo W, Gao Y, Li N, Shao F, Wang C, Wang P. et al. Exosomes: New players in cancer (Review) Oncology reports. 2017;38:665–75. doi: 10.3892/or.2017.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Chen JQ, Liu JL, Tian L. Exosomes in tumor microenvironment: novel transporters and biomarkers. Journal of translational medicine. 2016;14:297. doi: 10.1186/s12967-016-1056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. Journal of molecular medicine. 2013;91:431–7. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X, Hu S, Liu Q, Qian C, Liu Z, Luo D. Exosomal microRNA remodels the tumor microenvironment. PeerJ. 2017;5:e4196. doi: 10.7717/peerj.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer letters. 2015;368:7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 14.Ma XJ, Dahiya S, Richardson E, Erlander M, Sgroi DC. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast cancer research: BCR. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Developmental cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nature reviews Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 17.Ribelles N, Santonja A, Pajares B, Llacer C, Alba E. The seed and soil hypothesis revisited: current state of knowledge of inherited genes on prognosis in breast cancer. Cancer treatment reviews. 2014;40:293–9. doi: 10.1016/j.ctrv.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Justus CR, Sanderlin EJ, Yang LV. Molecular Connections between Cancer Cell Metabolism and the Tumor Microenvironment. International journal of molecular sciences. 2015;16:11055–86. doi: 10.3390/ijms160511055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBleu VS. Imaging the Tumor Microenvironment. Cancer journal. 2015;21:174–8. doi: 10.1097/PPO.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivey JW, Bonakdar M, Kanitkar A, Davalos RV, Verbridge SS. Improving cancer therapies by targeting the physical and chemical hallmarks of the tumor microenvironment. Cancer letters. 2016;380:330–9. doi: 10.1016/j.canlet.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo X, Wei Q, Exosome: emerging biomarker in breast cancer. Oncotarget; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy. Trends in molecular medicine. 2014;20:385–93. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.H. Rashed M, Bayraktar E, K. Helal G, Abd-Ellah M, Amero P, Chavez-Reyes A, et al. Exosomes: From Garbage Bins to Promising Therapeutic Targets. International Journal of Molecular Sciences. 2017;18:538. doi: 10.3390/ijms18030538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roma-Rodrigues C, Fernandes AR, Baptista PV. Exosome in Tumour Microenvironment: Overview of the Crosstalk between Normal and Cancer Cells. BioMed Research International. 2014;2014:1–10. doi: 10.1155/2014/179486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roma-Rodrigues C, Raposo LR, Cabral R, Paradinha F, Baptista PV, Fernandes AR. Tumor Microenvironment Modulation via Gold Nanoparticles Targeting Malicious Exosomes: Implications for Cancer Diagnostics and Therapy. International journal of molecular sciences; 2017. p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An T, Qin S, Xu Y, Tang Y, Huang Y, Situ B. et al. Exosomes serve as tumour markers for personalized diagnostics owing to their important role in cancer metastasis. Journal of extracellular vesicles. 2015;4:27522. doi: 10.3402/jev.v4.27522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Achreja A, Zhao H, Yang L, Yun TH, Marini J, Nagrath D. Exo-MFA - A 13C metabolic flux analysis framework to dissect tumor microenvironment-secreted exosome contributions towards cancer cell metabolism. Metabolic engineering; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney international. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 29.Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM. Exosome mediated communication within the tumor microenvironment. Journal of controlled release: official journal of the Controlled Release Society. 2015;219:278–94. doi: 10.1016/j.jconrel.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Han L, Xu J, Xu Q, Zhang B, Lam EW, Sun Y. Extracellular vesicles in the tumor microenvironment: Therapeutic resistance, clinical biomarkers, and targeting strategies. Medicinal research reviews. 2017;37:1318–49. doi: 10.1002/med.21453. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X. et al. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nature Communications. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Languino LR, Singh A, Prisco M, Inman GJ, Luginbuhl A, Curry JM. et al. Exosome-mediated transfer from the tumor microenvironment increases TGFbeta signaling in squamous cell carcinoma. American journal of translational research. 2016;8:2432–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Wu Q, Wu X, Ying X, Zhu Q, Wang X, Jiang L. et al. Suppression of endothelial cell migration by tumor associated macrophage-derived exosomes is reversed by epithelial ovarian cancer exosomal lncRNA. Cancer cell international. 2017;17:62. doi: 10.1186/s12935-017-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toth RK, Warfel NA. Strange Bedfellows: Nuclear Factor, Erythroid 2-Like 2 (Nrf2) and Hypoxia-Inducible Factor 1 (HIF-1) in Tumor Hypoxia. Antioxidants; 2017. p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini M. HIF-1alpha/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs) Breast cancer research: BCR. 2013;15:R64. doi: 10.1186/bcr3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell EJ, Vissers MC, Bozonet S, Dyer A, Robinson BA, Dachs GU. Restoring physiological levels of ascorbate slows tumor growth and moderates HIF-1 pathway activity in Gulo(-/-) mice. Cancer medicine. 2015;4:303–14. doi: 10.1002/cam4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collet G, Lamerant-Fayel N, Tertil M, El Hafny-Rahbi B, Stepniewski J, Guichard A. et al. Hypoxia-regulated overexpression of soluble VEGFR2 controls angiogenesis and inhibits tumor growth. Molecular cancer therapeutics. 2014;13:165–78. doi: 10.1158/1535-7163.MCT-13-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi S, Singh AR, Zulcic M, Durden DL. A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1alpha and HIF2alpha stability and tumor growth, angiogenesis, and metastasis. Molecular cancer research: MCR. 2014;12:1520–31. doi: 10.1158/1541-7786.MCR-13-0682. [DOI] [PubMed] [Google Scholar]
- 39.Hendry SA, Farnsworth RH, Solomon B, Achen MG, Stacker SA, Fox SB. The Role of the Tumor Vasculature in the Host Immune Response: Implications for Therapeutic Strategies Targeting the Tumor Microenvironment. Frontiers in immunology. 2016;7:621. doi: 10.3389/fimmu.2016.00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W, Li X, Liu S, Yang W, Pan F, Yang XY. et al. Gold nanoparticles attenuate metastasis by tumor vasculature normalization and epithelial-mesenchymal transition inhibition. International journal of nanomedicine. 2017;12:3509–20. doi: 10.2147/IJN.S128802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou R, Curry JM, Roy LD, Grover P, Haider J, Moore LJ. et al. A novel association of neuropilin-1 and MUC1 in pancreatic ductal adenocarcinoma: role in induction of VEGF signaling and angiogenesis. Oncogene. 2016;35:5608–18. doi: 10.1038/onc.2015.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okkenhaug K, Graupera M, Vanhaesebroeck B. Targeting PI3K in Cancer: Impact on Tumor Cells, Their Protective Stroma, Angiogenesis, and Immunotherapy. Cancer discovery. 2016;6:1090–105. doi: 10.1158/2159-8290.CD-16-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soler A, Angulo-Urarte A, Graupera M. PI3K at the crossroads of tumor angiogenesis signaling pathways. Molecular & cellular oncology. 2015;2:e975624. doi: 10.4161/23723556.2014.975624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidi S, Fremder E, Kan T, Raviv Z, Timaner M, Karin N. et al. The antiangiogenic role of the pro-inflammatory cytokine interleukin-31. Oncotarget. 2017;8:16430–44. doi: 10.18632/oncotarget.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rupp T, Langlois B, Koczorowska MM, Radwanska A, Sun Z, Hussenet T. et al. Tenascin-C Orchestrates Glioblastoma Angiogenesis by Modulation of Pro- and Anti-angiogenic Signaling. Cell reports. 2016;17:2607–19. doi: 10.1016/j.celrep.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Stantic M, Sakil HA, Zirath H, Fang T, Sanz G, Fernandez-Woodbridge A. et al. TAp73 suppresses tumor angiogenesis through repression of proangiogenic cytokines and HIF-1alpha activity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:220–5. doi: 10.1073/pnas.1421697112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar H, Choi DK. Hypoxia Inducible Factor Pathway and Physiological Adaptation: A Cell Survival Pathway? Mediators of inflammation. 2015;2015:584758. doi: 10.1155/2015/584758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu F. et al. HIF-1alpha promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. Journal of experimental & clinical cancer research: CR. 2017;36:60. doi: 10.1186/s13046-017-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li S, Wei Q, Li Q, Zhang B, Xiao Q. Down-regulating HIF-1alpha by lentivirus-mediated shRNA for therapy of triple negative breast cancer. Cancer biology & therapy. 2015;16:866–75. doi: 10.1080/15384047.2015.1040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang L, Ye SB, Li ZL, Ma G, Chen SP, He J. et al. Increased HIF-1alpha expression in tumor cells and lymphocytes of tumor microenvironments predicts unfavorable survival in esophageal squamous cell carcinoma patients. International journal of clinical and experimental pathology. 2014;7:3887–97. [PMC free article] [PubMed] [Google Scholar]
- 51.Kubo H, Kitajima Y, Kai K, Nakamura J, Miyake S, Yanagihara K. et al. Regulation and clinical significance of the hypoxia-induced expression of ANGPTL4 in gastric cancer. Oncology letters. 2016;11:1026–34. doi: 10.3892/ol.2015.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schilling D, Tetzlaff F, Konrad S, Li W, Multhoff G. A hypoxia-induced decrease of either MICA/B or Hsp70 on the membrane of tumor cells mediates immune escape from NK cells. Cell stress & chaperones. 2015;20:139–47. doi: 10.1007/s12192-014-0532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckert AW, Wickenhauser C, Salins PC, Kappler M, Bukur J, Seliger B. Clinical relevance of the tumor microenvironment and immune escape of oral squamous cell carcinoma. Journal of translational medicine. 2016;14:85. doi: 10.1186/s12967-016-0828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143:512–9. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palazon A, Aragones J, Morales-Kastresana A, de Landazuri MO, Melero I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:1207–13. doi: 10.1158/1078-0432.CCR-11-1591. [DOI] [PubMed] [Google Scholar]
- 56.Huang M, Wang L, Chen J, Bai M, Zhou C, Liu S. et al. Regulation of COX-2 expression and epithelial-to-mesenchymal transition by hypoxia-inducible factor-1alpha is associated with poor prognosis in hepatocellular carcinoma patients post TACE surgery. International journal of oncology. 2016;48:2144–54. doi: 10.3892/ijo.2016.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang R, Hou W, Zhang Q, Chen R, Lee YJ, Bartlett DL. et al. RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell death & disease. 2014;5:e1480. doi: 10.1038/cddis.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mace TA, Collins AL, Wojcik SE, Croce CM, Lesinski GB, Bloomston M. Hypoxia induces the overexpression of microRNA-21 in pancreatic cancer cells. The Journal of surgical research. 2013;184:855–60. doi: 10.1016/j.jss.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun W, Depping R, Jelkmann W. Interleukin-1beta promotes hypoxia-induced apoptosis of glioblastoma cells by inhibiting hypoxia-inducible factor-1 mediated adrenomedullin production. Cell death & disease. 2014;5:e1020. doi: 10.1038/cddis.2013.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hsieh CH, Lin YJ, Wu CP, Lee HT, Shyu WC, Wang CC. Livin contributes to tumor hypoxia-induced resistance to cytotoxic therapies in glioblastoma multiforme. Clinical cancer research: an official journal of the American Association for Cancer Research. 2015;21:460–70. doi: 10.1158/1078-0432.CCR-14-0618. [DOI] [PubMed] [Google Scholar]
- 61.Chen WL, Wang CC, Lin YJ, Wu CP, Hsieh CH. Cycling hypoxia induces chemoresistance through the activation of reactive oxygen species-mediated B-cell lymphoma extra-long pathway in glioblastoma multiforme. Journal of translational medicine. 2015;13:389. doi: 10.1186/s12967-015-0758-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez YI, Campos LE, Castro MG, Aladhami A, Oskeritzian CA, Alvarez SE. Sphingosine-1 Phosphate: A New Modulator of Immune Plasticity in the Tumor Microenvironment. Frontiers in oncology. 2016;6:218. doi: 10.3389/fonc.2016.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inacio Pinto N, Carnier J, Oyama LM, Otoch JP, Alcantara PS, Tokeshi F. et al. Cancer as a Proinflammatory Environment: Metastasis and Cachexia. Mediators of inflammation. 2015;2015:791060. doi: 10.1155/2015/791060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Q, Zhu B, Li Y. Resolution of Cancer-Promoting Inflammation: A New Approach for Anticancer Therapy. Frontiers in immunology. 2017;8:71. doi: 10.3389/fimmu.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serkova NJ. Nanoparticle-Based Magnetic Resonance Imaging on Tumor-Associated Macrophages and Inflammation. Frontiers in immunology. 2017;8:590. doi: 10.3389/fimmu.2017.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bent EH, Gilbert LA, Hemann MT. A senescence secretory switch mediated by PI3K/AKT/mTOR activation controls chemoprotective endothelial secretory responses. Genes & development. 2016;30:1811–21. doi: 10.1101/gad.284851.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu G, Zhang J, Frey L, Gang X, Wu K, Liu Q. et al. Prostate-specific IL-6 transgene autonomously induce prostate neoplasm through amplifying inflammation in the prostate and peri-prostatic adipose tissue. Journal of hematology & oncology. 2017;10:14. doi: 10.1186/s13045-016-0386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu S, Ye D, Wang T, Guo W, Song H, Liao Y. et al. Repression of GPRC5A is associated with activated STAT3, which contributes to tumor progression of head and neck squamous cell carcinoma. Cancer cell international. 2017;17:34. doi: 10.1186/s12935-017-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lang HL, Hu GW, Chen Y, Liu Y, Tu W, Lu YM. et al. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. European review for medical and pharmacological sciences. 2017;21:959–72. [PubMed] [Google Scholar]
- 71.Jung KO, Youn H, Lee CH, Kang KW, Chung JK. Visualization of exosome-mediated miR-210 transfer from hypoxic tumor cells. Oncotarget. 2017;8:9899–910. doi: 10.18632/oncotarget.14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu YL, Hung JY, Chang WA, Lin YS, Pan YC, Tsai PH. et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–42. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 73.Takasugi M, Okada R, Takahashi A, Virya Chen D, Watanabe S, Hara E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nature communications. 2017;8:15729. doi: 10.1038/ncomms15728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF. et al. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nature communications. 2017;8:14450. doi: 10.1038/ncomms14450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu Z, Wu J, Wu J, Luo D, Jiang C, Ding Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. Journal of experimental & clinical cancer research: CR. 2016;35:159. doi: 10.1186/s13046-016-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G. et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. Journal of experimental & clinical cancer research: CR. 2017;36:53. doi: 10.1186/s13046-017-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Au Yeung CL, Co NN, Tsuruga T, Yeung TL, Kwan SY, Leung CS. et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nature communications. 2016;7:11150. doi: 10.1038/ncomms11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kreger BT, Johansen ER, Cerione RA, Antonyak MA. The Enrichment of Survivin in Exosomes from Breast Cancer Cells Treated with Paclitaxel Promotes Cell Survival and Chemoresistance. Cancers; 2016. p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei Y, Li M, Cui S, Wang D, Zhang CY, Zen K, Shikonin Inhibits the Proliferation of Human Breast Cancer Cells by Reducing Tumor-Derived Exosomes. Molecules; 2016. p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang X, Zhang X, Hu S, Zheng M, Zhang J, Zhao J. et al. Identification of miRNA-7 by genome-wide analysis as a critical sensitizer for TRAIL-induced apoptosis in glioblastoma cells. Nucleic acids research. 2017;45:5930–44. doi: 10.1093/nar/gkx317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu Y, Li D, Wu A, Qiu X, Di W, Huang L. et al. TWEAK-stimulated macrophages inhibit metastasis of epithelial ovarian cancer via exosomal shuttling of microRNA. Cancer letters. 2017;393:60–7. doi: 10.1016/j.canlet.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Richards KE, Zeleniak AE, Fishel ML, Wu J, Littlepage LE, Hill R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–8. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yokoi A, Yoshioka Y, Yamamoto Y, Ishikawa M, Ikeda SI, Kato T. et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nature communications. 2017;8:14470. doi: 10.1038/ncomms14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y, Yi J, Chen X, Zhang Y, Xu M, Yang Z. The regulation of cancer cell migration by lung cancer cell-derived exosomes through TGF-beta and IL-10. Oncology letters. 2016;11:1527–30. doi: 10.3892/ol.2015.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H. et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nature communications. 2017;8:14041. doi: 10.1038/ncomms14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jin X, Chen Y, Chen H, Fei S, Chen D, Cai X, Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early stage non-small-cell lung cancer using next-generation sequencing. Clinical cancer research: an official journal of the American Association for Cancer Research; 2017. [DOI] [PubMed] [Google Scholar]
- 87.Wu H, Zhou J, Mei S, Wu D, Mu Z, Chen B. et al. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. Journal of cellular and molecular medicine. 2017;21:1228–36. doi: 10.1111/jcmm.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Casadei L, Calore F, Creighton CJ, Guescini M, Batte K, Iwenofu OH. et al. Exosome-Derived miR-25-3p and miR-92a-3p Stimulate Liposarcoma Progression. Cancer research. 2017;77:3846–56. doi: 10.1158/0008-5472.CAN-16-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hede K. Environmental protection: studies highlight importance of tumor microenvironment. Journal of the National Cancer Institute. 2004;96:1120–1. doi: 10.1093/jnci/96.15.1120. [DOI] [PubMed] [Google Scholar]
- 90.Soung YH, Nguyen T, Cao H, Lee J, Chung J. Emerging roles of exosomes in cancer invasion and metastasis. BMB reports. 2016;49:18–25. doi: 10.5483/BMBRep.2016.49.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li W, Li C, Zhou T, Liu X, Liu X, Li X. et al. Role of exosomal proteins in cancer diagnosis. Molecular cancer. 2017;16:145. doi: 10.1186/s12943-017-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. Journal of controlled release: official journal of the Controlled Release Society. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]